Abstract
Background and Aims
Thesium chinense is a hemiparasitic plant that is common in grassland habitats of eastern Asia. Although the physiology of Thesium has been well studied in attempts to control its weedy habit, there have been few ecological investigations of its parasitic life history. Thesium chinense is thought to parasitize species of Poaceae, but evidence remains circumstantial.
Methods
A vegetation survey was conducted to test whether any plant species occurs significantly more often in plots with T. chinense than expected. In addition, haustorial connections were examined directly by excavating the roots and post-attachment host selectivity was evaluated by comparing the observed numbers of haustoria on different hosts against those expected according to the relative below-ground biomass. Haustorium sizes were also compared among host species.
Key Results
Only two of the 38 species recorded, Lespedeza juncea and Eragrostis curvula, occurred more often in plots with Thesium than expected. In contrast to this, T. chinense parasitized 22 plant species in 11 families, corresponding to 57·9 % of plant species found at the study site. Haustoria were non-randomly distributed among host species, suggesting that there is some post-attachment host selectivity. Thesium chinense generally preferred the Poaceae, although haustoria formed on the Fabaceae were larger than those on other hosts.
Conclusions
This is the first quantitative investigation of the host range and selectivity of hemiparasitic plants of the Santalales. The preference for Fabaceae as hosts may be linked to the greater nutrient availability in these nitrogen-fixing plants.
Key words: Haustorium, hemiparasite, host range, host selectivity, Santalaceae, Thesium chinense
INTRODUCTION
Around 1 % of angiosperm species have evolved to abstract resources from other plants in the form of root parasitism (Press and Graves, 1995). Compared with stem parasites that grow upon other plants above ground, identifying the hosts of root parasites is not straightforward in the field (Piehl, 1963; Musselman and Mann, 1977; Gibson and Watkinson, 1989). The Santalales is primarily composed of hemi- and holoparasitic plants that have a variety of life forms. However, apart from stem parasites in the Viscaceae, Loranthaceae and Misodendraceae, host associations in the remaining root parasites are little understood.
Thesium (Santalaceae) is a genus of herbaceous and woody root hemiparasites that are widely distributed in temperate and tropical regions of the Old World (Pilger, 1935). Some species are well-known agricultural weeds. The physiology of Thesium has thus been studied in an agronomic context; for example, mannitol metabolism of Thesium has been particularly well studied for the purpose of controlling its weedy habit (Fer et al., 1993; Simier et al., 1993, 1994, 1998; Williamson et al., 2002). However, very little is known about its host range and selectivity in wild populations. For example, Thesium chinense is thought to parasitize species of Poaceae (Numata and Yoshizawa, 1975), but information is based on a limited number of observations and circumstantial evidence that these plants usually occur near plants from the family Poaceae. The situation is more or less the same in other members of Thesium, but the genus as a whole seems to be capable of using a wide range of angiosperms, including Themeda and Poa (Poaceae; Scarlett et al., 2003), Galium (Rubiaceae; Renaudin et al., 1981), barley and onion (Abu-Irmaileh, 1980) and grape (Dasgupta, 1988). Therefore, it is possible that each Thesium species parasitizes several host species within a population while having some preference for particular groups of hosts (e.g. Poaceae). Hence, it is necessary to examine root associations between Thesium and potential host plants in a given population and to quantify the strength of parasitism in order to understand the host range and post-attachment selectivity of Thesium. Knowledge of the parasitic life history not only provides basic biological information, but also contributes to a better understanding of how plants of the Santalales have evolved the current diversity of parasitic life forms.
The host range and selectivity of the root hemiparasite T. chinense were examined in a wild population situated on a riverside in central Japan. First, a vegetation survey was conducted to determine whether Thesium plants are significantly more likely to occur in association with particular plant species. Plants of Thesium as well as other root parasites take up host resources through a nodule-like structure called a haustorium. The abundance of haustoria in roots of all potential hosts was therefore directly examined and quantified and the host range and selectivity of T. chinense determined. This is the first quantitative analysis of host range and selectivity in root hemiparasitic plants of Santalales.
MATERIALS AND METHODS
Study plant
Thesium chinense (Santalaceae) is a perennial herb (Fig. 1) that is usually erect, but occasionally prostrate, and has branched stems up to 60 cm in length. The plant commonly occurs in disturbed habitats such as grasslands or riversides and quickly regenerates after fire. It has narrow, linear leaves, and the flowers are cylindrical, greenish-white, and approx. 2 mm long. Thesium chinense, in common with other root and stem parasites, takes up water and nutrients from its host by means of a specialized structure known as a haustorium (Fig. 1), which provides a physical as well as physiological bridge between parasite and host (Kuijt, 1969). The haustorium constitutes a hyaline body (the structure rich in nuclei involved in resource translocation and processing; Riopel and Timko, 1995), a penetration peg (the projection that enters host tissue; Tennakoon and Cameron, 2006) and a xylem element (the channel of nutrients and water to be absorbed from the host). Field studies were conducted by the riverside of the Kizu River, Kyoto Prefecture, Japan, during November and December 2007. During the study, T. chinense was mostly fruiting, but some plants were still in flower.
Fig. 1.

(A) Riverside vegetation with Thesium chinense. Tc, Thesium chinense; Ac, Artemisia capillaris; Ec, Eragrostis curvula; Gv, Galium verum. (B) Thesium chinense and its host (Artemisia princeps). (C) Root of T. chinense (white) entangled in the root of its host (Artemisia capillaris; dark brown). (D) Haustorial connection of T. chinense and host (Eragrostis curvula) roots. Scale bar = 1 mm.
Association analysis
In order to determine if any plant species are more likely than others to grow in close proximity to T. chinense, a line transect vegetation survey was conducted. A 50-m transect was randomly placed within the riverside vegetation and 30 × 30 cm2 quadrats established every 5 m, resulting in 11 quadrats per transect. This procedure was repeated for 19 transects for a total of 209 quadrats. A preliminary investigation indicated that the roots of T. chinense do not normally spread beyond 30 cm from the shoot; thus, the size of the quadrats was appropriate for inferring host–parasite associations. All plant species that occurred in each quadrat were recorded and a test was carried out to see if any species was more or less likely to occur in quadrats with T. chinense (Chuang and Heckard, 1971; Hodgson, 1973). The significance of species associations was assessed using Fisher's exact test. To avoid committing a type II error by testing small-sized samples, only species that occurred in >20 quadrats (the eight most-dominant species in the study site) were tested for association with T. chinense.
Examination of haustorial connections
The above association analysis is useful to determine if T. chinense is likely to occur in patches with particular plants at the population level. However, it is necessary to directly examine haustorial connections in order to correctly identify the host range. In addition, the host selectivity of T. chinense can be investigated by comparing the number of haustoria formed on the roots of each host species to that expected from the relative below-ground biomass of hosts (Gibson and Watkinson, 1989). Note that the host selectivity in this case more likely reflects the suitability of host after infection (post-attachment selectivity) and does not necessarily indicate the degree to which T. chinense actively chooses among potentially available hosts prior to infection. To examine haustorial connections for the above purposes, nine samples of 30 × 30 × 20 (depth) cm3 turf containing two to three T. chinense plants were randomly collected. Turf samples were taken to the laboratory, and the soil was loosened for 24 h in a waterbath; this reduced the risk of damaging haustorial connections when removing the soil and examining haustorial connections. The rhizobial nodules of legumes were easily distinguished from haustoria by their colour and shape. The host species was determined by tracing the root back to the shoot. After counting the number of haustoria formed on each host, the roots were oven-dried at 50 °C for 48 h and weighed to the nearest 0·01 g to calculate the relative below-ground biomass of each species. This was multiplied by the total number of haustoria found in the turf samples in order to obtain the expected distribution of haustoria across all of the potential hosts. The host selectivity of T. chinense was then tested using a χ2 test. All nine turf samples were combined for the analysis.
Haustorium size
Host selectivity is likely to be reflected in the number of haustorial connections, but the results of such an analysis should be interpreted cautiously if the size of haustoria differs among host species. Therefore all haustoria were classified into six size categories: <1 mm, 1–1·5 mm, 1·5–2·0 mm, 2·0–2·5 mm, 2·5–3·0 mm and 3·0–3·5 mm. Differences in haustorium size among taxa were tested using the Kruskal–Wallis test, and post hoc multiple tests of pairwise differences were done using Scheffe's test.
RESULTS
Association analysis
A total of 38 plant species were identified in the 209 plots surveyed. Of these, only Lespedeza juncea (P < 0·01) and Eragrostis curvula (P < 0·05) had significantly positive associations with T. chinense. Diodia teres (P < 0·05) had a significantly negative association with T. chinense (Table 1).
Table 1.
List of plant species at the study site with the frequency of occurrence in all plots and in Thesium plots
| Frequency of occurrence (%) |
|||||
|---|---|---|---|---|---|
| Species | Growth form‡ | Native species§ | Haustorial formation | All plots (n = 209) | Thesium plots (n = 60) |
| Artemisia capillaris (Asteraceae)# | p | n | Yes | 125 (59·8) | 36 (60·0) |
| Galium verum (Rubiaceae)# | p | n | Yes | 59 (28·2) | 20 (33·3) |
| Eragrostis curvula (Poaceae)# | p | a | Yes | 58 (27·8) | 24 (40·0)* |
| Diodia teres (Rubiaceae)# | p | a | Yes | 57 (27·3) | 9 (15·0)† |
| Lespedeza juncea (Fabaceae)# | p | n | Yes | 50 (23·9) | 23 (38·3)** |
| Rumex acetosella (Polygonaceae)# | p | n | Yes | 27 (12·9) | 8 (13·3) |
| Vicia sepium (Fabaceae)# | a | n | Yes | 26 (12·4) | 12 (20·0) |
| Viola mandshurica (Violaceae)# | p | n | Yes | 24 (11·5) | 5 (8·3) |
| Briza maxma (Poaceae) | a | a | Yes | 14 (6·7) | 4 (6·7) |
| Cerastium glomeratum (Caryophyllaceae) | b | a | 11 (5·3) | 6 (10·0) | |
| Oxalis corniculata (Oxialidaceae) | p | n | Yes | 10 (4·8) | 1 (1·7) |
| Andropogon virginicus (Poaceae) | p | a | Yes | 9 (4·3) | 1 (1·7) |
| Erigeron annuus (Asteraceae) | a | n | Yes | 8 (3·8) | 2 (3·3) |
| Fabaceae sp. (Fabaceae) | a | a | Yes | 7 (3·3) | 2 (3·3) |
| Artemisia princeps (Asteraceae) | p | n | Yes | 6 (2·9) | 1 (1·7) |
| Oenothera erythrosepala (Onagraceae) | b | a | 6 (2·9) | 0 (0·0) | |
| Dianthus superbus (Caryophyllaceae) | p | n | Yes | 5 (2·4) | 2 (3·3) |
| Bulbostylis barbata (Cyperaceae) | a | n | 5 (2·4) | 0 (0·0) | |
| Cymbopogon tortilis (Poaceae) | p | n | Yes | 5 (2·4) | 2 (3·3) |
| Leonurus sibiricus (Lamiaceae) | p | n | 4 (1·9) | 0 (0·0) | |
| Potentilla chinensis (Rosaceae) | p | n | Yes | 3 (1·4) | 1 (1·7) |
| Rubia argyi (Rubiaceae) | p | n | 3 (1·4) | 0 (0·0) | |
| Galium sprium (Rubiaceae) | b | n | 3 (1·4) | 0 (0·0) | |
| Agrostis sp. (Poaceae) | p | a | Yes | 3 (1·4) | 1 (1·7) |
| Sporobolus fertilis (Poaceae) | p | n | Yes | 3 (1·4) | 1 (1·7) |
| Pueraria lobata (Fabaceae) | p | n | Yes | 3 (1·4) | 1 (1·7) |
| Oenothera laciniata (Onagraceae) | b | a | 2 (1·0) | 1 (1·7) | |
| Carex sp. (Cyperaceae) | p | n | Yes | 2 (1·0) | 0 (0·0) |
| Rumex japonicus (Polygonaceae) | p | n | 2 (1·0) | 0 (0·0) | |
| Setaria faberi (Poaceae) | a | n | 2 (1·0) | 0 (0·0) | |
| Lamium purpureum (Lamiaceae) | b | a | 2 (1·0) | 0 (0·0) | |
| Ambrosia artemisiifolia (Asteraceae) | a | a | 2 (1·0) | 1 (1·7) | |
| Digitaria adscendens (Poaceae) | a | n | 1 (0·5) | 0 (0·0) | |
| Erigeron philadelphicus (Asteraceae) | p | n | 1 (0·5) | 1 (1·7) | |
| Arundinella hirta (Poaceae) | p | n | 1 (0·5) | 0 (0·0) | |
| Erigeron sumatrensis (Asteraceae) | b | a | 1 (0·5) | 0 (0·0) | |
| Sedum bulbiferum (Crassulaceae) | b | n | Yes | 1 (0·5) | 0 (0·0) |
| Akebia quinata (Lardizabalaceae) | p | n | 1 (0·5) | 1 (1·7) | |
‡ a, Annual; b, biennial; p, perennial.
§ n, Native; a, alien.
# Tested for significance of association using Fisher's exact test.
* P < 0·05, ** P < 0·01 positive association with Thesium plants.
† P < 0·05 negative association with Thesium plants.
Examination of haustorial connections
The direct examination of haustorial connections revealed 22 species belonging to 11 families as hosts of T. chinense (Table 1; including four species that were found associated with T. chinense in preliminary observations: Artemisia princeps, Diodia teres, Sporobolus fertilis and Sedum bulbiferum). The observed and expected numbers of haustoria were significantly different (χ2 test, P < 0·0001; Table 2), suggesting that there is post-attachment selectivity. Significant differences were also found when data were analysed according to host plant families (P < 0·0001; Table 3). Based on χ2 values, Andropogon virginicus was a highly preferred host, whereas Dianthus superbus and Potentilla chinensis were less preferred (Table 2). In the same manner, Poaceae was highly preferred, whereas Caryophyllaceae and Rosaceae were hardly parasitized (Table 3). However, it should be noted that the observed/expected numbers of haustoria were too small in some species/families to confidently infer the significance of preference.
Table 2.
Observed and expected numbers of Thesium haustoria on the roots of various host plants
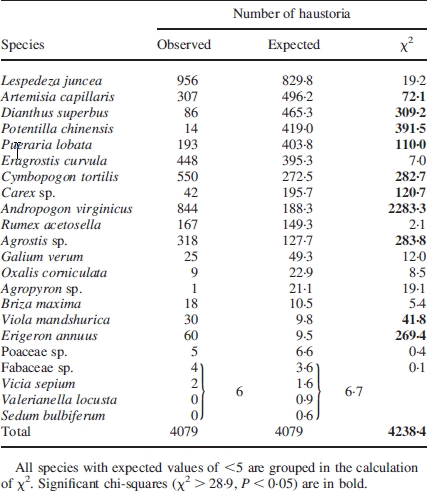 |
Table 3.
Observed and expected numbers of Thesium haustoria in various host-plant families
| Number of haustoria |
|||
|---|---|---|---|
| Family | Observed | Expected | χ2 |
| Fabaceae | 1155 | 1238·8 | 5·7 |
| Poaceae | 2184 | 1021·8 | 1321·9 |
| Asteraceae | 367 | 505·7 | 38·0 |
| Caryophyllaceae | 86 | 465·3 | 309·2 |
| Rosaceae | 14 | 419·0 | 391·5 |
| Cyperaceae | 42 | 195·7 | 120·7 |
| Polygonaceae | 167 | 149·3 | 2·1 |
| Rubiaceae | 25 | 49·3 | 12·0 |
| Oxialidaceae | 9 | 22·9 | 8·5 |
| Violaceae | 30 | 9·8 | 41·8 |
| Total | 4079 | 4079 | 2251·3 |
The expected numbers for Valerianaceae and Crassulaceae were less than five and were thus excluded from the calculation of χ2. Significant chi-squares (χ2 > 16·92, P < 0·05) are in bold.
Haustorium size
The majority of haustoria were <1 mm in most of the species examined. However, the haustorium size differed significantly among species (Kruskal–Wallis test, P < 0·0001; Table 4). The haustoria formed on Lespedeza juncea were significantly larger than those formed on Andropogon virginicus, Cymbopogon tortilis, Eragrostis curvula, Agrostis sp., Rumex acetosella and Dianthus superbus (Scheffe's test, P < 0·05). In addition, Pueraria lobata had significantly larger haustoria than did Rumex acetosella. Similarly, the size of haustoria in Fabaceae was significantly larger than that in other families, expect Rosaceae and Oxalidaceae. Asteraceae had significantly larger haustoria than did Polygonaceae.
Table 4.
Size distributions of Thesium haustoria found on the roots of each host species
| Size range (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Species no. | Species | <1·0 | 1·0–1·5 | 1·5–2·0 | 2·0–2·5 | 2·5–3·0 | >3·0 | Significance |
| 1 | Lespedeza juncea | 548 | 259 | 106 | 40 | 3 | 0 | 2, 3, 4, 5, 8, 9 |
| 2 | Andropogon virginicus | 659 | 136 | 41 | 7 | 1 | 0 | 1 |
| 3 | Cymbopogon tortilis | 410 | 111 | 18 | 8 | 2 | 1 | 1 |
| 4 | Eragrostis curvula | 368 | 75 | 4 | 1 | 0 | 0 | 1 |
| 5 | Agrostis sp. | 259 | 54 | 5 | 0 | 0 | 0 | 1 |
| 6 | Artemisia capillaris | 214 | 69 | 23 | 1 | 0 | 0 | |
| 7 | Pueraria lobata | 124 | 37 | 17 | 12 | 3 | 0 | 8 |
| 8 | Rumex acetosella | 149 | 16 | 2 | 0 | 0 | 0 | 1,7 |
| 9 | Dianthus superbus | 74 | 11 | 1 | 0 | 0 | 0 | 1 |
| 10 | Erigeron annuus | 48 | 12 | 0 | 0 | 0 | 0 | |
| 11 | Praecoces sp. | 38 | 4 | 0 | 0 | 0 | 0 | |
| 12 | Viola mandshurica | 28 | 2 | 0 | 0 | 0 | 0 | |
| 13 | Galium verum | 24 | 1 | 0 | 0 | 0 | 0 | |
| 14 | Briza maxima | 18 | 0 | 0 | 0 | 0 | 0 | |
| 15 | Potentilla chinensis | 12 | 2 | 0 | 0 | 0 | 0 | |
| 16 | Oxalis corniculata | 9 | 0 | 0 | 0 | 0 | 0 | |
| 17 | Poaceae sp. | 5 | 0 | 0 | 0 | 0 | 0 | |
| 18 | Fabaceae sp. | 4 | 0 | 0 | 0 | 0 | 0 | |
| 19 | Vicia sepium | 1 | 1 | 0 | 0 | 0 | 0 | |
| 20 | Agropyron sp. | 1 | 0 | 0 | 0 | 0 | 0 | |
The size distributions were significantly different among species (Kruskal–Wallis test, P < 0·0001). Pairs of species that had significant differences in the sizes of Thesium haustoria (Scheffe's test, P < 0·05) are indicated by species number.
DISCUSSION
For many root parasites, host range and selectivity in wild populations are poorly known and little studied because this requires a careful and extensive excavation of the root. Consequently, studies have concentrated on examination of parasite performance in pots with different host species as an alternative to excavation study (Malcom, 1966). However, pot-based studies are not suitable for a full understanding of host range and may yield misleading predictions regarding the pattern of host use in the wild (Marvier and Smith, 1997). Therefore, the present study was undertaken in order to explore the pattern of host association in T. chinense in a wild population. This is the first quantitative analysis of host associations in root hemiparasitic plants of Santalales.
The association analysis indicated that the majority of plants in the study population had neither positive nor negative associations with T. chinense. However, it should be noted that the sample sizes were too small for most species to make meaningful comparisons (Table 1), and it is possible that there is a hidden preference that it was not possible to detect. Among the eight species tested for significance, Lespedeza juncea and Eragrostis curvula occurred significantly more often in plots with T. chinense. There was also a significant negative association between Diodia teres and T. chinense, but direct examination of the roots indicated that Diodia teres is in fact parasitized, suggesting that our indirect method does not correctly reflect the actual patterns of host–parasite association in T. chinense. One of the positively associated species, Eragrostis curvula, belongs to the Poaceae; thus the present results confirm previous observations that T. chinense often occurs in proximity to grasses (Numata and Yoshizawa, 1975). The present study was conducted in natural grassland on a flood-prone riverside of the Kizu River, where many indigenous endangered plant species are found. However, the habitat has recently been invaded by various alien plant species, some of which are also parasitized by T. chinense, e.g. Eragrostis curvula, Diodia teres and Andropogon virginicus, further suggesting that there is limited specialization.
Direct observation of the roots revealed a previously unsuspected diversity of host plants for T. chinense. Overall, 57·9 % of the plant species that occurred in the population were parasitized by T. chinense (Table 1) and they belonged to 11 different families encompassing a broad range of angiosperms, suggesting that specialization to particular host taxonomic groups has not occurred in T. chinense. However, an analysis of hosts indicated that species such as Andropogon virginicus are strongly preferred and that the Poaceae had considerably more haustoria than expected from their root biomass. One factor that may influence such results is haustorium size because some species may have more but smaller haustoria than other species. The analysis of haustorium size in fact suggested that those that formed on Lespedeza juncea and Pueraria lobata were larger than those in some other species. However, haustorium size is generally very similar among host species, indicating that the strong preference for Andropogon virginicus and Poaceae plants is not associated with haustorium size
The two species that had larger haustoria, i.e. Lespedeza juncea and Pueraria lobata, both belong to the Fabaceae, the family that had the largest overall haustorium size (Table 5). The reason for the large haustoria sizes is unknown, but because these plants fix atmospheric nitrogen they may be of higher nutritional value, allowing haustoria to reach larger sizes. Thus, although these plants were not the preferred hosts as judged by the number of haustoria, they may be suitable hosts for T. chinense. However, Jiang et al. (2008) showed that in the root hemiparasitic Rhinanthus minor nitrogen fixation by the host legume is of no benefit to the parasite. It should therefore be tested explicitly whether T. chinense grown on legumes and on non-legumes differ in any significant ways that affect its growth performance, and whether such a difference, if any, is attributable to nitrogen fixation (Gibson and Watkinson, 1991; Marvier, 1996; Matthies, 1996).
Table 5.
Size distributions of Thesium haustoria for each host-plant family
| Size range (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Family no. | Family | <1·0 | 1·0–1·5 | 1·5–2·0 | 2·0–2·5 | 2·5–3·0 | >3·0 | Significance |
| 1 | Fabaceae | 1720 | 376 | 68 | 16 | 3 | 1 | 2 |
| 2 | Poaceae | 677 | 297 | 123 | 52 | 6 | 0 | 1, 3, 4, 5, 6, 7, 8 |
| 3 | Asteraceae | 262 | 81 | 23 | 1 | 0 | 0 | 2,4 |
| 4 | Polygonaceae | 149 | 16 | 2 | 0 | 0 | 0 | 2,3 |
| 5 | Caryophyllaceae | 74 | 11 | 1 | 0 | 0 | 0 | 2 |
| 6 | Cyperaceae | 38 | 4 | 0 | 0 | 0 | 0 | 2 |
| 7 | Violaceae | 28 | 2 | 0 | 0 | 0 | 0 | 2 |
| 8 | Rubiaceae | 24 | 1 | 0 | 0 | 0 | 0 | 2 |
| 9 | Rosaceae | 12 | 2 | 0 | 0 | 0 | 0 | |
| 10 | Oxalidaceae | 9 | 0 | 0 | 0 | 0 | 0 | |
The size distributions were significantly different among families (Kruskal–Wallis test, P < 0·0001). Pairs of families that had significant differences in the sizes of Thesium haustoria (Scheffe's test, P < 0·05) are indicated by family number.
Overall, the present results demonstrate that T. chinense uses various angiosperm hosts, some of which are more heavily infected than others. Several proximate factors are probably responsible for determining host range and selectivity in T. chinense, including the availability of chemical stimulants, strength of host defence, and level of osmotic pressure. Because species of Thesium are known to germinate in the absence of host chemical stimulants (Fer et al., 1993, 1994), such a signal is probably not required for seed germination in T. chinense. However, chemical stimulants are involved in haustorial formation in root parasites of Orobanchaceae (Musselman, 1980; Westwood, 2000; Yoder, 2001; Bouwmeester et al., 2003). Thus, it is possible that the availability of such signals may determine the success of root infection in Thesium. The strength of host defences such as induced lignification may also affect host quality. For example, some parasitic plants such as Rhinanthus minor (Cameron et al., 2006; Cameron and Seel, 2007; Rumer et al., 2007) and Orobanche crenata (Perez-de-Luque et al., 2005) are more likely to establish successful haustorial formation with less-defended plants. In addition, parasitic plants usually have higher root osmotic pressure than do their hosts, thereby facilitating water movement from host to parasite (Harris and Lawrence, 1916; Gworgwor and Weber, 1991; Simier et al., 1993; Williamson et al., 2002). Thus, it is possible that host plants vary greatly in these physiological attributes, which may be important for determining the pattern of host use in T. chinense.
Overall, the finding that T. chinense parasitizes an array of angiosperm hosts has broad general implication for patterns of host use in root parasites of Santalales. Although the present data suggest that T. chinense has strong host selectivity, it has obviously not become specialized to a limited number of preferred hosts. This is in marked contrast with some holoparasites such as Rafflesia, which has an extremely narrow host range (Ismail, 1988). Other members of Santalales are also known to use a broad range of angiosperms; for example, Osyris alba uses 23 host species belonging to 14 families (Jamal, 2006). Nevertheless, the host ranges of many Santalales species are still little understood, and many reported cases of host associations are probably fragmentary. For example, the holoparasitic Balanophora tobiracola (Balanophoraceae) is known to parasitize species of Pittosporum (Pittosporaceae), Ligustrum (Oleaceae), Eurya (Theaceae), Celastrus (Celastraceae) and Rhaphiolepis (Rosaceae; Akuzawa, 1982; Kawakita and Kato, 2002), but considering that these hosts belong to divergent families and are often dominant trees in the vegetation, it is possible that other plants are also used by B. tobiracola. Thus, the host ranges of Santalales species (especially the hemiparasitic species) are probably much broader than currently known, as evidenced by the present results for T. chinense, which was thought to parasitize only grasses. The evolutionary significance of a wide host range is yet unknown, but may be related to the generally large seed size in Santalales (Moles et al., 2005), which limits the number of seeds produced per plant and hence the chance of arriving at preferred hosts. Further studies of the life history and host associations in other members of the Santalales should broaden the perspectives on patterns of parasitic evolution in this intriguing plant lineage. Moreover, pot-based comparisons of performance on different hosts, or histological investigations of haustorial anatomy would provide further insights into the ecology of parasitic life style in plants of the Santalales.
ACKNOWLEDGEMENTS
We thank Dr Reiichi Miura and Mr Shota Sakaguchi for assistance with plant identification and useful discussion.
LITERATURE CITED
- Abu-Irmaileh BE. Thesium humile on onion. Haustorium. 1980;6:2. [Google Scholar]
- Akuzawa E. Balanophoraceae. In: Satake Y, Kitamura S, Oi J, Watari S, editors. Wild flowers of Japan: herbaceous plants. Vol. 2. Tokyo: Heibonsha; 1982. pp. 12–13. [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. Secondary metabolite signalling in host-parasitic plant interactions. Current Opinion in Plant Biology. 2003;6:358–364. doi: 10.1016/s1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Seel WE. Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytologist. 2007;174:412–419. doi: 10.1111/j.1469-8137.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Coats AM, Seel WE. Host and non-host resistance underlie variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany. 2006;98:1289–1299. doi: 10.1093/aob/mcl218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TI, Heckard LR. Observations of root-parasitism in Cordylanthus (Scrophulariaceae) American Journal of Botany. 1971;58:218–228. [Google Scholar]
- Dasgupta MK. Principles of plant pathology. New Delhi: Allied Publisher; 1988. [Google Scholar]
- Fer A, Simier P, Arnaud MC, Rey L, Renaudin S. Carbon acquisition and metabolism in a root-hemiparasitic angiosperm, Thesium humile (Santalaceae), growing on wheat (Triticum vulgare) Australian Journal of Plant Physiology. 1993;20:15–24. [Google Scholar]
- Fer A, Russo N, Simier P, Arnaud MC, Thalouarn P. Physiological changes in a root hemiparasitic angiosperm, Thesium humile (Santalaceae), before and after attachment to the host plant (Triticum vulgare) Journal of Plant Physiology. 1994;143:704–710. [Google Scholar]
- Gibson CC, Watkinson AR. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia. 1989;78:401–406. doi: 10.1007/BF00379116. [DOI] [PubMed] [Google Scholar]
- Gibson CC, Watkinson AR. Host selectivity and the mediation of competition by the root hemiparasite Rhinanthus minor. Oecologia. 1991;86:81–87. doi: 10.1007/BF00317393. [DOI] [PubMed] [Google Scholar]
- Gworgwor NA, Weber HC. Effect of N-application on sorghum growth, Striga infestaion and the osmotic pressure of the parasite in relation to the host. Journal of Plant Physiology. 1991;139:194–198. [Google Scholar]
- Harris JA, Lawrence JV. On the osmotic pressure of the tissue fluids of Jamaican Loranthaceae parasitic on various hosts. American Journal of Botany. 1916;3:438–455. [Google Scholar]
- Hodgson JF. Aspects of the carbon nutrition of angiospermous parasite. University of Sheffield.; 1973. PhD Thesis. [Google Scholar]
- Ismail G. Conservation of the giant Rafflesia, Sabah, Malaysia. Tree. 1988;3:316–317. [Google Scholar]
- Jamal RQ. Host range of the parasitic weed Osyris alba L. in Jordan. Weed Biology and Management. 2006;6:74–78. [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. Does legume nitrogen fixation underpin host quality for the parasitic plant Rhinanthus minor? Journal of Experimental Botany. 2008;59:917–925. doi: 10.1093/jxb/ern015. [DOI] [PubMed] [Google Scholar]
- Kawakita A, Kato M. Floral biology and unique pollination system of root holoparasites, Balanophora kuroiwai and B. tobiracola (Balanophoraceae) American Journal of Botany. 2002;89:1164–1170. doi: 10.3732/ajb.89.7.1164. [DOI] [PubMed] [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Malcom WM. Root parsitism of Castilleja coccinea. Ecology. 1966;47:179–186. [Google Scholar]
- Marvier M. Parasitic plant–host interactions: plant performance and indirect effects on parasite-feeding herbivores. Ecology. 1996;77:1398–1409. [Google Scholar]
- Marvier MA, Smith DL. Conservation implications for host use for rare parasitic plants. Conservation Biology. 1997;11:839–848. [Google Scholar]
- Matthies D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos. 1996;75:118–124. [Google Scholar]
- Musselman LJ. The biology of Striga, Orobanche, and other root-parasitic weeds. Annual Review of Phytopathology. 1980;18:463–489. [Google Scholar]
- Musselman LJ, Mann WF. Host plants of some Rhinanthoideae (Scrophulariaceae) of eastern North America. Plant Systematics and Evolution. 1977;127:45–53. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Westoby M. A brief history of seed size. Science. 2005;307:576–580. doi: 10.1126/science.1104863. [DOI] [PubMed] [Google Scholar]
- Numata S, Yoshizawa N. Weed flora of Japan. Tokyo: Zenkoku Noson Kyoiku Kyokai; 1975. [Google Scholar]
- Perez-de-Luque A, Rubiales D, Cubero JI, Press MC, Scholes J, Yoneyama K, et al. Interaction between Orobanche crenata and its host legumes: unsuccessful haustorial penetration and necrosis of the developing parasite. Annals of Botany. 2005;95:935–942. doi: 10.1093/aob/mci105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press MC, Graves JD. Parasitic plants. London: Chapman and Hall; 1995. [Google Scholar]
- Piehl MA. Mode of attachment, haustorium structure, and hosts of Pedicularis canadensis. American Journal of Botany. 1963;50:979–985. [Google Scholar]
- Pilger R. Engler A, Harms H, editors. Santalaceae. Die Naturlichen Pflanzen Familien. 1935;Vol. 16:52–91. [Google Scholar]
- Renaudin S, Cheguillaume N, Gallant DJ. Distribution and role of mineral compounds in the haustorium of a parasite of Galium arenarium, Thesium humifusum, before flowering. Canadian Journal of Botany. 1981;59:1998–2002. [Google Scholar]
- Riopel JL, Timko MP. Haustorial initiation and differentiation. In: Press MC, Graves JD, editors. Parasitic plants. London: Chapman and Hall; 1995. pp. 39–79. [Google Scholar]
- Rümer S, Cameron DD, Wacker R, Hartung W, Jiang F. An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora. 2007;202:194–200. [Google Scholar]
- Scarlett N, Bramwell M, Earl G. Austral toad flax. Flora and Fauna Guarantee Action Statement. 2003;56:1–5. [Google Scholar]
- Simier P, Fer A, Renaudin S. Identification of the main osmotically active solutes in the unstressed and water-stressed root-hemiparasitic angiosperm Thesium humile and its host Triticum vulgare. Australian Journal of Plant Physiology. 1993;20:223–230. [Google Scholar]
- Simier P, Renaudin S, Fer A. Characteristics of the mannitol pathway in a root hemiparasitic species, Thesium humile Vahl. (Santalaceae) Journal of Plant Physiology. 1994;143:33–38. [Google Scholar]
- Simier P, Robert S, Fer A. Mannitol metabolism in darkness in the leaves of the hemiparasitic angiosperm. Thesium humile. Plant Physiology and Biochemistry. 1998;36:237–245. [Google Scholar]
- Tennakoon KU, Cameron DD. The anatomy of Santalum album L. (sandalwood) haustoria. Canadian Journal of Botany. 2006;84:1608–1616. [Google Scholar]
- Westwood JH. Characterization of the Orobanche-Arabidopsis system for studying parasite-host interactions. Weed Science. 2000;48:742–748. [Google Scholar]
- Williamson JD, Jennings DB, Guo WW, Pharr DM, Ehrenshaft M. Sugar alcohols, salt stress, and fungal resistance: polyols-multifunctional plant protection? Journal of the American Society for Horticultural Science. 2002;127:467–473. [Google Scholar]
- Yoder JI. Host plant recognition by parasitic Scrophulariaceae. Current Opinion in Plant Biology. 2001;4:359–365. doi: 10.1016/s1369-5266(00)00185-0. [DOI] [PubMed] [Google Scholar]


