Abstract
HIV protease inhibitors are an important component of highly active antiretroviral therapy used to treat pregnant women infected with HIV. They have a low placental transfer and are highly plasma protein bound. This study was carried out to determine the unbound fraction of lopinavir in cord blood, and to characterize the binding of lopinavir to α1-acid glycoprotein (AAG) and human serum albumin (HSA), and displacement by ritonavir. Serum was obtained from cord blood from placentas obtained after cesarean section of healthy, non-HIV-infected women (n = 4). The unbound fraction of lopinavir in serum obtained from this cord blood was 0.022 ± 0.011%. The unbound fraction of lopinavir in separately obtained maternal serum samples (n = 4) was 0.89 ± 0.12%, which was not significantly different from that observed with cord serum samples. Varying concentrations of lopinavir, AAG, and HSA in buffer solutions were then used to characterize the lopinavir binding. The data were fit to obtain the number of binding sites (N) and equilibrium dissociation constant (KD). Binding of lopinavir to AAG (7–23 μM) was saturable with KD of 5.0 ± 1.1 μM and N of 1.2 ± 0.2. At low HSA concentrations (15–152 μM), lopinavir binding KD was 24.3 ± 8.7 μM and N was 1.1 ± 0.4; however, at 758 μM, lopinavir binding was essentially unsaturable. Lopinavir binding to AAG and HSA was not sensitive to ritonavir, and, thus, efforts to enhance fetal exposure to lopinavir should be focused on other issues such as efflux transporters.
Highly active antiretroviral therapy is used to treat patients infected with HIV, involving the administration of multiple antiretroviral drugs acting at different steps of the HIV life cycle. In treating pregnant patients infected with HIV, the aim of therapy is not only the treatment of the mother but also to prevent the transmission of the virus to the fetus. Among the antiretroviral drugs used, there are differences in the extent of transfer of these drugs across the placenta; HIV protease inhibitors are particularly poorly transferred.
The placenta separates maternal and fetal blood circulations, wherein the concentrations of drug-binding proteins are high in both circulations. However, they are unequal and dynamic over time. This protein concentration gradient favors partitioning of total drug on the maternal side for highly plasma protein-bound drugs like HIV protease inhibitors (most >98%). It has been shown that concentrations of plasma proteins such as α1-acid glycoprotein (AAG) and albumin change during the gestation period. Between 12 and 41 weeks of gestation, maternal serum albumin concentration ranges from 311 to 583 μM, whereas fetal serum albumin ranges from 114 to 603 μM (Krauer et al., 1984) and as high as 659 μM (Nation, 1981). Maternal serum AAG concentration, on the other hand, ranges from 7 to 24 μM (Krauer et al., 1984) and as high as 46 μMin the presence of acute or chronic inflammation (Chu et al., 1981). Fetal serum AAG concentrations range from ≤0.2 to 9 μM (Krauer et al., 1984). Similar plasma/serum protein concentration ranges have been observed in other studies as well (Laurell, 1968; Ganrot, 1972; Chu et al., 1981; Nation, 1981; Wood and Wood, 1981; Raynes, 1982; Denson et al., 1984; Krauer et al., 1984). Thus, the binding of highly plasma protein-bound drugs, such as HIV protease inhibitors, changes during gestation.
In addition, because of concentration differences of proteins in the maternal and fetal circulations, it is important to determine the unbound fraction of the protease inhibitors, not only in the maternal blood but also in the fetal/cord blood. Sudhakaran et al. (2007) observed a higher unbound fraction of indinavir and saquinavir in cord plasma compared with maternal plasma and suggested that this binding differential was due to the transplacental AAG concentration gradient. Therefore, the protein concentration gradient that exists in the maternal and fetal circulations during pregnancy may unfavorably affect partitioning of the drug into the fetal compartment.
Lopinavir was chosen as the drug to study because its combination with ritonavir is currently the drug combination of choice to treat HIV-infected pregnant women. Thus, the purpose of this study was to determine the unbound fraction of lopinavir in cord blood and to characterize the binding kinetics of lopinavir to human AAG and human serum albumin (HSA). Therefore, in this study, we determined the unbound fraction of lopinavir in serum obtained from cord blood. We also studied the binding of lopinavir to AAG and HSA. The AAG and HSA concentrations we used covered the concentration ranges occurring during gestation as discussed above. Because ritonavir is used clinically with lopinavir to enhance lopinavir exposure, we also determined the effect of ritonavir on lopinavir binding to these plasma proteins in a physiologic buffer. The role of ritonavir as a displacer of lopinavir protein binding for potential utility in increasing the maternal-to-fetal placental transfer was also investigated.
Materials and Methods
AAG was obtained from Thermo Fisher Scientific (Waltham, MA) and from Sigma-Aldrich (St. Louis, MO). HSA was obtained from Thermo Fisher Scientific. Lopinavir and [3H]lopinavir (specific activity of 100–139 mCi/mmol) were obtained from AK Scientific (Mountain View, CA) and Moravek Biochemicals (Brea, CA), respectively. Ritonavir was obtained from Sigma-Aldrich and Bosche Scientific (New Brunswick, NJ).
Placentas were obtained from four women (between the ages of 18–45 years, gestational length ≥36 weeks) from cesarean section deliveries following normal pregnancies at the VCU Medical Center Hospital. The study was approved by the VCU Institutional Review Board (protocol no. 4212), and informed consent was obtained from patients prior to delivery. Patients with hypertension, diabetes, preeclampsia, HIV infection or febrile illness, and patients with a history of smoking, alcohol, or drug abuse were excluded. Maternal blood samples were not available in this study. Cord blood was obtained from placentas within 30 min of birth, allowed to clot on ice, and centrifuged to obtain serum. Maternal serum samples were purchased from BioChemed Services (Winchester, VA). These samples were obtained from nonsmoking pregnant patients aged 19 to 34 years between 17 and 27 weeks gestation.
Rapid equilibrium dialysis (Thermo Scientific Pierce, Rockford, IL), with a molecular weight cutoff of 8,000 Da, was used to determine the protein binding of lopinavir in maternal serum and in serum obtained from cord blood. These protein-binding experiments with serum samples were followed by experiments with varying concentrations of lopinavir, AAG, and HSA. The protein solutions were prepared in phosphate-buffered saline (PBS), pH 7.4. The final concentrations of lopinavir were 0.1, 0.32, 1, 3, 10, 30, and 100 μM. Protein binding of lopinavir was determined in the presence of AAG (7 and 23 μM) and HSA (15, 152, and 758 μM).
The protein solution (500 μl) containing the drug was added to the sample (inner) chamber, and 750 μl of PBS was added in the buffer (outer) chamber. Nonspecific binding was determined by adding PBS in both the compartments with the solution in the sample chamber containing [3H]lopinavir alone. The Teflon base plate containing the inserts was covered and incubated at 37°C on an orbital shaker at 100 rpm. Samples (50 μl for serum experiments and 100 μl for the other experiments) were removed from each side at various times up to 16 h, and [3H]lopinavir in sample and buffer compartments was detected by liquid scintillation counting on a Packard TR2800 scintillation analyzer (Perkin-Elmer, Downers Grove, IL). Fraction unbound was determined by taking the ratio of mass of radioactivity observed in the buffer chamber to that in the sample chamber. All determinations were made in triplicates.
The equation for drug binding to a protein is given by:
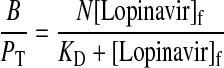 |
(1) |
where B is the bound drug concentration, PT is the total protein concentration, N is the number of binding sites per protein molecule, KD is the equilibrium dissociation constant, and [Lopinavir]f is the free drug concentration. Binding parameters N and KD were determined by nonlinear least-squares regression using eq. 1 (GraphPad Prism version 5; GraphPad Software Inc., San Diego, CA).
These studies were followed by selecting a physiologic maternal concentration of AAG (9 μM) or HSA (530 μM) and carrying out the determination of lopinavir protein binding at lopinavir concentrations ranging from 0.1 to 30 μM in the presence of different concentrations (0, 1, 10, 25, and 50 μM) of ritonavir. Lopinavir protein binding to HSA (530 μM) was also determined in the presence of a higher ritonavir concentration of 100 μM. Data from HSA and AAG experiments were analyzed by two-way analysis of variance with Bonferroni's post-test; significance was assessed at p < 0.05. Binding of lopinavir in cord and maternal serum was compared using an unpaired t test with Welch's correction (GraphPad Prism version 5).
Results and Discussion
Protein-binding equilibrium was achieved within 16 h, and nonspecific binding was negligible. Fraction unbound for lopinavir in serum obtained from cord from term placentas of healthy patients was 0.022 ± 0.011% (mean ± S.D., n = 4). Our results are similar to the data of Else et al. (2007) (0.017 ± 0.010%) who reported the protein binding of lopinavir in plasma from cord blood of HIV-positive pregnant women at term and on lopinavir/ritonavir therapy. Fraction unbound for lopinavir in maternal serum samples was 0.0089 ± 0.0012% (mean ± S.D., n = 4), which was not significantly different from that observed with cord serum obtained from term placentas (p = 0.099). It is noteworthy that the variances were significantly different (p < 0.01), which may imply more variability in unbound fraction of lopinavir in fetal blood.
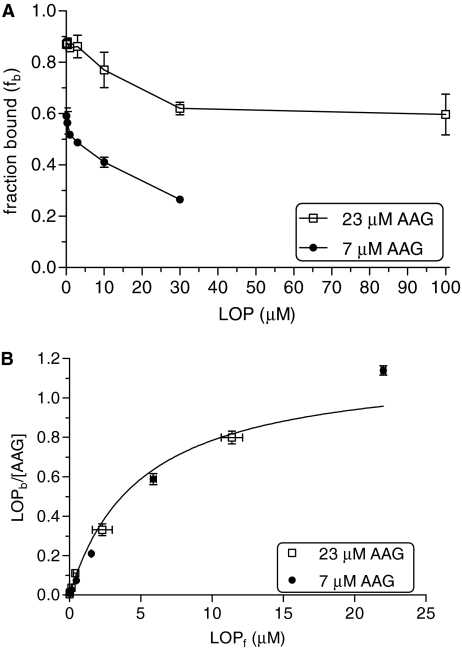
Lopinavir binding to 7 and 23 μM AAG at varying drug concentrations (0.1–30 μM) is shown in Fig. 1A. The binding of lopinavir to AAG was dependent on protein and drug concentrations. Fraction bound varied from 0.59 ± 0.03 to 0.27 ± 0.01 at 7 μM AAG and from 0.87 ± 0.01 to 0.62 ± 0.03 at 23 μM AAG over a lopinavir concentration range of 0.1 to 30 μM. It was not possible to determine the binding of lopinavir at 100 μM due to the low solubility of lopinavir. The data obtained for binding of lopinavir at both AAG concentrations were simultaneously fit to eq. 1 with weighting (1/y2). Figure 1B shows the saturation binding curve of lopinavir at 7 and 23 μM AAG concentrations in triplicate determinations. KD was 5.0 ± 1.1 μM and N was 1.2 ± 0.2. Binding of lopinavir to AAG thus appeared saturable.
Fig. 1.
Lopinavir binding to AAG. A, lopinavir binding at varying drug concentrations (0.1–30 μM) to 7 μM AAG (•) and at 0.1 to 100 μM to 23 μM AAG (□). B, bound lopinavir concentrations normalized for AAG concentration as a function of unbound lopinavir concentrations. AAG: 7 μM (•); 23 μM (□). LOP, lopinavir.
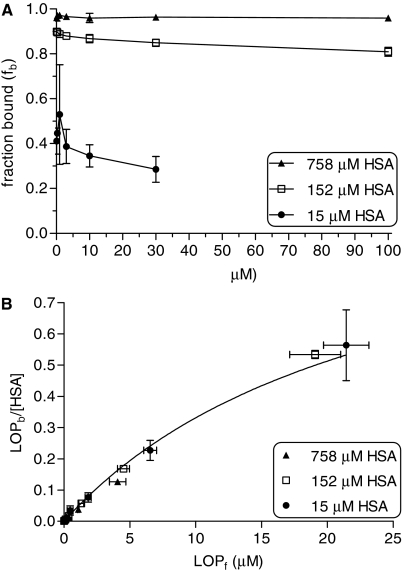
Similar triplicate experiments were carried out at several HSA concentrations (15, 152, or 758 μM). Lopinavir binding to 15, 152, and 758 μM HSA at varying drug concentrations (0.1–100 μM) is shown in Fig. 2A. In contrast to binding of lopinavir to AAG, binding to HSA was less dependent on lopinavir concentration; however, binding was dependent upon HSA concentration. With HSA, fraction bound ranged from 0.53 ± 0.22 to 0.29 ± 0.06 over a lopinavir concentration range of 0.1 to 30 μM at 15 μM HSA and from 0.90 ± 0.01 to 0.81 ± 0.02 over a lopinavir concentration range of 0.1 to 100 μM at 152 μM HSA. It was not possible to determine the binding of lopinavir at 100 μM in the presence of a low HSA concentration (15 μM) due to the low solubility of lopinavir. At 758 μM HSA, fraction bound of lopinavir (0.96 ± 0.02) was independent of lopinavir concentration (0.1–100 μM). The data obtained for binding of lopinavir to varying HSA concentrations were also simultaneously fit to eq. 1 with weighting (1/y2). Figure 2B shows the saturation binding curve of lopinavir at 15, 152, and 758 μM HSA concentrations. KD was estimated to be 24.3 ± 8.7 μM and N was 1.1 ± 0.4. However, at a physiologic concentration of 758 μM HSA, lopinavir binding was essentially nonsaturable.
Fig. 2.
Lopinavir binding to HSA. A, lopinavir binding at varying drug concentrations (0.1–30 μM) to 15 μM HSA (•) and at 0.1 to 100 μM lopinavir to 152 μM (□) and 758 μM HSA (▴). B, bound lopinavir concentrations normalized for HSA concentration as a function of unbound lopinavir concentrations. HSA: 15 μM (•); 152 μM (□); 758 μM (▴). LOP, lopinavir.
The KD values obtained in the case of experiments with AAG and HSA can be compared with the therapeutic concentrations of lopinavir to determine whether the binding is saturable or nonsaturable. Lopinavir Cmax following administration of 400 mg of lopinavir with 100 mg of ritonavir to healthy male volunteers is 13.5 μM (http://www.fda.gov/cder/foi/nda/2000/21–226_Kaletra_biopharmr_P1.pdf). However, the estimated unbound lopinavir concentration would be approximately 0.2 μM. In our study, KD for lopinavir binding to AAG and HSA of 5.0 ± 1.1 and 24.3 ± 8.7 μM, respectively, were much higher than the estimated unbound lopinavir concentration. Thus, lopinavir binding to both AAG and HSA would be nonsaturable at therapeutic concentrations.
Our data indicate that binding to HSA and AAG can account for the total protein binding of lopinavir by multiplying the expected unbound fractions of lopinavir at those protein concentrations. For the AAG and HSA concentration ranges previously discussed, the predicted lopinavir protein binding would vary between 0.96 to 0.99% in the maternal serum and 0.86 to 0.98% in the fetal serum during the course of pregnancy, because protein concentrations in maternal and fetal serum change between 12 and 41 weeks gestation. The range of these predictions includes our observed unbound fractions in both fetal and maternal serum. The trend in the present results toward a higher unbound fraction for lopinavir in cord serum is consistent with the higher unbound fraction in cord blood reported for indinavir and saquinavir (Sudhakaran et al., 2007).
This study shows that protein binding of lopinavir is characterized by saturable binding to AAG and nonsaturable binding to albumin at physiologic protein concentrations. Although HSA binds lopinavir with a somewhat lower affinity and both proteins appear to bind lopinavir at a single site, the physiologic concentration of HSA is much greater resulting in a much higher binding capacity. As a result, HSA would contribute more to the high degree of total plasma protein binding of lopinavir.
The concentration of serum proteins, the number of binding sites, and the apparent KD determine the extent to which drugs such as lopinavir are bound to proteins. It is anticipated that in the absence of active transport processes, equilibrium would occur for unbound concentrations in fetal and maternal blood for drugs with a long half-life. Thus, changes in fraction unbound (due to alterations in binding kinetics) in either circulation would change the fetal to maternal serum total drug concentration ratios, whereas the unbound concentration ratios would presumably remain constant.
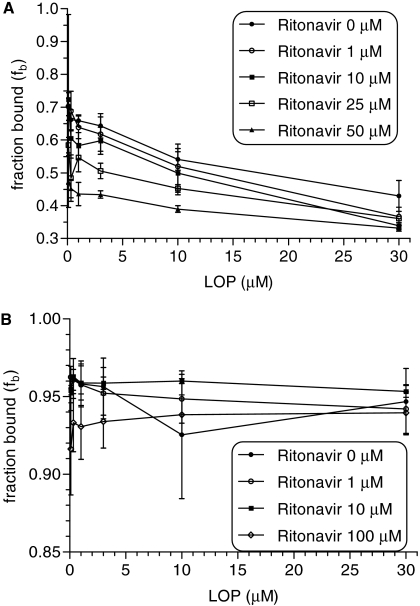
Effect of Ritonavir on Lopinavir Binding to AAG and HSA. Figure 3A shows the binding of lopinavir to 9 μM AAG at various-lopinavir concentrations (0.1–30 μM) in the presence of ritonavir (0, 1, 10, 25, or 50 μM). Lopinavir binding to AAG was significantly displaced by ritonavir only at 50 μM but not lower concentrations; it was not possible to obtain a reliable estimate of Ki. Figure 3B shows lopinavir binding to 530 μM HSA at various lopinavir concentrations (0.1–30 μM) in the presence of ritonavir (0, 1, 10, or 100 μM). Ritonavir (1 and 10 μM) had no apparent effect on unbound fraction of lopinavir in the presence of 530 μM HSA. In the presence of 100 μM ritonavir (but not lower concentrations), lopinavir binding to HSA was significantly altered.
Fig. 3.
Effect of ritonavir on lopinavir binding to AAG and HSA. A, lopinavir binding to 9 μM AAG at various concentrations of lopinavir (0.1–30 μM) in the presence of ritonavir (0, 1, 10, 25, or 50 μM; •, ○, ▪, □, ▴, respectively). B, lopinavir binding to 530 μM HSA at various concentrations of lopinavir (0.1–30 μM) in the presence of ritonavir (0, 1, 10, or 100 μM; •, ○, ▪, □, respectively).
In addition, ritonavir that is coadministered with lopinavir to pregnant women infected with HIV may not act to displace lopinavir from binding to AAG or HSA at its therapeutically relevant concentrations (Cmax = 0.83 μM, Ctrough = 0.30 μM) (Murphy et al., 2001). Therefore, efforts to enhance fetal exposure to lopinavir should probably focus on other mechanisms such as ATP-binding cassette transporters present on the apical (maternal-facing) syncytiotrophoblast. Moreover, mechanistic studies of transplacental transfer of drugs should consider unbound fetal to maternal concentration ratios, especially for highly protein bound drugs.
This work was supported in part by the National Institutes of Health National Center on Minority Health and Health Disparities [Grant 1P60-MD002256]; the Higher Education Equipment Trust Fund of Virginia; and the VCU School of Pharmacy, Department of Pharmaceutics.
This work was previously presented as follows: Gulati A, Boudinot FD, and Gerk PM (2008) Binding of lopinavir to human serum albumin and alpha-1 acid glycoprotein. Annual Meeting of the American Association of Pharmaceutical Scientists; 2008 Nov 16–20; Atlanta, GA. Virginia Commonwealth University, Richmond, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.026708.
ABBREVIATIONS: AAG, α1-acid glycoprotein; HSA, human serum albumin; PBS, phosphate-buffered saline.
References
- Chu CY, Singla VP, Wang HP, Sweet B, and Lai LT (1981) Plasma alpha 1-acid glycoprotein levels in pregnancy. Clin Chim Acta 112 235–240. [DOI] [PubMed] [Google Scholar]
- Denson D, Coyle D, Thompson G, and Myers J (1984) Alpha 1-acid glycoprotein and albumin in human serum bupivacaine binding. Clin Pharmacol Ther 35 409–415. [DOI] [PubMed] [Google Scholar]
- Else L, Dickinson L, Bell C, Douglas M, Back D, Khoo S, and Taylor G (2007) Total and unbound lopinavir pharmacokinetics during pregnancy and at delivery in HIV-infected women. 8th International Workshop on Clinical Pharmacology of HIV Infection; 2007 April 16–18; Budapest, Hungary. Department of Pharmacology, University of Liverpool, Liverpool, UK and St. Mary's NHS Trust, Imperial College, London, UK.
- Ganrot PO (1972) Variation of the concentrations of some plasma proteins in normal adults, in pregnant women and in newborns. Scand J Clin Lab Invest Suppl 124 83–88. [DOI] [PubMed] [Google Scholar]
- Krauer B, Dayer P, and Anner R (1984) Changes in serum albumin and alpha 1-acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. Br J Obstet Gynaecol 91 875–881. [DOI] [PubMed] [Google Scholar]
- Laurell CB (1968) Orosomucoid and alpha 1-antitrypsin in maternal and fetal sera at parturition. Scand J Clin Lab Invest 21 136–138. [DOI] [PubMed] [Google Scholar]
- Murphy RL, Brun S, Hicks C, Eron JJ, Gulick R, King M, White AC Jr, Benson C, Thompson M, Kessler HA, et al. (2001) ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15 F1–F9. [DOI] [PubMed] [Google Scholar]
- Nation RL (1981) Meperidine binding in maternal and fetal plasma. Clin Pharmacol Ther 29 472–479. [DOI] [PubMed] [Google Scholar]
- Raynes J (1982) Variations in the relative proportions of microheterogeneous forms of plasma glycoproteins in pregnancy and disease. Biomed Pharmacother 36 77–86. [PubMed] [Google Scholar]
- Sudhakaran S, Rayner CR, Li J, Kong DC, Gude NM, and Nation RL (2007) Differential protein binding of indinavir and saquinavir in matched maternal and umbilical cord plasma. Br J Clin Pharmacol 63 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M and Wood AJ (1981) Changes in plasma drug binding and alpha 1-acid glycoprotein in mother and newborn infant. Clin Pharmacol Ther 29 522–526. [DOI] [PubMed] [Google Scholar]