Abstract
Background and Aims
Dormancy in seeds of Cuscuta (Convolvulaceae, tribe Cuscuteae) is due to a water-impermeable seed coat (physical dormancy). In nondormant seeds of several species of this family, bulges adjacent to the micropyle have been identified as the initial route of water entry into seeds (water gap). However, there are claims that water enters seeds of Cuscuta spp. via the entire seed coat. Although several studies have been done on seed coat anatomy of Cuscuta, none has identified and/or characterized the morphology/anatomy of a water gap. Thus, the primary aim of this research was to identify and describe the morphology and anatomy of the water gap in seeds of Cuscuta australis. It was also determined if sensitivity cycling to dormancy-breaking treatments occurs in seeds of this species.
Methods
Light microscopy, scanning electron microscopy, tissue-sectioning and dye-tracking and blocking experiments were used to investigate the morphology and anatomy of the water gap. Treatments simulating natural conditions were used to break seed dormancy. Storage of seeds at different temperatures was tested for their effect on sensitivity to dormancy-breaking treatment.
Key Results
Dormancy-breaking treatments caused the tightly closed hilar fissure to open. Staining was observed in cells below the hilum area but not in those below the seed coat away from the hilum. Sensitivity to dormancy-breaking treatment was induced by storing seeds dry and reduced by storing them wet.
Conclusions
Whereas bulges adjacent to the micropyle act as the water gap in other species of Convolvulaceae with physical dormancy, the hilar fissure serves this function in Cuscuta. Cuscuta australis can cycle between insensitivity ↔ sensitivity to dormancy-breaking treatments.
Key words: Convolvulaceae, Cuscuta, hilar fissure, palisade layer, physical dormancy, seed coat anatomy, seed dormancy, seed germination, sensitivity cycling, water gap
INTRODUCTION
The water gap is a place on the seed (or fruit) coat of physically dormant (PY) seeds that differs morphologically and anatomically from the rest of the seed coat. In seeds of many species with PY, it serves as the environmental signal detector for germination and, once dormancy is broken, as the initial route of water entry into the seed (Baskin and Baskin, 2000; Baskin et al., 2000). Thus, ecologically the water gap is an important component of seeds with PY. A water gap has been identified and documented in 12 of the16 families with PY including Convolvulaceae (Baskin et al., 2000, 2006; Jayasuriya et al., 2007), the only family in the evolutionary-advanced Asterid clade (Angiosperm Phylogeny Group, 1998, 2003) with this class of dormancy. However, a water gap has not been identified in the genus Cuscuta (Convolvulaceae), the only holoparasitic taxon with PY (Baskin et al., 2000). Thus, it is important to study the morphological and anatomical aspects of seed dormancy and germination in Cuscuteae in order to be able to make inferences about the evolution of PY both within this family and within angiosperms in general. Some taxonomists have classified Cuscuta in its own family Cuscutaceae in orders Convolvulales (Takhtajan, 1980, 1997), Polemoniales (Cronquist, 1968) and Solanales (Cronquist, 1988; Dahlgren, 1991), whereas Thorne (1992, 2000, 2007), Austin (1998) and molecular taxonomists (APG, 1998, 2003; Neyland, 2001; Stefanovic et al., 2003; Stefanovic and Olmstead, 2004) placed Cuscuta in Convolvulaceae.
In seeds of Ipomoea lacunosa (Convolvulaceae, tribe Ipomoeeae) (Jayasuriya et al., 2007) and in those of species in other tribes of Convolvulaceae (K. M. G. G. Jayasuriya et al., unpubl. res.), bulges adjacent to the micropyle constitute the water gap. However, Hutchison and Ashton (1979) used petroleum gel to block the putative area of the water gap in seeds of Cuscuta campestris and concluded that nondormant (made nondormant by treating seeds with conc. H2SO4) C. campestris seeds take up water throughout the entire seed coat. Hutchison and Ashton (1979) observed damaged papillae in the seed coat, and thus they concluded that water enters the seed through these structures. Lyshede (1984, 1992) compared electron micrographs of nondormant seeds of C. pedicellata and of dormant and nondormant (made nondormant by percussion) seeds of C. campestris and also concluded that papillae on the seed surface are responsible for water uptake by nondormant seeds of Cuscuta. However, papillae are formed superficially from bulging cells of the epidermal layer (Hamed and Mourad, 1994), which lies above the palisade layer that is normally considered to be the water-impermeable layer in water-impermeable seeds (Baskin et al., 2000).
Extensive studies have been done on seed anatomy and embryology of Cuscuta (Tiagi, 1951; Johri and Tiagi, 1952; Teryokhin and Nikiticheva, 1982; Sampathkumar and Ayyangar, 1982; Hamed and Mourad, 1994). However, most of these studies concentrated on taxonomy. Hamed and Mourad (1994) described seed coat anatomy and surface morphology of the seed coat of C. gronovii and C. chinensis away from the hilum. Johri and Tiagi (1952) described seed development, including the hilum, of C. reflexa but from a taxonomic aspect. There have been no studies focusing on the anatomy or morphology of the water gap of any Cuscuta species.
Cycling of dormancy is a common phenomenon in seeds with PD (Baskin and Baskin, 1985), and there are claims that dormancy cycling can also occur in seeds with PY (Rolston, 1978; Norsworthy and Oliviera, 2007). However, seeds with PY become nondormant by formation of an opening in a specific morpho-anatomical area in the seed coat referred to as the water gap. Once it opens, resealing of this structure would not seem to be possible. To explain the cyclic germinability patterns of physically dormant seeds of some legumes (Taylor, 1981, 2005; Taylor and Revell, 1999; Van Assche et al., 2003) and Ipomoea lacunosa (Jayasuriya et al., 2008), a sensitivity cycling model has been proposed in which seeds cycle between sensitive and insensitive stages. In the sensitive stage, seeds respond to dormancy-breaking treatments, and in the insensitive stage they fail to respond to dormancy-breaking treatments. Sensitivity cycling has been documented in only two of 16 families that produce seeds with PY: Convolvulaceae (tribe Ipomoeeae) and Fabaceae (subfamily Papilionoideae).
Gaertner (1950) studied seed germination of 16 species of Cuscuta and came to the conclusion that a water impermeable seed coat (along with PD in some species) was responsible for dormancy in all of them. However, germination of freshly collected seeds of C. epilinum and C. epithymum was not facilitated by scarification with concentrated sulfuric acid, which worked well for the other 14 species. Meulebrouck et al. (2008) found that scarified seeds of C. epithymum require a period of cold stratification to break physiological dormancy (PD) of the embryo. Thus, they concluded that seeds of this species have combinational dormancy (PY + PD). Germination of C. europea seeds was not facilitated by mechanical scarification or by complete removal of the seed coat (Gaertner, 1956), and a significant percentage of these seeds germinated only after they were cold stratified at –8 °C and 5 °C. This suggests that seeds of C. europea also have combinational dormancy. Tingey and Allred (1961) clearly showed that seeds of C. approximata have combinational dormancy since scarified seeds required 2 weeks stratification at (5 °C) to germinate. PY has been reported for seeds of several Cuscuta species (Drummitt, 1946): C. campestris (Hutchison and Ashton, 1980; Lados, 1999; Benvenuti et al., 2005), C. trifolii (Lados, 1999), C. monogyna and C. planiflora (Salimi and Shahraeen, 2000), C. chinensis (Marambe et al., 2002), C. gronovii, C. umbrosa, C. epithymum and C. epilinum (Costea and Tardif, 2006). However, the percentages of hard seeds at dispersal can vary from plant to plant [e.g. C. campestris (Hutchison and Ashton 1979) and C. chinensis (Marambe et al., 2002)]. Lyshede (1984) claimed that seeds of C. pedicellata were nondormant at the time of dispersal; however, she did not present any data to support her conclusion.
Cuscuta australis belongs to tribe Cuscuteae in family Convolvulaceae, and it, like all of the about 145 species of Cuscuta, is a holoparasitic vine (Mabberley, 1997). Cuscuta australis occurs throughout southern Europe, in south–south-east Asia and in Australia (Liao et al., 2000). Holm et al. (1997) also reported this species in the USA. Cuscuta australis is morphologically similar to the Chinese medicinal plant C. chinensis, although there are chemical differences between the two species. Cuscuta australis also is a medicinal plant in China (Fang and Staples, 1995). This species is a weed in soybean fields in Australia, but it is not as problematic as other introduced Cuscuta species in that country (Parson and Cuthbertson, 2001). There does not appear to be any information on seed germination of Cuscuta australis, although such information is very important in formulating a strategy to control it in agricultural systems.
The objective of the present research on the seed biology of C. australis was 3-fold: (1) to determine the class of seed dormancy [i.e. do seeds have PY, PD or (PY + PD)?]; (2) to identify the initial route of water entry (water gap) into nondormant seeds and describe it morphologically and anatomically; and (3) to determine if sensitivity cycling to dormancy break occurs in seeds of this holoparasitic angiosperm.
MATERIALS AND METHODS
Seed collection
Mature fruits containing mature seeds were collected from numerous C. australis vines in Kenting (21 °57′N, 120 °47′E), Pingtung County, Southern Taiwan, Taiwan. Seeds were air dried and then mailed immediately to the University of Kentucky, USA. They were stored in plastic bottles until used. Experiments were started within 1 month after collection.
Documenting PY in C. australis using imbibition of non-treated and manually scarified seeds
Twenty-five samples of three manually scarified (individually with a scalpel) seeds each and 25 samples of five non-treated (intact) seeds each were weighed at time 0 using a digital balance. Each three-seed sample was placed on moistened filter paper in Petri dishes. At various time intervals for 52 h, seeds were removed from the filter paper, blotted dry, weighed to the nearest 0·1 mg and returned to the Petri dishes. Seeds also were monitored for 4 more days for germination and swelling.
Germination of manually scarified and non-treated seed
Five samples of four replicates of 25 scarified seeds and of four replicates of 25 nonscarified seeds were incubated on moist sand in 5·5-cm-diameter Petri dishes in light/dark (14/10 h) (approx. 40 µmol m−2 s−1, 400–700 nm, cool white fluorescent light) and in the dark at 35/20, 30/15, 25/15, 20/10 and 15/6 °C (12/12 h) temperature regimes. The 14-h photoperiod extended from 1 h before the beginning of the high temperature period to 1 h after the beginning of the low temperature period. Dark was provided by wrapping the dishes with aluminium foil. The number of germinated seeds in light/dark experiments was counted at 2-d intervals, while those in dark experiments were counted only after 2 weeks, at the end of the experiments. Radicle emergence was the criterion for germination. Percentage of swollen seeds in non-treated (fresh) seed treatments was also determined.
Breaking dormancy in intact seeds
The following treatments were applied to break dormancy of C. australis seeds: seeds dipped in boiling water for 5, 10, 15 or 20 s; seeds dry-heated at 90 °C for 5, 10, 20 or 30 min; and seeds stored dry or wet at ambient laboratory temperatures for 2 months. Following treatments, seeds were germinated at 35/20 °C in the light/dark regime described above.
Morphological changes during dormancy break
Dormant and nondormant seeds (made nondormant by dipping in boiling water for 10 s) were coated with gold palladium in a Technics Hummer VI sputter coater and scanned with a Hitachi H-800 FE scanning electron microscope. Micrographs of dormant and nondormant seeds were compared to identify changes in morphology associated with breaking PY.
Dye tracking of imbibition pathway
Ten nondormant seeds (made nondormant by dipping in boiling water for 10 s), ten dormant seeds (non-treated) and ten nondormant seeds (made nondormant by dipping in boiling water for 10 s) with the hilum painted with Thompson's Water Seal (Thompson and Formby, Inc., Memphis, TN, USA), were each placed in a saturated aqueous solution of aniline blue. Samples were removed from the dye at 15-min intervals for 90 min, blotted dry and transverse cuts made through the hilar slit by hand using a razor blade. Cuts were photographed using a Canon EOS 30 D camera with a Canon 95-mm macro lens.
Water gap anatomy
Hand and vibratome (Vibratome 1500, St Louis, MO, USA) sections (25 µm) of seeds at the hilar area were prepared. The sections were observed under an Olympus (Model BX 50) light microscope, and photographs were taken in the hilar area using a Canon EOS 30 D camera.
Effect of dry and wet storage on sensitivity
Thirty-two samples of four replicates, each containing 25 seeds, were stored on dry or wet sand at 35/20, 15/6, 5/1 °C and ambient laboratory temperatures (22–24 °C) for 1, 2, 3 or 6 months. Following storage, seeds were incubated on wet sand in Petri dishes at the 35/20 °C light/dark regime. The number of germinated and imbibed seeds was counted at 2-d intervals for 30 d. Different proportions of seeds germinated during wet storage at the different temperatures before testing germination. Therefore, final germination was corrected using the following equation.
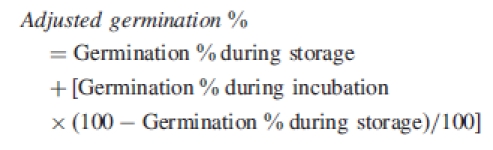 |
This adjusted germination was used in data analysis.
Ten samples of four replicates containing 25 seeds each were stored dry or wet at ambient laboratory temperature for 2 mo, after which they were incubated at 35/20, 30/15, 25/15, 20/10 and 15/6 °C in light/dark. Number of germinated seeds was counted at 2-d intervals for 30 d.
Reversing sensitivity
Four samples of four replicates, each containing 25 seeds, were stored dry at ambient laboratory temperature for 2 months, after which two of these samples each were stored dry or wet for 1 and 2 weeks at 35/20 °C. These seeds were then incubated at 35/20 °C in light/dark. The number of germinated seeds was counted at 2-d intervals for 30 d. Seeds that germinated during the 1 and 2 weeks of storage at 35/20 °C were also added to the germination observed during the 30-d period of incubation.
Four samples of four replicates, each containing 25 seeds, were stored dry at ambient laboratory temperature for 2 months, after which seeds were stored at 35/20 °C on dry sand or in sealed Petri dishes at 100 % RH for 2 or 4 weeks. An RH of 100 % was maintained in the sealed Petri dish by keeping a vial containing distilled water inside the sealed Petri dish. Seeds were incubated on wet sand at 25/15 °C after the storage. Germinated seeds were counted in 2-d intervals for 30 d.
Germination of seeds buried in soil
Two samples of three replicates of 100 seeds each were placed in nylon mesh (0·25-mm mesh diameter) bags and buried approx. 10 cm deep in glasshouse potting media in 15-cm-diameter pots. The pots were kept in a nonheated glasshouse at the University of Kentucky. One sample was watered when necessary to keep it wet, and the other sample was kept alternately wet/dry by watering it once per month. Seeds were evaluated for germination 7 d following watering. Temperature in the nonheated glasshouse was recorded using an electric thermograph. Bags were exhumed at various times over a 7-month period, and the number of germinated and of swollen seeds counted and removed from the sample. Then, the bags containing nonimbibed seeds were reburied.
Analysis of data
A completely randomized design was used in all experiments. Arcsine-transformed germination data were analysed using one-way ANOVA with SAS statistical software. Duncan's mean separation procedure was used to compare treatments.
RESULTS
Documenting PY in C. australis using imbibition of non-treated and manually scarified seeds
The mass of manually scarified seeds increased >175 % in 52 h, whereas that of non-treated (intact) seeds increased by only about 40 %, since only 13 (17 %) of the 75 non-treated seeds imbibed during the 6-d incubation period at ambient laboratory temperature. Imbibition curves for manually scarified seeds of C. australis were similar to typical seed imbibition curves.
Germination of C. australis seeds
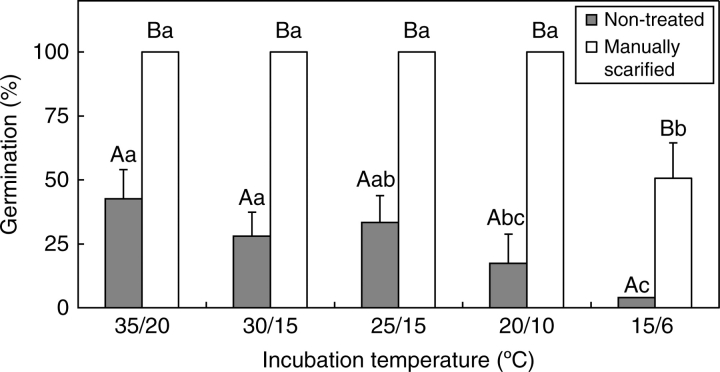
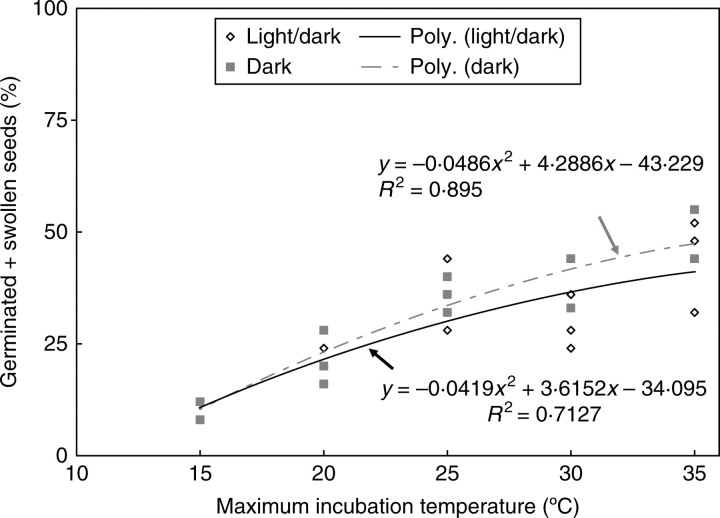
Manually scarified seeds germinated to 100 % within 30 d in all temperature regimes except 15/6 °C in light/dark (Fig. 1) and 15/6 °C and 20/10 °C in dark (data not shown). At 15/6 °C, 100 % of the seeds imbibed within 30 d, but 100 % germination was achieved only after 75 d (data not shown). Non-treated seeds germinated >30 % at 35/20, 30/15 and 25/15 °C and <25 % at 20/10 and 15/6 °C in both light/dark (Fig. 1) and dark (data not shown). All swollen seeds germinated quickly except at 15/6 °C. There is a significant 2nd degree polynomial correlation between swelling (release from PY) and maximum incubation temperature in both light/dark and dark (Fig. 2).
Fig. 1.
Germination of manually scarified and of non-treated C. australis seeds in light/dark after 30 d incubation at five temperature regimes. Different upper-case letters indicate significant differences between treatments within same temperature regimes and different lower-case letters significant differences between temperature regimes within the same treatment. UT, Non-treated; MS, manually scarified. Bars are +1 s.e.
Fig. 2.
Correlation between swollen seeds (germinated + swollen) and maximum incubation temperature in light/dark and in dark.
Breaking dormancy in intact seeds
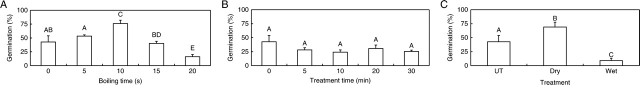
Seeds germinated >75 % at 35/20 °C (Fig. 3A) and at 25/15 °C (data not shown) after dipping in boiling water for 10 s. Germination percentage was lower after dipping seeds in boiling water for 15 and 20 s. Seeds exposed to 90 °C dry heat for 5–30 min germinated <30 % (Fig. 3B) at 35/20 °C. Seeds stored dry at ambient laboratory temperature for 2 months germinated >65 %, whereas wet-stored seeds germinated to only 8·9 % (Fig. 3C)
Fig. 3.
Germination (= imbibition) of C. australis seeds at 35/20 °C following (A) boiling, (B) dry-heat treatment and (C) dry and wet storage at ambient laboratory temperature for 2 months. Different upper-case letters indicate significant differences between treatments. UT, Non-treated. Bars are +1 s.e.
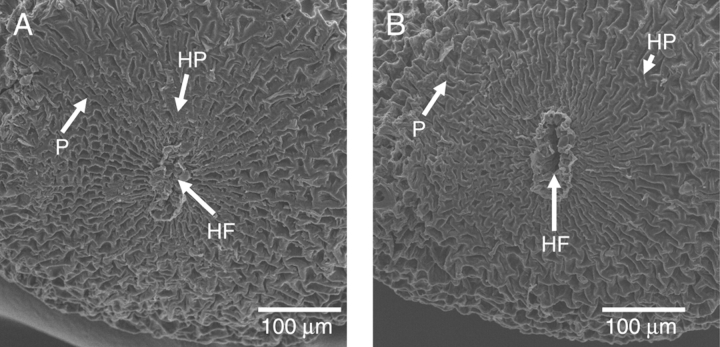
Morphological changes during dormancy break
No cracks or slits were observed in the seed coat of dormant seeds. A flat, disc-shaped hilar pad was present on one side of the seed (Fig. 4A). In the centre of the hilar pad, a tightly closed, linear-shaped hilar fissure was present. In both dormant and nondormant seeds, small polygonal-shaped papillae could be seen all over the seed coat (Fig. 4A, B). No cracks or slits were seen in the seed coat or in the papillae of nondormant seeds (made nondormant by dipping in boiling water for 10 s). However, the hilar fissure in nondormant seeds was fully open, and it was the only opening observed in these seeds (Fig. 4B).
Fig. 4.
Electron micrographs of (A) non-treated (dormant) and of (B) treated (nondormant) seeds. HF, Hilar fissure; HP, hilar pad; P, papillae.
Dye tracking of imbibition pathway
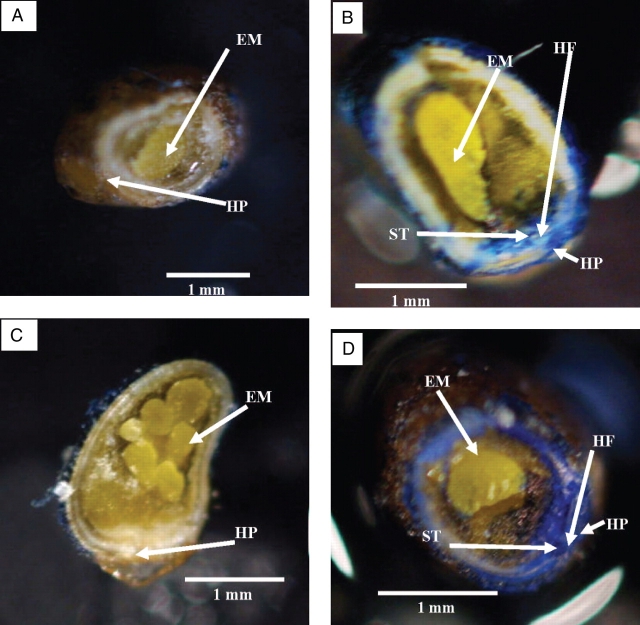
No stain had penetrated the seed coat of dormant seeds after 1 h (Fig. 5A), whereas stain was observed in tissues under the hilar fissure in nondormant seeds soaked for 1 h in aniline blue (Fig. 5B). No staining could be seen in tissues under the seed coat away from the hilar fissure. After soaking for 2 h (Fig. 5D) in aniline blue, stain was observed throughout the embryo and seed coat of nondormant seeds, whereas in dormant seeds (not shown) and in hilum-painted nondormant seeds (Fig. 5C) no stain was observed.
Fig. 5.
Photographs of (A) dormant seed, (B) nondormant seed, (C) hilum-painted nondormant seed, each of which was immersed in aniline blue for 1 h, and (D) nondormant seed immersed in aniline blue for 2 h. EM, Embryo; HF, hilar fissure; HP, hilar pad; ST, stain.
Water gap anatomy
Seed coat away from the hilum
In C. australis, the seed coat away from the hilum consists of three or four cell layers (Fig. 6A, B): one layer of bulging epidermal cells, one layer of palisade cells in the centre and one or two layers of sclereid cells. The bulging epidermal cell layer forms the papillae on the seed coat. These cells and papillae can easily fall off the seed coat. Palisade cells contain a light line (linea lucida) similar to that in seeds of other taxa with water-impermeable seed coats. Below the seed coat, remnants of the nucellus can be seen. It consists of remnants of large parenchyma cells. The innermost layer of the integument may also be crushed and mixed with the nucellar remnants.
Fig. 6.
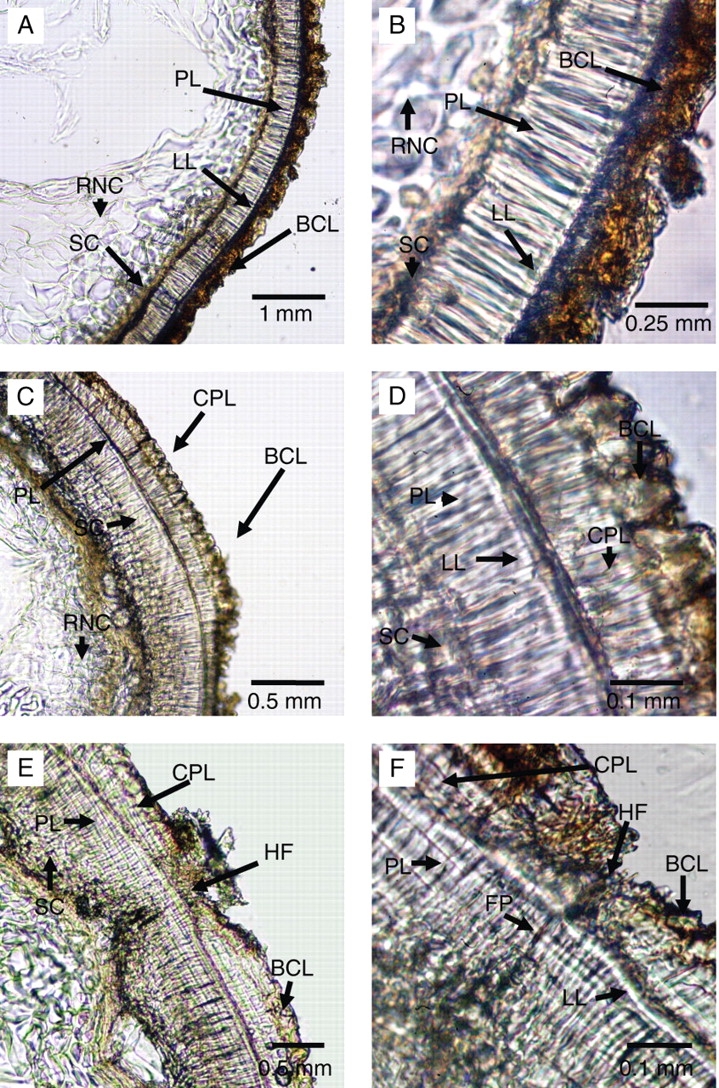
Cross-sections through seeds of C. australis showing (A and B) seed coat away from the hilar pad; (C and D) hilar pad; and (E and F) hilar fissure. BCL, Bulging cell layer; CPL, counter palisade; FP, fissure through palisade layer; HF, hilar fissure; LL, light line; PL, palisade layer; RNC, remnants of nucellar cells; SC, sclereid cells.
Hilar pad
In the hilar pad, the seed coat is thicker (Fig. 6C, D) than the seed coat away from hilar pad and contains two palisade layers. The inner palisade layer contains the light line and appears to originate from the same ontogenetical layer as the palisade layer in the seed coat away from the hilum. The outer palisade layer does not have a light line and appears to originate from the epidermal layer. On this outer (counter palisade) layer, the bulging epidermal cell layer can be seen. This layer also forms papillae on the hilar pad. Below the inner palisade layer, three or four layers of thick sclereids are present. The sclereid layer under the hilum is thicker than it is in seed coat away from the hilum. The sclereid layer consists of square-shaped cells. Remnants of the nucellus also can be seen below the sclereid layer.
In the centre of the hilar pad, a linear-shaped fissure can be seen (Fig. 6E, F). Formation of this fissure primarily is due to discontinuation of the counter palisade layer and of the bulging epidermal cells. However, there also is a suture-type discontinuity in the inner palisade layer. The inner palisade cells in the fissure region are much shorter than those in the rest of the seed coat. The size of sclereid cells under the hilar fissure is also shorter than they are in other parts of the hilar pad. There is only one layer of sclereid cells in the hilar fissure. The inner part of the fissure is filled with remnants of cells, which may be those of the nucellus and/or of the innermost layers of the integument. No remnants of the micropyle were observed in the mature seeds.
Effect of dry and wet storage on sensitivity
Germination and imbibition percentages at 35/20 °C of seeds stored at ambient laboratory temperature increased gradually to >70 % after 3 months of storage, compared with 42 % in non-treated control (data not shown). However, the germination then declined rapidly from >70 % to 42 % after 6 months of dry storage. Germination of seeds stored dry at 35/20 °C for 1 or 2 months did not differ from that of the control, but after 6 months the germination had declined to 30 %.The germination at 35/20 ° C of seeds stored dry at 15/6 °C increased gradually, and after 6 months of storage they germinated >60 %. The germination of seeds stored at 5/1 °C declined rapidly, from 40 % in the control to <10 % after 6 months of dry storage. None of the wet storage treatments increased the germination percentage, and in fact the germination of wet stored seeds at 15/6 and 5/1 °C decreased.
More than 70 % of seeds stored dry for 2 months at ambient laboratory temperature imbibed and germinated at 35/20 °C (data not shown). However, germination percentages of seeds incubated at 30/15, 25/15, 20/10 and 15/6 °C were <25.
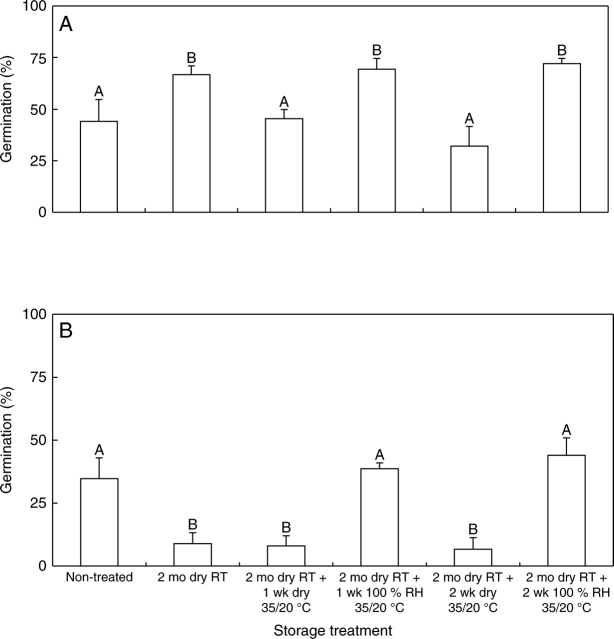
Reversing sensitivity
After 2 months of dry storage at ambient laboratory temperature, 65 % of the seeds germinated at 35/20 °C within 30 d, but after further dry storage at 35/20 °C for 1 and 2 weeks germination percentage had decreased to 48 and 32, respectively (Fig. 7A). However, germination of seeds stored wet at 35/20 °C for 1 and 2 weeks was >65 % and 72 %, respectively; the difference between 1 week and 2 weeks of wet storage was not significant.
Fig. 7.
Effect of wet vs. dry storage at 35/20 °C on germination (= imbibition) of sensitive seeds of C. australis at (A) 35/20 °C and (B) 25/15 °C. Seeds were made sensitive by storing them dry at ambient laboratory temperature for 2 months, after which they were stored wet or dry at 35/20 °C for 1 and 2 weeks. Different upper-case letters indicate significant differences between treatments. RT, Ambient laboratory temperature. Bars are +1 s.e.
Germination of non-treated seeds at 25/15 °C was 33 %, and during 2 months dry storage at ambient laboratory temperature it decreased to 20 %. Germination did not change significantly after subsequent dry storage at 35/20 °C (Fig. 7B), whereas germination of seeds stored wet at 35/20 °C for 1 and 2 weeks increased again to 38 % and 44 %, respectively. The difference between 1-week and 2-week wet-storage treatment was not significant.
Germination of seeds buried in soil
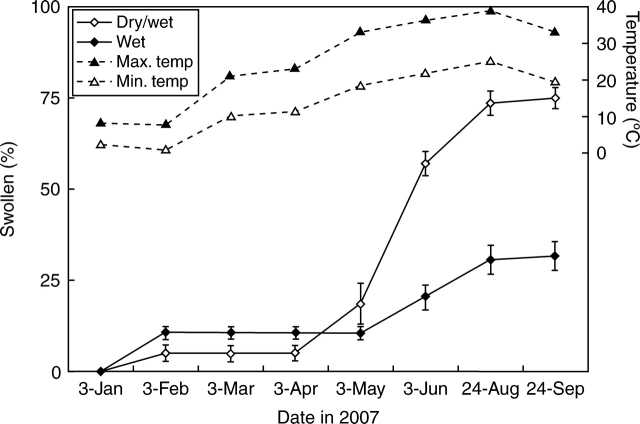
More than 5 % of seeds in both wet and dry/wet treatments had swollen after 1 month of burial (7 February; Fig. 8). As temperatures increased in the nonheated glasshouse in spring, so did the percentage of swollen seeds in both wet and dry/wet treatments. However, swelling percentage of the dry/wet treatment was significantly higher than that of the wet treatment (P < 0·001, F = 256·2, R2 = 0·98) from June to September 2007. There are significant 2nd degree polynomial correlations between the swelling percentage and temperature in both wet (P < 0·0001, F = 81·67, R2 = 0·79) and dry/wet (P < 0·0001, F = 99·04, R2 = 0·91) treatments.
Fig. 8.
Swelling of C. australis seeds buried on 3 January 2007 in continuously wet and in alternately wet/dry soil in a nonheated glasshouse. Mean daily minimum and maximum air temperatures are shown for each month of the study. Bars are ±1 s.e.
DISCUSSION
Only 17 % of non-treated C. australis seeds took up water, and thus only 17 % of the seeds germinated during a 6-d incubation period. However, all manually scarified seeds took up water rapidly and germinated. Thus, it is concluded that a high percentage (>80 %) of the fresh seeds of C. australis used in this study were physically dormant (Baskin and Baskin, 2004). Scarified seeds germinated to high percentages in all temperature regimes tested (except 15/6 °C), whereas a comparatively low percentage of intact seeds did so. Higher proportions (>30 %) of non-treated seeds germinated at high temperatures (35/20, 30/15, 25/15 °C) than at low temperatures [(≤16 %) 20/10, 15/6 °C]. Further, there was a highly significant (P < 0·0001) second-degree polynomial correlation between the percentage of swollen seeds and maximum incubation temperature. Thus, high temperature acts as the dormancy-breaking treatment for C. australis seeds. Since no cracks were observed in the seed coat of nondormant seeds and the only opening was the hilar fissure, which was tightly closed in dormant seeds, we suspected that the hilar fissure was the only route for water entry into nondormant seeds of C. australis. This hypothesis was confirmed by results of the dye-tracking experiment. Thus, stain was observed only in cells under the hilar area in nondormant seeds after they were soaked for 1 h in saturated aniline blue solution, whereas no stain penetrated the seed coat of dormant seeds or of nondormant seeds with the hilum painted.
Hutchison and Ashton (1979) blocked the hilum area of nondormant seeds (made nondormant by treating with conc. H2SO4) of C. campestris with petroleum gel and observed no difference in germination between blocked and non-treated seeds. Hence, they concluded that nondormant seeds take up water throughout the entire seed coat. Further, these authors observed damaged papillae on seed coats of nondormant seeds under the scanning electron microscope and suggested that these were sites of water entry into the seeds. They also stated that the impermeable layer in the seed coat of this species is located above the light line. However, when conc. H2SO4 was used to break PY the entire seed coat was damaged (Liu et al., 1981). Cuscuta campestris seeds would not be exposed to such high concentrations of H2SO4 in nature. Therefore, acid scarification is not the natural way by which dormancy is broken in seeds of this species. However, when C. australis seeds were dipped in boiling water for 10 s no cracks or damage were observed in the seed coat away from the hilum. The only opening was the hilar fissure. Dipping in boiling water for 10 s also is not a natural treatment for breaking PY. However, opening of the hilar fissure also occurred in seeds stored dry for 2 months at ambient laboratory temperature followed by incubation at 35/20 °C, and this condition is similar to that to which seeds of C. australis might be exposed in the field.
Lyshede (1984) studied nondormant seeds of C. pedicellata and compared them with nondormant and dormant seeds of C. campestris. She also came to the conclusion that nondormant seeds take up water through the entire seed coat. Further, Lyshede (1984) suggested that swollen papillae in nondormant seeds of C. pedicellata and swollen and damaged papillae in those of C. campestris are responsible for water uptake. However, in the present study swelling of papillae was observed in both dormant and nondormant seeds of C. australis (data not shown), yet water entered nondormant seeds of this species only via the hilar fissure. A papilla is a structure formed by bulging cells of the epidermis (Fig. 6A, B), and it lies above the impermeable layer in the seed coat, which in most instances is the palisade layer. Therefore, damaged papillae are not the route for water uptake into seeds of Cuscuta.
The water gap of Ipomoea lacunosa (tribe Ipomoeeae, Convolvulaceae) is made up of two bulges adjacent to the micropyle (Jayasuriya et al., 2007). Slits form around one or both bulges during dormancy break, thus allowing water to pass into the seed. This same type of water gap has been observed in physically dormant seeds of species of seven other tribes of Convolvulaceae (K. M. G. G. Jayasuriya et al., unpubl. res.). The water gap of C. australis (tribe Cuscuteae) differs from that of the other seven tribes in the family that have been studied. In C. australis, there are no bulges in the seed coat. However, the hilar fissure in seeds of I. hederacea and I. lacunosa are involved in the formation of slits around the bulges during dormancy break by absorbing (I. lacunosa) or losing (I. hederacea) water vapour (K. M. G. G. Jayasuriya et al., unpubl. res.). The hilar fissure acting as the water gap in Cuscuteae may be a primitive trait that was present in the common ancestor of the other tribes with PY, including Cuscuteae.
The hilum appears to be the water gap in Cercis canadensis (Caesalpinioideae, Fabaceae; Jones and Geneve, 1995). The bixoid chalazal plug in seeds of Cistaceae (Thanos and Georghiou, 1988), Bixaceae (Chopra and Kaur, 1965) and Malvaceae (Egley et al., 1986) are modified structures of the hilar pad. However, the anatomy of the bixoid chalazal plug is much more complex (Nandi, 1998) than that of the hilum of C. australis. In breaking of dormancy in seeds of Sida spinosa (Malvaceae), the chalazal plug is removed from the seed coat (Egley and Paul, 1981; Seal and Gupta, 2000), whereas in seeds of C. australis palisade cells in the hilar suture separate and the hilar fissure opens. This mechanism is somewhat similar to that suggested for dormancy break in seeds of Cercis canadensis (Jones and Geneve, 1995).
Dry storage of seeds at ambient laboratory temperature did not break PY. Instead, it increased the sensitivity of seeds to the dormancy-breaking treatment, namely incubation at 35/20 °C under wet conditions. Sensitivity was reduced in seeds stored dry at temperatures ≥35 °C or at ≤5 °C. Wet storage of seeds at 15/6 and 5/1 °C also reduced their sensitivity, whereas wet storage at 35/20 °C or at ambient laboratory temperature had no effect on sensitivity. However, after sensitivity is increased, seeds require wet incubation at a high temperature for PY to be broken. Dry storage at 35/20 °C decreased the sensitivity of seeds. Further, results of germination experiments with non-treated seeds suggest that they can become nondormant gradually at temperatures ≥25 °C. However, dry storage of non-treated seeds at ambient laboratory temperature reduced sensitivity of seeds to 25 °C but increased sensitivity to 35 °C. This means that pre-storage treatment increased the sensitivity of seeds to a narrow range of dormancy-breaking temperatures.
Jayasuriya et al. (2008) developed a conceptual model for the seed dormancy-breaking behaviour of I. lacunosa, and it can be used to explain the sensitivity dynamics of seeds of this species in their natural environment. Sensitive seeds of I. lacunosa respond quickly to dormancy-breaking treatment (Jayasuriya et al., 2008), requiring only 3 h at 35 °C and RHs >90 % to become nondormant. In contrast, seeds of C. australis require a period >2 weeks of dormancy-breaking treatment to release them from dormancy, as found for seeds of clover species in Australia (Taylor, 1981; Revell et al., 1999).
Sensitivity cycling has been observed in seeds of only two of the 16 families known to produce seeds with PY. In Fabaceae, several species have been identified that produce seeds capable of sensitivity cycling (Taylor, 1981, 2005; Taylor and Revell, 1999; Van Assche et al., 2003), whereas only one species, in the Convolvulaceae (I. lacunosa), has heretofore been reported to produce seeds showing sensitivity cycling (Jayasuriya et al., 2008). The present research provides a second example of a species in Convolvulaceae that can undergo sensitivity cycling and the first demonstration of this phenomenon in the only holoparasitic genus whose seeds have PY.
Benvenuti et al. (2005) reported cycling of dormancy in initially physically dormant seeds of C. campestris. Their interpretation of dormancy cycling in this species seems to be that seeds cycle between PD and nondormancy (PD ↔ ND) after breaking of PY. Thus, the two annual cycles of dormancy (their Fig. 2) reported for seeds of C. campestris would be as follows: PY (fresh seeds in autumn) → ND (1st spring) → PD (2nd autumn) → ND (2nd spring) → PD (3rd autumn). However, Benvenuti et al. (2005) did not present convincing evidence to support their claim that primary dormancy (PY) was broken. Thus, we suggest that the cyclic pattern of germinability of C. campestris seeds may be explained by sensitivity cycling. Thus, seeds of C. campestris may have undergone cycling of sensitivity during burial in soil, and their dormancy-breaking requirements may have been fulfilled when they were incubated at 30 °C. Hence, seeds showed a cyclic pattern of germination at 30 °C. Further support for this suggestion is that apparently seeds of C. campestris have only PY (e.g. Hutchison and Ashton, 1980; Lados, 1999). Thus, the embryo is nondormant at seed maturity. In which case, it probably does not have the capacity to change dormancy states, and as such cannot undergo dormancy cycling (see Baskin and Baskin, 2004).
Cycling of sensitivity allows seeds with PY to sense the appropriate environmental conditions for germination and subsequent seedling establishment. If seeds do not receive the appropriate environmental conditions at a time of year for both germination and subsequent completion of the life cycle, then they can revert to the insensitive stage. As such, they avoid germination in an environment that may be favourable for germination but not for completion of the life cycle. Cycling of sensitivity serves the same function in controlling timing of germination in plants whose seeds have PY as does dormancy cycling in plants whose seeds have PD (Baskin and Baskin, 1998).
ACKNOWLEDGEMENTS
We thank Mr Henry H. ‘Pete’ Southgate, College of Agriculture, University of Kentucky, for helping with scanning electron microscopy.
LITERATURE CITED
- Angiosperm Phylogeny Group. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Austin DF. Parallel and convergent evolution in the Convolvulaceae. In: Mathiews P, Sivadasan M, editors. Biodiversity and taxonomy of tropical flowering plants. Culicut, India: Mentor Books; 1998. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. BioScience. 1985;35:492–498. [Google Scholar]
- Baskin JM, Baskin CC. Evolutionary considerations of claims for physical dormancy-break by microbial action and abrasion by soil particles. Seed Science Research. 2000;10:409–413. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Benvenuti S, Dinelli G, Bonetti A, Catizone P. Germination ecology, emergence and host detection in Cuscuta campestris. Weed Research. 2005;45:270–278. [Google Scholar]
- Chopra RN, Kaur H. Embryology of Bixa orellana linn. Phytomorphology. 1965;15:211–214. [Google Scholar]
- Costea M, Tardif FJ. The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Canadian Journal of Plant Science. 2006;86:293–316. [Google Scholar]
- Cronquist A. The evolution and classification of flowering plants. Boston, MA: Houghton Mifflin; 1968. [Google Scholar]
- Cronquist A. The evolution and classification of flowering plants. 2nd edn. Bronx, NY: New York Botanical Garden; 1988. [Google Scholar]
- Dahlgren G. Steps towards a natural system of the dicotyledons embryological characters. Aliso. 1991;13:107–165. [Google Scholar]
- Drummitt M. The germination of dodder seed occurring in lespedeza. Proceedings of the Association of Official Seed Analysts. 1946;36:125–131. [Google Scholar]
- Egley GH, Paul RN. Morphological observations on the early imbibition of water by Sida spinosa (Malvaceae) seed. American Journal of Botany. 1981;68:1056–1065. [Google Scholar]
- Egley GH, Paul RN, Lax AR. Seed coat imposed dormancy: histochemistry of the region controlling onset of water entry into Sida spinosa seeds. Physiologia Plantarum. 1986;67:320–327. [Google Scholar]
- Fang R, Staples G. Convolvulaceae. In: Wu ZY, Raven PH, editors. Flora of China. Vol. 16. Beijing: Science Press; 1995. Gentianaceae through Boraginaceae. [Google Scholar]
- Gaertner EE. Studies of seed germination, seed identification, and host relationships in dodders, Cuscuta spp. Memoirs of the Cornell University Agricultural Experiment Station No. 1950:3–56. 294. [Google Scholar]
- Gaertner EE. Dormancy in the seeds of Cuscuta europea. Ecology. 1956;32:389. [Google Scholar]
- Hamed KA, Mourad MM. Seed exomorphic and anatomical characters of some species of Convolvulaceae. Egyptian Journal of Botany. 1994;34:1–16. [Google Scholar]
- Holm L, Doll J, Holm E, Pancho J, Herberger J. World weeds, natural histories and distribution. New York, NY: John Wiley & Sons; 1997. [Google Scholar]
- Hutchison JM, Ashton FM. Effect of desiccation and scarification on the permeability and structure of the seed coat of Cuscuta campestris. American Journal of Botany. 1979;66:40–46. [Google Scholar]
- Hutchison JM, Ashton FM. Germination of field dodder (Cuscuta campestris) Weed Science. 1980;28:330–333. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. Morphology and anatomy of physical dormancy in Ipomoea lacunosa: identification of the water gap in seeds of Convolvulaceae (Solanales) Annals of Botany. 2007;100:13–21. doi: 10.1093/aob/mcm070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Baskin CC. Cycling of sensitivity to physical dormancy-break in seeds of Ipomoea lacunosa (Convolvulaceae) and ecological significance. Annals of Botany. 2008;101:341–352. doi: 10.1093/aob/mcm285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri MB, Tiagi B. Floral morphology and seed formation in Cuscuta reflexa. Phytomorphology. 1952;2:162–180. [Google Scholar]
- Jones RO, Geneve RL. Seed coat structure related to germination in eastern redbud (Cercis canadensis) Journal of the American Society for Horticultural Science. 1995;120:123–127. [Google Scholar]
- Lados M. Effect of temperature, pH and host plant extract on the germination of Cuscuta trifolii and C. campestris seeds. Novenytermeles. 1999;48:367–376. [Google Scholar]
- Liao GI, Chen MY, Kuoh CS. Cuscuta L. (Convolvulaceae) in Taiwan. Taiwania. 2000;45:226–234. [Google Scholar]
- Liu NY, Khatamain H, Fretz TA. Seed coat structure of three woody legume species after chemical and physical treatments to increase seed germination. Journal of the American Society for Horticultural Science. 1981;106:691–694. [Google Scholar]
- Lyshede OB. Seed structure and germination in Cuscuta pedicellata with some notes on C. campestris. Nordic Journal of Botany. 1984;4:669–674. [Google Scholar]
- Lyshede OB. Studies on mature seeds of Cuscuta pedicellata and C. campestris by electron microscopy. Annals of Botany. 1992;69:365–371. [Google Scholar]
- Mabberley OJ. The plant book: a portable dictionary of the vascular plants. 2nd edn. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Marambe B, Wijesundara S, Tennekoon K, Pindeniya D, Jayasinghe C. Growth and development of Cuscuta chinensis Lam. and its impact on selected crops. Weed Biology and Management. 2002;2:79–83. [Google Scholar]
- Meulebrouck K, Ameloot E, Van Assche JA, Verheyen K, Hermy M, Baskin CC. Germination ecology of the holoparasite Cuscuta epithymum. Seed Science Research. 2008;18:25–34. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- Neyland R. A phylogeny inferred from large ribosomal subunit (26S) rDNA sequences suggests that Cuscuta is a derived member of Convolvulaceae. Brittonia. 2001;53:108–115. [Google Scholar]
- Norsworthy JK, Oliveira MJ. Role of light quality and temperature on pitted morningglory (Ipomoea lacunosa) germination with after-ripening. Weed Science. 2007;55:111–118. [Google Scholar]
- Parson WT, Cuthbertson EG. Noxious weeds of Australia. Collingwood, Australia: CSIRO Publishing; 2001. [Google Scholar]
- Revell CK, Taylor GB, Cocks PS. Effect of length of growing season on development of hard seeds in yellow serradella and their subsequent softening at various depths of burial. Australian Journal of Agricultural Research. 1999;50:1211–1223. [Google Scholar]
- Rolston MP. Water impermeable seed dormancy. The Botanical Review. 1978;44:365–390. [Google Scholar]
- Salimi H, Shahraeen N. Study on comparison of seed dormancy and germination of three species of dodder. Rostaniha. 2000;1:33–36. [Google Scholar]
- Sampathkumar R, Ayyangar KR. Seed morpho-anatomy of Convolvulaceae. Journal of the Indian Botanical Society. 1982;61:346–354. [Google Scholar]
- Seal S, Gupta K. Chalazal regulation of seed coat imposed dormancy in Sida species. Journal of Medicinal and Aromatic Plant Sciences. 2000;22:200–205. [Google Scholar]
- Stefanovic S, Olmstead R. Testing the phylogenetic position of a parasitic plant (Cuscuta, Convolvulaceae, Asteridae): Bayesian inference and the parametric bootstrap on data drawn from three genomes. Systematic Biology. 2004;53:384–399. doi: 10.1080/10635150490445896. [DOI] [PubMed] [Google Scholar]
- Stefanovic S, Austin DF, Olmstead RG. Classification of Convolvulaceae: a phylogenetic approach. Systematic Botany. 2003;28:791–806. [Google Scholar]
- Takhtajan A. Outline of the classification of flowering plants (Magnoliophyta) The Botanical Review. 1980;46:225–359. [Google Scholar]
- Takhtajan A. Diversity and classification of flowering plants. New York, NY: Columbia University Press; 1997. [Google Scholar]
- Taylor GB. Effect of constant temperature treatments followed by fluctuating temperatures on the softening of hard seeds of Trifolium subterraneum L. Australian Journal of Plant Physiology. 1981;8:547–558. [Google Scholar]
- Taylor GB. Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research. 2005;56:645–661. [Google Scholar]
- Taylor GB, Revell CK. Effect of pod burial, light, and temperature on seed softening in yellow serradella. Australian Journal of Agricultural Research. 1999;50:1203–1209. [Google Scholar]
- Teryokhin ES, Nikiticheva ZI. Biology and evolution of embryo and endosperm in parasitic flowering plants. Phytomorphology. 1982;32:335–339. [Google Scholar]
- Thanos CA, Georghiou K. Ecophysiology of fire stimulated germination in Cistus incanus ssp. creticus (L.) Heywood and C. salvifolius L. Plant, Cell and Environment. 1988;11:841–849. [Google Scholar]
- Thorne RF. Classification and geography of flowering plants. The Botanical Review. 1992;58:225–348. [Google Scholar]
- Thorne RF. The classification and geography of the flowering plants: dicotyledons of the class Angiospermae. The Botanical Review. 2000;66:441–647. [Google Scholar]
- Thorne RF. An updated classification of the class Magnoliopsida (“Angiospermae”) The Botanical Review. 2007;73:67–182. [Google Scholar]
- Tiagi DC. Contribution to the morphology and embryology of Cuscuta hyaline Roth and C. planiflora Lenore. Phytomorphology. 1951;1:9–21. [Google Scholar]
- Tingey DC, Allred KR. Breaking dormancy in seeds of Cuscuta approximata. Weeds. 1961;9:429–436. [Google Scholar]
- Van Assche JA, Debucquoy LA, Rommens AF. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae) New Phytologist. 2003;158:315–323. [Google Scholar]