Abstract
Metabolism of the heterocyclic amine carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) was evaluated in mice with and without 40 mg/kg β-naphthoflavone (BNF). Following an oral dose of 40 mg/kg 14C-IQ, a 24-h urine sample was collected. Metabolism was assessed by high-performance liquid chromatography, and metabolites were identified by electrospray ionization mass spectrometry. Three new metabolites were identified as 1,2-dihydro-2-amino-5-hydroxy-3-methylimidazo[4,5-f]quinoline (m/z 217, [M + H]+), 1,2-dihydro-2-amino-5-O-glucuronide-3-methylimidazo[4,5-f]quinoline (m/z 393, [M + H]+), and 1,2-dihydro-2-amino-5,7-dihydroxy-3-methylimidazo[4,5-f]quinoline (m/z 233, [M + H]+). These metabolites represented 21% of the total urinary radioactivity recovered. For BNF-treated mice, the abundance of metabolites observed was 5-O-glucuronide > m/z 217 > m/z 393 > 5-sulfate > m/z 233 > N-glucuronide > demethyl-IQ > sulfamate. In control mice, metabolite urinary abundance was 5-O-glucuronide > demethyl-IQ > sulfamate > N-glucuronide > m/z 217 > 5-sulfate. In liver slices from BNF-treated mice, synthesis of m/z 217 and 5-O-glucuronide was significantly reduced by ellipticine, a cytochrome P450 (P450) inhibitor, whereas sulfamate synthesis was significantly increased and demethyl-IQ was unchanged. Liver microsomes from BNF-treated mice produced m/z 217 and demethyl-IQ, with the former inhibited by ellipticine and furafylline, a selective 1A2 inhibitor, and the latter by ellipticine only. Injection (intraperitoneal) of demethyl-IQ into BNF-treated mice resulted in only a 30% conversion to three metabolites that were not observed in urine from animals receiving IQ. Results from BNF-treated mice showed significant IQ metabolism by hepatic P450s. Therefore, differences in metabolism between mice treated with and without BNF may affect IQ tumorigenicity.
Cancer etiology and human risk estimates suggest that our diet contains mutagenic and carcinogenic chemicals that are agents in human cancer. Examples of these agents are aflatoxin B1, which is formed by fungi growing on poorly stored grain and associated with liver cancer (Groopman et al., 1988). Benzo[a]pyrene, a product of incomplete combustion of organic material, can deposit on food from fat dripping onto the coals during cooking or as a result of charring of food. These and other polycyclic aromatic hydrocarbons have been associated with several types of tumors (IARC, 1983). Heterocyclic amines (HCAs) are another class of dietary carcinogens (Felton and Knize, 1990). These chemicals are produced by high-temperature cooking of meat and derived from amino acids, creatine, and glucose present in meat. More than 17 different HCAs have been isolated from cooked meat. Studies have identified breast, colon, and bladder as possible target organs of HCAs and recently designated 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) as “reasonably anticipated to be a human carcinogen” (National Toxicology Program Report on Carcinogens: Background Document for Heterocyclic Amines: MeIQ, MeIQx, IQ, and PhIP, 2005; http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s092vhca.pdf).
IQ is an HCA. Studies in nonhuman primates given IQ showed a 95% incidence of hepatocarcinomas (Adamson et al., 1994). Additional studies in mice and rats have shown tumor formation in multiple tissues (Sugimura, 2000). Exposure to HCAs varies depending on cooking techniques, temperature, time, and the type and amount of meat consumed with individuals potentially exposed to micrograms of these amines per day (Felton and Knize, 1990; Pais et al., 1999). Current knowledge underlying the mechanism of HCA carcinogenesis indicates interplay of activation and detoxification pathways.
IQ activation requires N-oxidation by cytochrome P450 (P450), followed by O-acetylation with subsequent formation of a reactive intermediate, nitrenium ion, which binds DNA and initiates carcinogenesis (Turesky et al., 1991). N-OH-IQ is detected as a microsomal oxidation product (Turesky et al., 1991) but is not detected in urine because of its lability. Bacteria expressing human P450 1A2 and N-acetyltransferase activate IQ to a mutagen(s), mimicking the in vivo pathways (Josephy et al., 1998). Rat IQ inactivation involves P450-catalyzed ring oxidation at the 5-position followed by conjugation with sulfate or glucuronic acid (Fig. 1) (Inamasu et al., 1989; Luks et al., 1989; Armbrecht et al., 2007). Oxidation at the 7-position is reported in monkeys and humans (Snyderwine et al., 1992; Langouët et al., 2001). Direct conjugation of the exocyclic amine to form N2-glucuronide or sulfamate is another important pathway (Turesky et al., 1986; Inamasu et al., 1989; Luks et al., 1989; Armbrecht et al., 2007).
Fig. 1.
Structures of IQ and its urinary metabolites in mouse.
Although the rat is the primary species used to study HCA metabolism, significant differences are reported between HCA metabolism in humans and rats. For example, several studies in humans have detected HCA metabolites, which are not present in rats and have not yet been identified (Turesky et al., 1998; Garner et al., 1999; Langouët et al., 2001). In addition, N-demethylation is a primary pathway for HCA in humans (Langouët et al., 2001) and monkeys (Snyderwine et al., 1992) but is not detected in rats (Armbrecht et al., 2007; Lakshmi et al., 2008). 2-Amino-imidazo[4,5-f]quinoline (demethyl-IQ) is mutagenic (Barnes et al., 1985). Because recent studies by our laboratory have identified demethyl-IQ as a major urinary metabolite in the mouse, we have further evaluated IQ metabolism in mice after inducing phase I and II enzymes by administration of β-naphthoflavone (BNF). In this study, we show the formation of three new IQ metabolites, which represent approximately 21% of the radioactivity excreted in urine. These metabolites have not been reported in rats and show another pathway common to both mice and humans.
Materials and Methods
Materials. All the chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. [2-14C]IQ (10 mCi/mmol, >98% radiochemical purity) and IQ were purchased from Toronto Research Chemicals (Toronto, ON, Canada). Ultima-Flo AP was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Synthetic N-acetyl-2-amino-3-methylimidazo[4,5-f]quinoline (N-acetyl-IQ) was prepared by adding IQ to acetic anhydride in pyridine for 15 min at 60°C. The product was purified and identified by electrospray ionization/mass spectrometry (ESI/MS) in the positive-ion mode. The product exhibited [M + H]+, [M + Na]+, and [M + K]+ ions at m/z 241, 263, and 279, respectively. The [M + H]+ at m/z 241 gave rise to prominent ions at m/z 199, representing a protonated IQ, and at m/z 184 and 157, arising from consecutive losses of CH3 and HCN, respectively. The results indicated that the product is N-acetyl-IQ.
Animals and Dosing. Six-week-old C57BL/6 female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were fed AIN-76A from Bio-Serv (Frenchtown, NJ) for 8 weeks before use. Experiments were performed according to the National Institutes of Health standards for care and use of experimental animals and the St. Louis Veterans Affairs Medical Center Animal Care and Use Committee. Animals were group-housed, maintained on a 12-h light/dark cycle, and had access to food and water ad libitum. To induce hepatic phase I and II enzymes, animals were treated with 40 mg/kg BNF (0.2 ml/20 g injected i.p.; 13% dimethyl sulfoxide/87% corn oil) for 3 consecutive days. To assess IQ metabolism, mice were administered 40 mg/kg 14C-IQ (20 μCi; 0.2 ml/20 g oral dose; 20% dimethyl sulfoxide/80% 0.05 N HCl), placed in metabolic cages, and a 24-h urine sample was collected. The recovery of radioactivity in the 24-h urine from mice treated with and without BNF was 40 ± 6 and 53 ± 6% of the total radioactivity administered, respectively.
Processing Urine and Analysis. The 24-h urine samples were treated with 4 volumes of methanol/acetone (1:1), mixed, allowed to stand for 60 min, and centrifuged to precipitate protein and debris. The supernatants were transferred to silanized glass tubes, evaporated, and dissolved in a small volume of methanol. Urine from all the animals was analyzed by high-performance liquid chromatography (HPLC) using a Beckman Coulter (Fullerton, CA) HPLC with System Gold software and a 5-μm, 4.6 × 150-mm C-18 ultrasphere column attached to a guard column. The mobile phase contained 20 mM ammonium acetate, pH 5.0, in 2% acetonitrile from 0 to 5 min; 2 to 5% acetonitrile from 5 to 23 min; 5 to 10% acetonitrile from 24 to 29 min; 10 to 40% acetonitrile from 35 to 40 min; and 40 to 2% acetonitrile from 40 to 45 min at a flow rate of 1 ml/min (solvent system 1). Radioactivity in HPLC eluants was continuously measured using a FLO-ONE (PerkinElmer Life and Analytical Sciences) radioactive flow detector. Data are expressed as a percentage of total radioactivity or nanomole after HPLC.
HPLC Purification of Metabolites. Mouse IQ urinary metabolites identified earlier were purified using HPLC solvent systems described previously (Lakshmi et al., 2008). Purification of the new early eluting metabolites uses the following HPLC solvent systems at a flow rate of 1 ml/min: solvent system 2, the mobile phase contained 20 mM ammonium formate, pH 3.1, in 0% acetonitrile from 0 to 10 min, 0 to 40% acetonitrile from 10 to 15 min, and 40 to 0% acetonitrile from 20 to 25 min; solvent system 3, the mobile phase contained 20 mM ammonium acetate, pH 7.8, in 0% methanol from 0 to 2 min, 0 to 2% methanol from 2 to 7 min, 2 to 3.2% from 10 to 17 min, 3.2 to 35% methanol from 27 to 32 min, and 35 to 0% from 35 to 40 min; solvent 4, the mobile phase contained 0.1% glacial acetic acid, pH 3.0, in 0% methanol from 0 to 2 min, 0 to 2.5% methanol from 2 to 7 min, 2.5 to 50% methanol from 10 to 15 min, and 50 to 0% methanol from 15 to 20 min; solvent 5, the mobile phase contained 20 mM ammonium acetate, pH 7.8, in 0% methanol from 0 to 15 min, 0 to 0.15% methanol from 15 to 30 min, 0.15 to 40% methanol from 30 to 35 min, and 40 to 0% methanol from 40 to 45 min; solvent 6, the mobile phase contained 20 mM ammonium acetate, pH 7.8, in 0% acetonitrile from 0 to 15 min, 0 to 0.15% acetonitrile from 15 to 30 min, 0.15 to 40% acetonitrile from 30 to 35 min, and 40 to 0% acetonitrile from 40 to 45 min; and solvent 7, the mobile phase contained 0.1% glacial acetic acid, pH 3.0, in 0% acetonitrile from 0 to 15 min, 0 to 40% acetonitrile from 15 to 20 min, and 40 to 0% acetonitrile from 25 to 30 min. Silanized glass tubes were used in all the purification procedures.
To purify the new metabolites, urine was first separated using solvent system 1. Individual fractions were collected, concentrated, and applied to the next solvent system for further purification. The m/z 217 was purified with solvent systems 2, 3, and 4; m/z 233 was purified with systems 5 and 7; and m/z 393 was purified with systems 5, 6, and 7.
Qualitative Identification of Urinary and Liver Slice Metabolites. Metabolites were identified by their HPLC elution time relative to authentic rat products previously identified by us (Armbrecht et al., 2007; Lakshmi et al., 2008) and susceptibility to specific treatments (Luks et al., 1989; Snyderwine et al., 1992). Urinary metabolites were purified before assessing their susceptibility. Slice media were treated with 4 volumes of methanol/acetone (1:1), processed as described above, and then susceptibility was assessed. The 5-O-glucuronide is susceptible to Escherichia coli β-glucuronidase (type VII-A) in 50 mM sodium acetate, pH 6.8, buffer at 37°C for 4 h. 5-Sulfate is susceptible to abalone sulfatase (type VIII) in 50 mM sodium acetate, pH 5.0, buffer at 37°C for 72 h. N2-Glucuronide and sulfamate are hydrolyzed with 1 N HCl at 60°C for 2 h.
Liver Slice IQ Incubation. Mouse liver slices were prepared with a Stadie-Riggs microtome as previously described (Lakshmi et al., 1995b) and incubated with Dulbecco's modified Eagle's medium with high glucose containing 3.7 μg/ml NaHCO3 and 6 μg/ml Hepes. Approximately 100 to 120 mg of liver was placed in a 20-ml scintillation vial with 1 ml of medium, gassed with 5% O2/95% CO2 for 1 min, and incubated at 37°C for 60 min. Medium contained 0.06 mM IQ, and where indicated medium also contained 0.5 mM ellipticine. To stop incubations, 1 ml of cold methanol was added, and samples were frozen. The samples were thawed, sonicated, and spun to remove protein and cell debris, and the supernatant was evaporated to concentrate and redissolved in N,N-dimethylformamide/methanol (1:6) for analysis by HPLC using solvent system 1. Activity is expressed as percentage of radioactivity after HPLC.
Microsomal IQ Incubation. To identify specific pathways for IQ metabolism, liver microsomes were prepared using a previously described procedure (Zenser et al., 1978). Samples were stored in small aliquots at –70°C for the assays; unused material was discarded after one freeze-thaw cycle. Microsomes (1 mg/ml) were incubated in sodium phosphate buffer, pH 7.4, containing 0.1 mM diethylenetriaminepentaacetic acid, 1 mM NADPH, 5 mM MgCl2, and 0.06 mM 14C-IQ for 30 min at 37°C. Furafylline was preincubated with the reaction mixture containing microsomes for 10 min before 14C-IQ addition. The reaction was linear with respect to protein concentration and time and was stopped by addition of an equal volume of methanol with 1 mM ascorbic acid. Samples were centrifuged to remove the precipitated protein, and the supernatants were analyzed by HPLC. The mobile phase contained 20 mM ammonium acetate, pH 5.0, in 2% acetonitrile with 2 to 3.3% acetonitrile from 2 to 7 min, 3.3 to 10% acetonitrile from 10 to 15 min, 10 to 50% acetonitrile from 22 to 24 min, and 50 to 2% acetonitrile from 30 to 35 min at a flow rate of 1 ml/min (solvent system 8). The reaction product is measured as nanomoles or percentage of radioactivity after HPLC separation.
Mass Spectral Analysis. Metabolites were identified by ESI/MS. Analyses were performed with a Finnigan TSQ-7000 triple-stage quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA) equipped with a Finnigan ESI source and controlled by Finnigan ICIS software operated on a Hewlett Packard (Palo Alto, CA) alpha workstation. Methanol was continuously infused onto the ESI source with a Harvard Apparatus Inc. (Holliston, MA) syringe pump at a flow rate of 5 μl/min, and samples were loop-injected. The skimmer was at ground potential, and the electrospray needle was at 4.5 kV. The heated capillary temperature was 250°C. To obtain collisionally activated dissociation tandem mass spectra, the collision energy was set at 22 eV, and argon (2.3 mTorr) was used as target gas. The product ion spectra were acquired in the profile mode at the scan rate of 1 scan/3 s.
Statistical Analysis. Data are expressed as a mean ± S.E., and significant differences were evaluated using a Student's unpaired t test with p < 0.05.
Results
Analysis of IQ Metabolites in Mouse Urine by HPLC. The HPLC profiles of IQ metabolites in urine from mice treated with and without BNF were evaluated. The elution profile of IQ metabolites in control (–BNF) mice is illustrated in Fig. 2, top, and the quantitative distribution of metabolites associated with these HPLC peaks is provided in Table 1. All these metabolites were also present in BNF-treated mice (Fig. 2, bottom), but only small amounts were observed, except 5-O-glucuronide, which is the most abundant. Three new metabolites of m/z 233, 393, and 217 were also observed by ESI/MS in the positive-ion mode (data not shown). The metabolite of m/z 217 was also present in control mice but not previously reported (Lakshmi et al., 2008). In control mice, the relative abundance of excreted metabolites was 5-O-glucuronide > demethyl-IQ > sulfamate > N-glucuronide > m/z 217 > 5-sulfate, similar to previously reported (Lakshmi et al., 2008). In BNF-treated mice, the abundance of the metabolites excreted was 5-O-glucuronide > m/z 217 > m/z 393 > 5-sulfate > m/z 233 > N-glucuronide > demethyl-IQ > sulfamate. The new metabolites represented approximately 21% of the total radioactivity recovered in urine. Whereas no IQ was recovered in BNF-treated mice, the amount of IQ remaining in control urine was 2.1 ± 0.4%. N-Acetyl-IQ was not detected in urine from either group.
Fig. 2.
Urinary excretion of 14C-IQ from control and BNF-treated mice. Mice were administered an oral dose of 40 mg/kg 14C-IQ, and a 24-h urine sample was collected. A representative HPLC profile from each treatment group is shown using solvent system 1. The peaks were tentatively identified by retention times and susceptibility to specific treatments.
TABLE 1.
IQ metabolites in urine from control and BNF-treated mice
Mice were administered radiolabeled IQ and placed in metabolic cages for a 24-h urine collection. Table entries are the mean ± S.E. of six mice. Urine from mice was analyzed by HPLC as illustrated in Figure 2.
| Condition | m/z 233 | m/z 393 | m/z 217 | N-Glucuronide | 5-O-Glucuronide | Sulfamate | 5-Sulfate | Demethyl-IQ |
|---|---|---|---|---|---|---|---|---|
| % of total urine radioactivity | ||||||||
| Control | N.D. | N.D. | 4.5 ± 0.3 | 7.5 ± 0.5 | 33 ± 1 | 13.6 ± 0.8 | 2.4 ± 0.2 | 29 ± 1 |
| + BNF | 3.5 ± 0.4 | 5.2 ± 0.7 | 12.7 ± 1.5* | 2.8 ± 0.4* | 56 ± 3* | 1.0 ± 0.1* | 4.2 ± 0.4* | 1.4 ± 0.4* |
N.D., not detected.
p < 0.05 compared with control.
Identification of Urinary IQ Metabolites. The structures of the five metabolites eluting before IQ in control urine (Fig. 2, top) were identified by the product-ion spectra and were further confirmed by their HPLC retention times, which are identical to those arising from authentic standards (Lakshmi et al., 2008). The structural assignments are also consistent with those observed by enzymatic and chemical reactions, in which the 5-O-glucuronide and 5-sulfate metabolites were susceptible to β-glucuronidase and sulfatase treatments, respectively, whereas the N-glucuronide and sulfamate were susceptible to treatment with 1 N HCl at 60°C.
The metabolites isolated from BNF-treated mice (Fig. 2, bottom) and the feature ions leading to their structural characterization by mass spectrometry have been previously reported (Lakshmi et al., 2008). When subjected to ESI in the positive-ion mode, the 12.2-min peak (Fig. 2) exhibited an [M + H]+ at m/z 375 and gave prominent ions at m/z 241 (protonated N-acetyl-IQ), 199 (protonated IQ), and 184 (loss of CH3), which are typical ions reflecting that the metabolite is IQ-N2-glucuronide. In the positive-ion mode, the 17.8-min peak gave the [M + H]+, [M + Na]+, and [M + K]+ ions at m/z 391, 413, and 429, respectively, consistent with the observation of [M – H]–, the ion at m/z 389 in negative-ion mode. The product-ion spectrum of the ion at m/z 389 contained prominent ions at m/z 213, representing a deprotonated 5-OH-IQ anion, and at m/z 198 arising from loss of CH3 residue, indicating that the 17.8-min peak is IQ-5-O-glucuronide. In the negative-ion mode, the 19.7-min peak yielded an [M – H]– ion at m/z 277, which gave rise to an MS2 spectrum that is dominated by the ions at m/z 197 (deprotonated IQ anion) and 182 (loss of CH3), along with ion at m/z 80, representing an SO –3 ion. The results indicated that the compound eluting at 19.7 min is IQ-sulfamate. The 27.1-min peak yielded the [M – H]– ion at m/z 293, which gave prominent product ions at m/z 213 (deprotonated 5-OH-IQ anion) and 198 (loss of CH3), along with the ions at m/z 80 (SO –3) and 97 (HSO –4), indicating that the peak represents IQ-5-sulfate. The 31.9-min peak exhibited an [M + H]+ at m/z 185, which gave prominent product ions at m/z 143 (loss of NH2-CN), 116 (143 – HCN), and 89 (116 – HCN), as well as ions at m/z 158 (185 – HCN) and 131 (158 – HCN), consistent with the structure of demethyl-IQ, which was recently identified (Lakshmi et al., 2008).
The three new metabolites observed in urine from BNF-treated mice were identified by their ESI/MS spectra (Fig. 3) and by specific treatments with enzymes. The tandem mass spectrometric approaches leading to the structural identification are detailed elsewhere (F.-F. Hsu, M. Lakshmi, and T. V. Zenser, unpublished data). In brief, the structure of 1,2-dihydro-2-amino-5-hydroxy-3-methylimidazo[4,5-f]quinoline assigned for the 10.6-min peak is based on the observation of the [M + H]+ ion at m/z 217, which yielded prominent product ions at m/z 200 (loss of NH3), 186 (loss of CH3-NH2), and 161 (loss of HN = C = N-CH3), along with ions at m/z 185 (200 – CH3), 171 (200 – CH2 = NH), 159 (200 – H2C = C = NH), and m/z 157 (185 – CO). The 7.1-min peak exhibited an [M + Na]+ ion at m/z 415 and [M + H]+ ion at m/z 393. The m/z 393 ion yielded a prominent product ion at m/z 217 by loss of glucuronide residue, whereas further dissociation of the ion of m/z 217 with tandem quadrupole instrument via source collisionally activated dissociation gave a product-ion spectrum identical to that seen in Fig. 3A, which is consistent with the compound being 1,2-dihydro-2-amino-5-O-glucuronide-3-methylimidazo[4,5-f]quinoline. The structure assignment is further supported by β-glucuronidase treatment, which resulted in an HPLC peak that corresponds to the peak of m/z 217. The 3.8-min peak showed [M + H]+ at m/z 233 consistent with the observation of the [M – H]– ion at m/z 231. The tandem quadrupole product-ion spectrum (Fig. 3C) contained analogous ions that are 16 Da heavier than those seen for m/z 217 (Fig. 3A), and the profiles of the two spectra are also similar, indicating the presence of an additional hydroxyl group at C(7). The results are consistent with the notion that the metabolite is 1,2-dihydro-2-amino-5,7-dihydroxy-3-methylimidazo[4,5-f]quinoline (F.-F. Hsu, M. Lakshmi, and T. V. Zenser, unpublished data). The product-ion spectrum of the ion of m/z 217 seen at 10.6 min with control urine (Fig. 2, top) was also identical to that of m/z 217 arising from BNF-treated mouse urine.
Fig. 3.
Positive-ion ESI tandem MS for new urinary IQ metabolites from BNF-treated mice. Metabolite [M + H]+ ions at m/z 217, 393, and 233 are represented in A, B, and C, respectively.
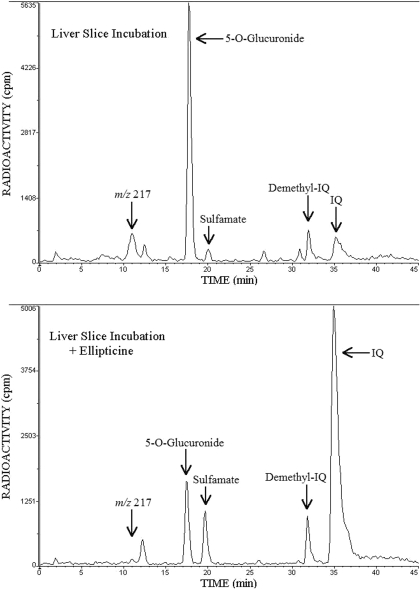
IQ Metabolism by Liver Slices from BNF-Treated Mice. When IQ was metabolized in liver slices, 5-O-glucuronide was the main metabolite along with smaller amounts of demethyl-IQ, sulfamate, and m/z 217 (Fig. 4, top). The 5-O-glucuronide and sulfamate were susceptible to hydrolysis by β-glucuronidase and acid treatment, respectively. With 0.5 mM ellipticine, a P450 inhibitor (Fig. 4, bottom), IQ metabolism was significantly reduced, and its presence in medium increased from 10.6 ± 3.7 to 64 ± 3% of the total recovered radioactivity (Table 2). The synthesis of 5-O-glucuronide and m/z 217 was significantly decreased by ellipticine, whereas sulfamate formation was more than doubled, and demethyl-IQ formation remained unchanged.
Fig. 4.
BNF-treated mouse liver slice IQ metabolism. Slices were incubated with 0.06 mM 14C-IQ for 60 min at 37°C in the presence and absence of 0.5 mM ellipticine. A representative HPLC profile from each treatment group is shown using solvent system 1. Peaks were identified by retention times and susceptibility to specific treatments.
TABLE 2.
IQ metabolites in liver slices from BNF-treated mice
Slices were incubated with 0.06 mM IQ for 60 min at 37°C in the presence and absence of 0.5 mM ellipticine. Samples were analyzed by HPLC and data recorded as percentage of total radioactivity after HPLC. Entries represent mean ± S.E. of determinations from four mice.
| Condition | m/z 217 | 5-O-Glucuronide | Sulfamate | Demethyl-IQ | IQ |
|---|---|---|---|---|---|
| % of total radioactivity | |||||
| Control | 8.9 ± 0.2 | 45 ± 1 | 2.6 ± 0.2 | 5.2 ± 1.0 | 10.6 ± 3.7 |
| +500 μM Ellipticine | 0.8 ± 0.4* | 9.9 ± 2.2* | 6.7 ± 0.7* | 4.3 ± 0.6 | 64 ± 3* |
p < 0.05 compared with control.
IQ Metabolism by Liver Microsomes from BNF-Treated Mice. To further evaluate metabolism of IQ in liver, microsomes were prepared. Demethyl-IQ and m/z 217 were the major metabolites observed, as evidenced by their elution profile (Fig. 5) and by their ESI/MS spectra (data not shown).
Fig. 5.
BNF-treated mouse liver microsomal IQ metabolism. Microsomes were incubated with 0.06 mM 14C-IQ for 30 min at 37°C. A representative HPLC profile is shown using solvent system 8. The m/z 217 and demethyl-IQ peaks were tentatively identified by their retention time and confirmed by mass spectrometry.
P450 Inhibition of IQ N-Demethylation by Liver Microsomes from BNF-Treated Mice. To determine the P450 enzymes involved in metabolism, a variety of P450 inhibitors were tested (Table 3). Ellipticine was the most effective inhibitor at a concentration of 0.02 mM, and no demethyl-IQ formation was observed at this concentration. Inhibition at 0.02 mM was also observed with ketoconazole (65% of total nanomoles; 3A inhibitor). However, significant inhibition was not observed with 0.02 mM furafylline (85%; a selective 1A2 inhibitor), SKF-525A (73%; a nonspecific inhibitor), sulfaphenazole (73%; a 2C inhibitor), or α-naphthoflavone (77%; 1A inhibitor). To further assess N-demethylation, a range of ellipticine concentrations was assessed, and a Dixon plot determined the Ki to be 0.008 mM.
TABLE 3.
P450 inhibition of demethyl-IQ formation by liver microsomes from BNF-treated mice
Incubations were conducted for 30 min at 37°C with 0.06 mM IQ. Furafylline was preincubated for 10 min before addition of IQ. For those incubations, the control microsomal activity was 0.25 ± 0.01 nmol, which is not different from the control value above. Data are expressed as mean nanomole of demethyl-IQ ± S.E. with n = 3.
| Test Agents | Type of P450 Inhibitor | Concentration | Activity | % of Total |
|---|---|---|---|---|
| μM | nmol | |||
| Control | 0.26 ± 0.04 | 100 | ||
| Ellipticine | 1A | 10 | 0.03 ± 0.01* | 12 |
| 20 | N.D. | 0 | ||
| Furafylline | 1A2 | 20 | 0.22 ± 0.01 | 85 |
| α-Naphthoflavone | 1A | 20 | 0.20 ± 0.01 | 77 |
| 100 | 0.15 ± 0.02* | 58 | ||
| SKF-525A | Nonspecific | 20 | 0.19 ± 0.04 | 73 |
| 100 | 0.12 ± 0.02* | 46 | ||
| Ketoconazole | 3A | 20 | 0.17 ± 0.02* | 65 |
| 100 | 0.07 ± 0.04* | 27 | ||
| Sulfaphenazole | 2C | 20 | 0.19 ± 0.05 | 73 |
| 100 | 0.20 ± 0.03 | 77 |
N.D., not detected.
p < 0.05 compared with control.
P450 Inhibition of m/z 217 Formation by Liver Microsomes from BNF-Treated Mice. The inhibitor profile for m/z 217 was different from that observed with N-demethylation (Table 4). At a concentration of 0.02 mM, significant inhibition was observed with furafylline (55% of total nanomoles), ketoconazole (59%), and SKF-525A (66%). The m/z 217 was not detected with either ellipticine or α-naphthoflavone. As was the case for N-demethylation, 0.1 mM sulfaphenazole did not alter m/z 217 formation. A Dixon plot determined the Ki for ellipticine to be 0.0014 mM.
TABLE 4.
P450 inhibition of m/z 217 formation by liver microsomes from BNF-treated mice
Incubations were conducted for 30 min at 37°C with 0.06 mM IQ. Furafylline was preincubated for 10 min before addition of IQ. For those incubations, the control microsomal activity was 0.31 ± 0.02 nmol, which is not different from the control value above. Data are expressed as mean nanomole of m/z 217 ± S.E. with n = 3 to 6.
| Test Agents | Type of P450 Inhibitor | Concentration | Activity | % of Total |
|---|---|---|---|---|
| μM | nmol | |||
| Control | 0.29 ± 0.02 | 100 | ||
| Ellipticine | 1A | 10 | N.D. | 0 |
| 20 | N.D. | 0 | ||
| Furafylline | 1A2 | 20 | 0.16 ± 0.04* | 55 |
| α-Naphthoflavone | 1A | 20 | 0.07 ± 0.04* | 24 |
| 100 | N.D. | 0 | ||
| SKF-525A | Nonspecific | 20 | 0.19 ± 0.01* | 66 |
| 100 | 0.18 ± 0.01* | 62 | ||
| Ketoconazole | 3A | 20 | 0.17 ± 0.01* | 59 |
| 100 | 0.07 ± 0.04* | 24 | ||
| Sulfaphenazole | 2C | 20 | 0.27 ± 0.02 | 93 |
| 100 | 0.26 ± 0.01 | 90 |
N.D., not detected.
p < 0.05 compared with control.
In Vivo Metabolism of Demethyl-IQ by BNF-Treated Mice. To determine whether demethyl-IQ is further metabolized in vivo, purified material (80 μg) from control mouse urine was injected intraperitoneally into two BNF-treated mice, and a 24-h urine sample was collected. Demethyl-IQ was metabolized with peak times (solvent system 1; Fig. 2) and percentage of the total recovered radioactivity as follows (average of n = 2): 11.0 min and 7.8%, 16.2 min and 9%, and 31 min and 7.8% (data not shown). Treatment of urine with β-glucuronidase hydrolyzed the 16.2-min peak. This is consistent with oxidation and subsequent O-glucuronidation of demethyl-IQ by BNF-treated mouse. However, 70% of demethyl-IQ was excreted unchanged.
Discussion
This report identifies three new urinary metabolites of IQ in mice with BNF-induced phase 1 and phase 2 enzymes. Metabolites were identified by ESI/tandem MS as 1,2-dihydro-2-amino-5-hydroxy-3-methylimidazo[4,5-f]quinoline (m/z 217), 1,2-dihydro-2-amino-5-O-glucuronide-3-methylimidazo[4,5-f]quinoline (m/z 393), and 1,2-dihydro-2-amino-5,7-dihydroxy-3-methylimidazo[4,5-f]quinoline (m/z 233). These new metabolites represent a large portion of the total recovered radioactivity (21%) and have not been reported in rats with or without BNF treatment (Armbrecht et al., 2007; Lakshmi et al., 2008). Demethyl-IQ is also present in mice (Fig. 2) but not in rats. The N-demethylated and C-7 hydroxylated metabolites are reported in monkeys and humans (Snyderwine et al., 1992; Langouët et al., 2001). Four of the mouse metabolites—N-glucuronide, 5-O-glucuronide, sulfamate, and 5-sulfate—exhibited MS spectra identical to the corresponding metabolites in rats. One of the newly identified metabolites, m/z 217, was also present in control mouse urine (4.5 ± 0.3%) but not previously reported (Lakshmi et al., 2008). All five previously observed metabolites in urine from control mice were present in treated mice. However, these products represented only a small amount of total metabolites observed (9.4%), except 5-O-glucuronide. The latter was the most prevalent metabolite in both groups. Consistent with the results seen in urine, m/z 217 was a major metabolite in BNF-treated mouse liver slices and microsomes. Demethyl-IQ was also produced by slices and microsomes and was recently reported to be a major metabolite in control mouse urine and a product of P450 metabolism (Lakshmi et al., 2008). Mice may have a high capacity for metabolizing IQ because IQ was not detected in the urine from BNF-treated mice, and only 2.1 ± 0.4% was observed from control mice after administration of 40 mg/kg IQ. The results showed major differences in the IQ metabolic profile in BNF-treated compared with control mice and as many as four products not detected in rat (Inamasu et al., 1989; Luks et al., 1989; Armbrecht et al., 2007; Lakshmi et al., 2008). Similarities between mouse and human metabolism were shown.
Because N-acetylation plays an important role in aromatic amine metabolism (Lakshmi et al., 1995a), the presence of N-acetyl-IQ was assessed. Because this product coelutes with IQ (Fig. 2, bottom) (Lakshmi et al., 2008), the lack of detection of radioactivity associated with IQ indicates that N-acetyl-IQ is absent in the urine of BNF-treated mice. In previous studies by us and others, urinary N-acetylated HCAs were not detected in mice, rats, monkeys, or humans (Luks et al., 1989; Snyderwine et al., 1992; Kestell et al., 1999; Langouët et al., 2001; Armbrecht et al., 2007; Lakshmi et al., 2008). The liver cytosol also failed to catalyze N-acetylation, suggesting that this reaction is not involved in mouse HCA metabolism. This is consistent with the studies showing that HCAs are poor substrates for N-acetylation by N-acetyltransferases but good substrates for O-acetylation (Turesky et al., 1991; Minchin et al., 1992). Because HCAs are thought to be activated by N-oxidation followed by O-acetylation to form DNA adducts, the combined rapid CYP1A2/rapid acetyltransferase-2 (NAT-2) phenotype has been associated with an increased risk of colon polyps (Lang et al., 1994).
Demethyl-IQ represented a significant amount of metabolism in both liver slices and microsomes from BNF-treated mice, but only a small amount of this metabolite was observed in urine. After intraperitoneal administration, the majority of demethyl-IQ (70%) was excreted unchanged. Therefore, increased metabolism may not explain the low level of demethyl-IQ excretion in IQ-dosed BNF-treated mice. In control mice, demethyl-IQ is not further metabolized (Lakshmi et al., 2008). Because the demethyl-IQ metabolite at 11.0 min elutes close to m/z 217, the quantitation of this latter compound as shown in Table 1 may have been overestimated. However, a chromatographic separation used for purification (solvent system 2) indicated that the demethyl-IQ peak eluting at 11.0 min is absent in urine from BNF-treated mice (data not shown). There was no evidence that a significant amount of any demethyl-IQ metabolite is present in the urine from BNF-treated mice. The observation of a low level of sulfamate and high level of 5-O-glucuronide in BNF-treated mouse urine (Table 1) suggests a rapid hepatic conversion of IQ to 5-OH-IQ, and the level of IQ substrate is perhaps too low for demethyl-IQ synthesis in vivo.
Several distinct P450 inhibitors were used to determine the enzyme(s) responsible for demethyl-IQ formation (Table 3). Ellipticine was the most effective inhibitor of microsomal metabolism. Whereas our previous studies in mice (Lakshmi et al., 2008) suggested that ellipticine was a 1A1 inhibitor, rat and human studies have suggested otherwise (Draper et al., 1997; Aimová and Stiborová, 2005). Furafylline, a 1A2 inhibitor, did not inhibit synthesis. Ketoconazole is an effective inhibitor of 1A2 in rats (Kobayashi et al., 2003) and was the only other agent that significantly inhibited demethyl-IQ synthesis at a concentration of 0.02 mM. Additional studies are needed to distinguish the specific P450(s) involved.
For BNF-treated mice, the inhibitor response to IQ oxidation to form m/z 217 was different from that observed with N-demethylation (Table 4). Although 0.02 mM furafylline had no significant effect on IQ N-demethylation, it reduced m/z 217 formation by approximately 50%. Ellipticine and α-naphthoflavone, a P450 1A inhibitor, completely prevented oxidation. As observed with demethyl-IQ (Table 3), sulfaphenazole (2C) did not reduce the synthesis of m/z 217. Mutagenic activation of IQ by human 1A2 was inhibited by α-naphthoflavone (Josephy et al., 1998). To further evaluate 1A inhibitors, metabolism of 1 mM phenacetin, a 1A substrate, was assessed. Microsomal acetaminophen formation was significantly reduced by both 0.01 mM ellipticine (30% of control) and furafylline (64% of control), with the former more effective. Using ethoxyresorufin (0.002 mM), a 1A1 selective substrate, metabolism was evaluated over a range of concentrations of ellipticine and furafylline. A 50% inhibition of 7-ethoxyresorufin-O-deethylation was observed with 0.0005 mM ellipticine and 0.0087 mM furafylline. More selective inhibition of O-deethylation by ellipticine than by furafylline has also been reported with control mouse microsomes (Lakshmi et al., 2008). Additional studies with inhibitory antibodies could help distinguish a more prominent role for either 1A1 or 1A2. Results suggest that m/z 217 formation by BNF-treated mice is mediated by P450 1A enzymes.
In liver slice incubations, ellipticine elicited a 90% reduction in m/z 217 formation. However, no significant change in demethyl-IQ formation occurred (Fig. 4, bottom; Table 2). Our microsomal inhibitor studies (Dixon plot) indicate ellipticine is approximately 6-fold more effective in inhibiting m/z 217 than demethyl-IQ formation. In addition, the ellipticine concentration in slices would be considerably reduced by P450 metabolism (Stiborová et al., 2004) and/or restriction of its transport into liver slices. Together these factors could explain the lack of demethyl-IQ inhibition by ellipticine in liver slices.
5-OH-IQ is labile and not detected in microsomal incubation. However, slice incubations provide evidence of its formation by the presence of the 5-O-glucuronide. Formation was inhibited approximately 80% by ellipticine in liver slice incubations (Fig. 4, bottom). A similar inhibition was observed with m/z 217. Thus, P450 1A enzymes may be responsible for the formation of m/z 217, 5-OH-IQ, and demethyl-IQ.
The reactions responsible for m/z 217 formation need to be determined. P450s produce mono-oxidation products, which should add OH rather than add H2O to IQ. This and previous studies have shown mouse P450-catalyzed IQ oxidation at C-5 (Lakshmi et al., 2008). Therefore, m/z 217 formation may first involve P450-mediated formation of 5-OH-IQ. Slice incubations emphasize that this is a major hepatic P450-dependent pathway for IQ metabolism. If 5-OH-IQ is reduced to m/z 217, inhibition of m/z 217 formation by ellipticine would not necessarily mean the reduction is catalyzed by P450 1A. The structure of m/z 217 is not consistent with known products of imidazole reduction, i.e., imidazoline and imidazolidine. Whether this reduction is nonenzymatic or enzymatic remains unclear. The m/z 217 is stable compared with 5-OH-IQ. Urinary excretion of m/z 233 and 393 further substantiates the in vivo formation of m/z 217 and is consistent with formation of these metabolites by either oxidation or O-glucuronidation of m/z 217.
Differences between mouse and rat IQ metabolism were also found in the proportion of 5-sulfate metabolite shared by both species. In control rats receiving the same dose of IQ (40 mg/kg), 5-sulfate was nearly 4-fold greater in rats than mice (Lakshmi et al., 2008). With BNF treatment, mouse urinary 5-sulfate increased from 2.4 ± 0.2 to 4.2 ± 0.4% of total urine radioactivity (Table 1). The latter value is still considerably less than that from the young rats (31 ± 2%) treated with a similar dose of BNF (Armbrecht et al., 2007).
In conclusion, our studies of IQ metabolism by BNF-treated mice have identified three previously unknown metabolites and have shown the presence of four metabolites not observed in rats. The N-demethylated and C-7 hydroxylated metabolites of aminoimidazole HCAs are reported in monkeys and humans (Snyderwine et al., 1992; Langouët et al., 2001). Human enzymes responsible for these products have not been studied. Mouse studies show involvement of P450 in N-demethylation and offer a resource for further evaluation of this pathway and that for C-7 hydroxylation. Demethyl-IQ is a weak mutagen (Barnes et al., 1985), representing approximately 30% of the total IQ metabolism in control mice. In BNF-treated mice, the new metabolite m/z 217 appears to be derived from 5-OH-IQ, and when considered along with 5-O-glucuronide, this pathway accounts for nearly 80% of IQ metabolites. Formation of m/z 217 and its metabolites appear to have increased polarity relative to IQ. The mouse model described here identified pathways for HCA metabolism present in humans and provides a rationale for future studies, including assessment of tumorigenicity.
Acknowledgments
We thank Priscilla Jones for excellent technical assistance. Mass spectrometry was performed at Washington University School of Medicine supported by the National Institutes of Health.
This work was supported in part by the Department of Veterans Affairs; the National Institutes of Health National Cancer Institute [Grant CA72613]; the National Institutes of Health National Center for Research Resources [Grant P41-RR00954]; and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants P30-DK56341, P60-DK20579].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.027342.
ABBREVIATIONS: HCA, heterocyclic amine; IQ, 2-amino-3-methylimidazo[4,5-f]quinoline; P450, cytochrome P450; demethyl-IQ, 2-amino-imidazo[4,5-f]quinoline; BNF, β-naphthoflavone; N-acetyl-IQ, N-acetyl-2-amino-3-methylimidazo[4,5-f]quinoline; ESI/MS, electrospray ionization/mass spectrometry; HPLC, high-performance liquid chromatography.
References
- Adamson RH, Takayama S, Sugimura T, and Thorgeirsson UP (1994) Induction of hepatocellular carcinoma in nonhuman primates by the food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline. Environ Health Perspect 102 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimová D and Stiborová M (2005) Antitumor drug ellipticine inhibits the activities of rat hepatic cytochromes P450. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149 437–440. [PubMed] [Google Scholar]
- Armbrecht HJ, Lakshmi VM, Wickstra J, Hsu FF, and Zenser TV (2007) Metabolism of a heterocyclic amine colon carcinogen in young and old rats. Drug Metab Dispos 35 633–639. [DOI] [PubMed] [Google Scholar]
- Barnes WS, Lovelette CA, Tong C, Williams GM, and Weisburger JH (1985) Genotoxicity of the food mutagen 2-amino-3-methylimidazo-[4,5-f]quinoline (IQ) and analogs. Carcinogenesis 6 441–444. [DOI] [PubMed] [Google Scholar]
- Draper AJ, Madan A, and Parkinson A (1997) Inhibition of coumarin 7-hydroxylase activity in human liver microsomes. Arch Biochem Biophys 341 47–61. [DOI] [PubMed] [Google Scholar]
- Felton JS and Knize MG (1990) Heterocyclic-amine mutagens/carcinogens in foods, in Handbook of Experimental Pharmacology (C.S. Cooper and P.L. Grover eds) pp 471–502, Springer-Verlag, Berlin.
- Garner RC, Lightfoot TJ, Cupid BC, Russell D, Coxhead JM, Kutschera W, Priller A, Rom W, Steier P, Alexander DJ, et al. (1999) Comparative biotransformation studies of MeIQx and PhIP in animal models and humans. Cancer Lett 143 161–165. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Cain LG, and Kensler TW (1988) Aflatoxin exposure in human populations: measurements and relationship to cancer. Crit Rev Toxicol 19 113–145. [DOI] [PubMed] [Google Scholar]
- IARC (1983) Benzo[a]pyrene, in Monographs on the Evaluation on the Carcinogenic Risk of Chemicals to Humans. Polynuclear Aromatic Compounds, Part 1: Chemical, Environmental, and Experimental Data (IARC ed) pp 211–224, International Agency for Research on Cancer, Lyon, France. [PubMed]
- Inamasu T, Luks H, Vavrek MT, and Weisburger JH (1989) Metabolism of 2-amino-3-methylimidazo[4,5-f]quinoline in the male rat. Food Chem Toxicol 27 369–376. [DOI] [PubMed] [Google Scholar]
- Josephy PD, Evans DH, Parikh A, and Guengerich FP (1998) Metabolic activation of aromatic amine mutagens by simultaneous expression of human cytochrome P450 1A2, NADPH-cytochrome P450 reductase, and N-acetyltransferase in Escherichia coli. Chem Res Toxicol 11 70–74. [DOI] [PubMed] [Google Scholar]
- Kestell P, Zhao L, Zhu S, Harris PJ, and Ferguson LR (1999) Studies on the mechanism of cancer protection by wheat bran: effects on the absorption, metabolism and excretion of the food carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline (IQ). Carcinogenesis 20 2253–2260. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Urashima K, Shimada N, and Chiba K (2003) Selectivities of human cytochrome P450 inhibitors toward rat P450 isoforms: study with CDNA-expressed systems of the rat. Drug Metab Dispos 31 833–836. [DOI] [PubMed] [Google Scholar]
- Lakshmi VM, Bell DA, Watson MA, Zenser TV, and Davis BB (1995a) N-Acetylbenzidine and N,N'-diacetylbenzidine formation by rat and human liver slices exposed to benzidine. Carcinogenesis 16 1565–1571. [DOI] [PubMed] [Google Scholar]
- Lakshmi VM, Hsu FF, and Zenser TV (2008) N-Demethylation is a major route of 2-amino-3-methylimidazo[4,5-f]quinoline metabolism in mouse. Drug Metab Dispos 36 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi VM, Zenser TV, Goldman HD, Spencer GG, Gupta RC, Hsu FF, and Davis BB (1995b) The role of acetylation in benzidine metabolism and DNA adduct formation in dog and rat liver. Chem Res Toxicol 8 711–720. [DOI] [PubMed] [Google Scholar]
- Lang NP, Butler MA, Massengill J, Lawson M, Stotts RC, Hauer-Jensen M, and Kadlubar FF (1994) Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomarkers Prev 3 675–682. [PubMed] [Google Scholar]
- Langouët S, Welti DH, Kerriguy N, Fay LB, Huynh-Ba T, Markovic J, Guengerich FP, Guillouzo A, and Turesky RJ (2001) Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem Res Toxicol 14 211–221. [DOI] [PubMed] [Google Scholar]
- Luks HJ, Spratt TE, Vavrek MT, Roland SF, and Weisburger JH (1989) Identification of sulfate and glucuronic acid conjugates of the 5-hydroxy derivative as major metabolites of 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Cancer Res 49 4407–4411. [PubMed] [Google Scholar]
- Minchin RF, Reeves PT, Teitel CH, McManus ME, Mojarrabi B, Ilett KF, and Kadlubar FF (1992) N- and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochem Biophys Res Commun 185 839–844. [DOI] [PubMed] [Google Scholar]
- Pais P, Salmon CP, Knize MG, and Felton JS (1999) Formation of mutagenic/carcinogenic heterocyclic amines in dry-heated model systems, meats, and meat drippings. J Agric Food Chem 47 1098–1108. [DOI] [PubMed] [Google Scholar]
- Snyderwine EG, Welti DH, Fay LB, Würzner HP, and Turesky RJ (1992) Metabolism of the food mutagen 2-amino-3-methylimidazo[4,5-f]quinoline in nonhuman primates undergoing carcinogen bioassay. Chem Res Toxicol 5 843–851. [DOI] [PubMed] [Google Scholar]
- Stiborová M, Sejbal J, Borek-Dohalská L, Aimová D, Poljaková J, Forsterová K, RupertováM, Wiesner J, Hudecek J, Wiessler M, et al. (2004) The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res 64 8374–8380. [DOI] [PubMed] [Google Scholar]
- Sugimura T (2000) Nutrition and dietary carcinogens. Carcinogenesis 21 387–395. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Garner RC, Welti DH, Richoz J, Leveson SH, Dingley KH, Turteltaub KW, and Fay LB (1998) Metabolism of the food-borne mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in humans. Chem Res Toxicol 11 217–225. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Lang NP, Butler MA, Teitel CH, and Kadlubar FF (1991) Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis 12 1839–1845. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Skipper PL, Tannenbaum SR, Coles B, and Ketterer B (1986) Sulfamate formation is a major route for detoxification of 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Carcinogenesis 7 1483–1485. [DOI] [PubMed] [Google Scholar]
- Zenser TV, Mattammal MB, and Davis BB (1978) Differential distribution of the mixed-function oxidase activities in rabbit kidney. J Pharmacol Exp Ther 207 719–725. [PubMed] [Google Scholar]