Abstract
In vitro biosynthesis using pooled human liver microsomes was applied to help identify in vivo metabolites of ketamine by liquid chromatography (LC)-tandem mass spectrometry. Microsomal synthesis produced dehydronorketamine, seven structural isomers of hydroxynorketamine, and at least five structural isomers of hydroxyketamine. To aid identification, stable isotopes of the metabolites were also produced from tetra-deuterated isotopes of ketamine or norketamine as substrates. Five metabolites (three hydroxynorketamine and two hydroxyketamine isomers) gave chromatographically resolved components with product ion spectra indicating the presence of a phenolic group, with phenolic metabolites being further substantiated by selective liquid-liquid extraction after adjustments to the pH. Two glucuronide conjugates of hydroxynorketamine were also identified. Analysis by LC-coupled ion cyclotron resonance mass spectrometry gave unique masses in accordance with the predicted elemental composition. The metabolites, including the phenols, were subsequently confirmed to be present in urine of subjects after oral ketamine administration, as facilitated by the addition of deuterated metabolites generated from the in vitro biosynthesis. To our knowledge, phenolic metabolites of ketamine, including an intact glucuronide conjugate, are here reported for the first time. The use of biologically synthesized deuterated material as an internal chromatographic and mass spectrometric marker is a viable approach to aid in the identification of metabolites. Metabolites that have particular diagnostic value can be selected as candidates for chemical synthesis of standards.
The development of procedures in analytical, clinical, and forensic toxicology is usually restricted to compounds that are commercially available as analytical standards. Because many drugs are extensively metabolized, it is desirable to target metabolites in the hope of improving sensitivity and specificity of suspected drug ingestion. Custom synthesis of metabolites of interest, known or putative, to aid drug metabolism studies, can be prohibitively expensive. Alternatively, in-house synthesis of metabolites in laboratories dedicated to analysis is usually hindered by a lack of expertise and equipment. The use of human microsomes to produce metabolites on a small scale, which can then be characterized, may offer a simple, rapid, and economical solution. Although this strategy is no substitute for authenticated chemically synthesized material, it does offer an important intermediate solution until such material becomes commercially available. In addition, it provides the prospect of using analogs of the drug as a substrate, particularly those labeled with stable isotopes that can then be converted to labeled metabolites suitable for use as internal “standards” in analysis by mass spectrometry.
Ketamine was chosen as a model substrate because not all of its reference metabolites are available. In addition, because it has been used as an anesthetic since the 1960s, its uses are widespread and include veterinary medicine and pediatrics. It is also used recreationally as a “club drug” (Degenhardt et al., 2005; Kenyon et al., 2005; Leong et al., 2005), and there is a concern that ketamine may be used to facilitate sexual assault. Methods developed to analyze ketamine and norketamine in various biological matrices are widespread (Bolze and Boulieu, 1998; Yanagihara et al., 2003; Lua and Lin, 2004; Adamowicz and Kala, 2005; Leong et al., 2005; Negrusz et al., 2005; Wang et al., 2005; Apollonio et al., 2006; Xiang et al., 2006) but the analysis of the secondary metabolite dehydronorketamine, and hydroxylated metabolite (both intact and conjugated), is much less common (Adams et al., 1981; Bolze and Boulieu, 1998; Hijazi et al., 2001). At the time of this work, the lack of commercial standards has hindered analysis of these metabolites, whose identification requires custom synthesis (Huang et al., 2005; Wu et al., 2007). Procedures for identifying novel metabolites of ketamine are of interest, not least for forensic purposes.
Often the enzymes responsible for metabolism are unknown, hindering the use of recombinant enzymes to produce specific metabolites (Peters et al., 2007). The N-demethylation of ketamine is performed by CYP2B6 (Yanagihara et al., 2001), but the enzymes for other stages of metabolism have not been characterized. However, the in vivo metabolism of a drug can be mimicked by the use of microsomes. Human microsomes are used in drug discovery for in vitro assays to elucidate metabolic pathways, to predict drug-drug interactions, to determine inhibition or induction potentials of enzymes by the new drug, and to help identify possible toxic metabolites.
In this study, we report the approach of using commercial human microsomes to produce metabolites of ketamine and norketamine, which, for the first time, have been used as analytical markers in their crude form. Furthermore, microsomes were also used to synthesize metabolites of deuterated substrates to assist in the assignment of analytical properties of the metabolites, such as retention time and MS/MS spectra. Using this in vitro approach, we have identified novel metabolites, these being phenolic isomers of hydroxyketamine and hydroxynorketamine, and two phase II metabolites. This, in turn, led to the identification of metabolites in urine after ketamine administration to volunteers, using the synthesized deuterated metabolites as reference compounds.
Materials and Methods
Chemicals and Reagents. Methanolic standards of ketamine HCl, norketamine HCl (1 mg/ml as the free base), and 3,4,5,6-tetradeuterophenyl-ketamine HCl and 3,4,5,6-tetradeuterophenyl-norketamine HCl (0.1 mg/ml as the free base) were purchased from LGC Promochem (Teddington, UK). Infusions of these standards into a quadrupole mass spectrometer yielded protonated molecules that were consistent with these compounds. The only impurities observed were trideuterated ketamine in the tetradeuterated ketamine and trideuterated norketamine in the tetradeuterated ketamine, these being 14 and 3%, respectively (comparing peak heights of the protonated molecules). Water was purified (resistivity 18.2 MΩ-cm) by a Maxima HPLC ultra-pure water system (Elga, High Wycombe, UK). Acetonitrile, acetic acid, propan-2-ol (gas-liquid chromatography pesticide residue grade), dichloromethane (HPLC grade), hydrochloric acid (analytical reagent grade), sodium hydroxide pellets, potassium hydroxide pellets, potassium dihydrogen orthophosphate, disodium hydrogen orthophosphate dihydrate, and sodium dihydrogen orthophosphate dihydrate were from Fisher Scientific (Loughborough, UK). Ammonia solution was obtained from BDH Chemicals (Poole, UK). Formic acid was obtained from VWR International (Lutterworth, UK). 1-Chlorobutane (Chromasolv) was obtained from Sigma-Aldrich (Poole, UK). NADPH-regenerating system A (consisting of 31 mM NADP+, 66 mM glucose 6-phosphate, and 66 mM MgCl2 in water), NADPH-regenerating system B (consisting of 40 U/ml glucose-6-phosphate dehydrogenase in 5 mM sodium citrate), UDP reaction mix solution A (consisting of 25 mM UDP-glucuronic acid in water), and UDP-regenerating system B (consisting of 250 mM Tris-HCl, 40 mM MgCl2, and 0.125 mg/ml alamethicin in water) were obtained from BD Biosciences (Oxford, UK), as were pooled human liver microsomes (20 mg/ml in 250 mM sucrose). Bond Elute Certify I (mixed-mode; C8 with strong cation exchange) solid-phase extraction (SPE) cartridges were obtained from Varian (Walton-on-Thames, UK).
In Vitro Metabolism of Ketamine or Norketamine by Microsomes. For phase I metabolism, to either 10 μg of ketamine, ketamine-d4, norketamine, or norketamine-d4, the following were added and then mixed: 500 μl of 0.2 M phosphate buffer (pH 7.4), 50 μl of the microsome preparation (to give a final concentration of 1 mg/ml protein), 50 μl of NADPH-regenerating system A, 10 μl of NADPH-regenerating system B, and 390 μl of water to make up to a 1 ml volume in total. The combined components, with the exception of the microsomes, were preincubated at 37°C for 10 min, after which the microsomes were added to initiate metabolism. The mixture was subsequently incubated for 2 h at 37°C, with vortex mixing every 30 min, after which metabolism was terminated with the addition of 250 μl of acetonitrile on ice, and centrifuged at 12,000g for 4 min to precipitate and remove the protein. Phase II metabolism was also performed in conjunction with phase I, the methodology being that described above, with the exception that instead of 380 μl of water being added, 125 μl of UDP reaction mix solution A and 200 μl of UDP-regenerating system B were added with 65 μl of water. Ten incubations of each deuterated substrate were performed to synthesize a sufficient quantity of metabolites as our investigation progressed, amounting in total to 100 μg of deuterated ketamine and 100 μg of deuterated norketamine.
pH-Dependent Extraction of Metabolites. To help identify novel phenolic metabolites produced in microsomal incubates, the solution was extracted with 1-chlorobutane (3 by 5 ml extractions) after pH adjustment to 8.4 using 0.1 M NaOH. After vortexing, shaking on a rotary mixer for 5 min, and centrifugation at 2000g for 5 min, the 1-chlorobutane layers were combined and evaporated at 60°C under nitrogen and reconstituted in 250 μl of acetonitrile-water (10:90, v/v). In addition, a separate triplicate extraction was performed after adjustment to pH 12 to confirm the absence of phenolic metabolites; because they are amphoteric (having a phenol and amine function), they should not be extracted at this pH. The aqueous portion of this extract was readjusted back to pH 8.4 and extracted again three times to aid in phenolic metabolite identity.
Ultra-High-Performance Liquid Chromatography-Mass Spectrometry. Analysis was performed using an Acquity ultra-performance liquid chromatography system coupled to a Quattro Premier triple quadrupole mass spectrometer (Waters, Elstree, UK). A BEH C18 2.1 × 150 mm column with 1.7-μm particle size (Waters) was used with gradient mobile phase conditions of 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B) with a flow rate of 0.35 ml/min and a column temperature of 45°C. A run time of 8 min was used, starting at 10% B for 0.5 min, ramping to 50% B over 7 min, and ramping back down to the original conditions over 0.5 min. Incubates were injected in aliquots of 20 μl onto the HPLC system in either extracted or crude form.
Full-scan MS/MS (m/z 50–260) experiments and selected reaction monitoring (SRM) were performed at cone voltages of 25 V. Total scan times were typically 1.2 s for MS/MS and SRM experiments. MS/MS experiments were typically performed using collision energies of 15 to 22 eV. The most prominent ions obtained from MS/MS experiments were used for SRM in the positive electrospray ionization mode after further optimization of collision energies for each transition. The appropriate SRM transitions and the collision energies used were as follows: 238 → 115 (42 eV), 238 → 125 (27 eV), and 238 → 163 (25 eV) for ketamine; 242 → 129 (27 eV) for ketamine-d4; 224 → 115 (42 eV), 224 → 125 (27 eV), and 224 → 207 (16 eV) for norketamine; 228 → 211 (42 eV) for norketamine-d4; 222 → 125 (27 eV), 222 → 177 (30 eV), and 222 → 205 (30 eV) for dehydronorketamine; 226 → 209 (30 eV) for dehydronorketamine-d4; 240 → 125 (30 eV), 240 → 151 (25 eV), 240 → 177 (25 eV), 240 → 179 (25 eV), 240 → 195 (25 eV), 240 → 205 (25 eV), and 240 → 141 (30 eV) for hydroxynorketamine isomers, 242 → 127 (30 eV), 242 → 143 (25 eV), and 242 → 179 (25 eV) for 37Cl isotopes of hydroxynorketamine isomers; 244 → 129 (25 eV) and 246 → 131 (25 eV) for hydroxynorketamine-d4 and its 37Cl isotope (cyclohexanone ring-hydroxylated); 243 → 144 (30 eV) and 245 → 146 (30 eV) for hydroxynorketamine-d3 and its 37Cl isotope (chlorobenzene ring-hydroxylated); 254 → 125 (30 eV), 254 → 141 (22 eV), 254 → 151 (25 eV), 254 → 163 (25 eV), 254 → 179 (25 eV), and 254 → 195 (25 eV) for hydroxyketamine isomers; 256 → 127 (30 eV), 256 → 143 (30 eV), and 256 → 181 (25 eV) for 37Cl isotopes of hydroxyketamine isomers; 258 → 129 (30 eV) and 260 → 131 (30 eV) for hydroxyketamine-d4 and its 37Cl isotope; 257 → 144 (30 eV) and 259 → 146 (30 eV) for hydroxyketamine-d3 and its 37Cl isotope; 416 → 240 (20 eV), 416 → 222 (35 eV), 416 → 125 (25 eV), and 416 → 207 (25 eV) for hydroxynorketamine glucuronide; and 420 → 129 (20 eV) for hydroxynorketamine glucuronide-d4. Electrospray, extractor, and radiofrequency lens voltages were 3.5 kV, 4 V, and 0 V, respectively. MS experiments used the same conditions as mentioned above except for the collision energy, 0 eV, and entrance voltage, 50 V. All data were collected in the centroid mode.
Volunteer Study and Collection of Samples. Six volunteers, three male and three female, were administered an oral dose of 50 mg of ketamine in the form of a quarter-vial (5 ml) of a 200 mg ketamine (Ketalar) dose (AAH Pharmaceuticals Ltd., Romford, UK), diluted with 125 ml of water. The rationale for this dose was that, taking into account that the oral bioavailability of ketamine is approximately 20%, a 50 mg oral administration would not be expected to cause a loss of consciousness and would have a minimal risk of psychedelic effects in volunteers with a normal body mass index. Indeed, with this dose, the volunteers were found to be only mildly sedated. Urine was collected 10 min before administration of the dose and at designated time points over a period of 11 days (0–2, 2–4, 4–6, 6–8, 8–12, and 12–24 h and spot collections at 30 h and 2–10 days postdose with morning and afternoon samples collected on days 4, 7, and 10). Ethics approval for the study was granted by our institution (King's College Research Ethics Committee), and written informed consent was obtained from the volunteers.
Preparation and Extraction of Urine Samples from the Volunteer Study. To 4 ml of each urine sample, 6 ml of phosphate buffer (10 mM, pH 6.0) together with 200 μl of deuterated incubate was added, and the solution was vortex mixed for 30 s. Mixed-mode SPE cartridges were preconditioned by slowly passing under vacuum 2 ml of propan-2-ol followed by 2 ml of phosphate buffer. The buffered urine samples were then loaded onto the SPE cartridges and allowed to slowly pass through the bed without vacuum. Empty urine tubes were washed with 1 ml of phosphate buffer, and this wash was also added to the cartridge. Loaded SPE cartridges were then rinsed sequentially with 2 ml of water and 2 ml of acetate buffer (0.1 mol/l, pH 4.0). Retained drug and metabolites were eluted under gravity with dichloromethane:propan-2-ol: ammonium hydroxide (78:20:2 v/v) in four sequential aliquots of 1 ml (4 ml total). The eluent was evaporated under nitrogen at 60°C. The dried extracts were reconstituted in 250 μl of water-acetonitrile-formic acid (89.9:10:0.1 v/v/v) and vortex-mixed for 30 s, and 20 μl was injected into the chromatographic system.
Accurate Mass Measurement (LC-FT-ICR-MS). The chromatographic conditions were achieved using a 1200 series quaternary LC system (Agilent, Wokingham, UK). An XBridge C18 column with dimensions of 150 × 2.1 mm and 3 μm particle size (Waters) was used, without temperature control, with a gradient program consisting of formic acid (0.1%) in water and acetonitrile with formic acid (0.1%) as mobile phases A and B, respectively. The gradient involved maintaining a composition of 10% B for 5 min followed by ramping up to 17% over 7 min, maintaining this composition for 5 min and ramping up to 50% B over 1 min, maintaining 50% B for 3 min, ramping back down to 10% B over 1 min, and reequilibration for 19 min to give a total run time of 40 min. The flow rate was 0.25 ml/min, and the volume of sample injected was 50 μl.
An LTQ Fourier transform-ion cyclotron resonance-mass spectrometer (Thermo Fisher Scientific, Waltham, MA) was used to determine accurate masses of metabolites. Electrospray ionization source parameters were as follows: sheath gas flow rate, 25; auxiliary gas flow rate, 5; sweep gas flow rate, 0; electrospray voltage, 4 kV; capillary temperature, 275°C; capillary voltage, 13 V; and tube lens voltage, 30 V. Data were collected in profile mode.
Results
In Vitro Metabolism of Ketamine and Norketamine and Their Deuterated Analogs. Incubation of ketamine and norketamine and their deuterated analogs with microsomes resulted in a number of metabolites. Metabolites of the substrates ketamine and norketamine from the microsomal incubate were identified by liquid chromatography-mass spectrometry. The structures of the metabolites are given in Table 1, as interpreted from the data presented here.
TABLE 1.
Properties of ketamine and metabolites that were monitored in microsomal preparations and in urine
Retention times and m/z ratios targeted for the metabolites of ketamine showing all the isomers of hydroxynorketamine and hydroxyketamine are included. Accurate masses are shown where obtained (with ppm error from theoretical).
| Structure | Abbreviation | LC tR | m/z (Deuterated Analog) | Measured m/z (ppm) |
|---|---|---|---|---|
| min | ||||
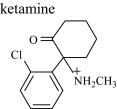 |
K | 3.7 | 238 (242) | 238.09923 (0.084) |
 |
NK | 3.5 | 224 (228) | 224.08365 (0.089) |
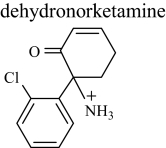 |
DHNK | 3.0 | 222 (226) | 222.06801 (0.045) |
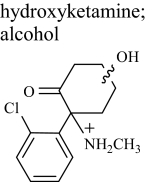 |
HK 1 | 2.0 | 254 (258) | 254.09418 (0.275) |
| HK 2 | 2.6 | 254 (258) | 254.09418 (0.275) | |
| HK 5 | 5.8 | 254 (258) | —a | |
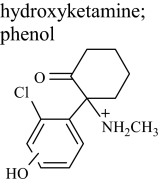 |
HK 3 | 3.2 | 254 (257) | 254.09418 (0.275) |
| HK 4 | 3.4 | 254 (257) | — | |
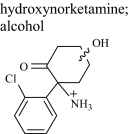 |
HNK 1 | 1.7 | 240 (244) | 240.07858 (0.042) |
| HNK 2 | 2.2 | 240 (244) | 240.07857 (0.042) | |
| HNK 3 | 2.4 | 240 (244) | 240.07858 (0.042) | |
| HNK 7 | 5.8 | 240 (244) | 240.07858 (0.042) | |
| Glucuronide conjugate | 5.3 | 416 (420) | 416.11075 (0.192) | |
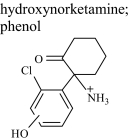 |
HNK 4 | 2.8 | 240 (243) | 240.07863 (0.208) |
| HNK 5 | 3.1 | 240 (243) | 240.07858 (0.042) | |
| HNK 6 | 3.3 | 240 (243) | 240.07870 (0.499) | |
| Glucuronide conjugate | 1.2 | 416 (419) | — |
—, insufficient metabolite for accurate mass determination.
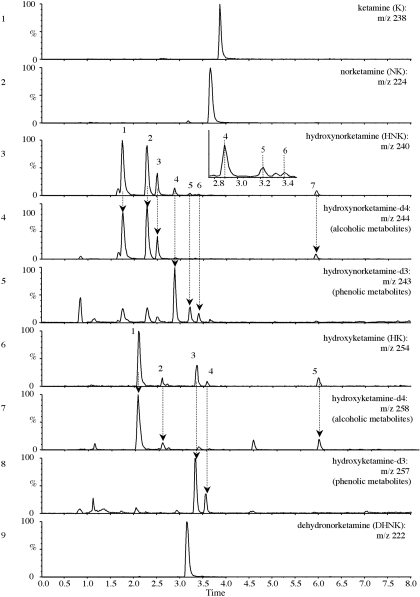
After centrifugation of the incubate, a portion of the supernatant was analyzed, which resulted in a number of peaks in the total ion chromatogram that were not observed in the absence of substrate. It was assumed that these peaks were dehydro and hydroxylated metabolites, and therefore extracted ion chromatograms were generated based on the predicted monoisotopic (containing the 35Cl isotope) protonated molecule [M + H]+, as presented in Fig. 1. These were m/z 222 for dehydronorketamine, m/z 240 for hydroxynorketamine, and m/z 254 for hydroxyketamine. Isotopic doublets 35Cl/37Cl of each metabolite were observed at the expected 3:1 ratio. An extracted ion chromatogram of m/z 236 showed no peak, indicating that dehydroketamine was not present in significant amounts. No evidence of hydroxylated dehydronorketamine (m/z 238) or dihydroxynorketamine metabolites (m/z 256) was obtained from microsomal incubates of ketamine or norketamine.
Fig. 1.
Extracted ion chromatograms of selected metabolites obtained from microsomal incubates: ketamine (m/z 238), norketamine (m/z 224), hydroxynorketamine (m/z 240), tetradeuterated hydroxynorketamine (m/z 244), trideuterated hydroxynorketamine (m/z 243), hydroxyketamine (m/z 254), tetradeuterated hydroxyketamine (m/z 258), trideuterated hydroxyketamine (m/z 257), and dehydronorketamine (m/z 222). Dotted lines are drawn through the peaks discussed in the main text.
When norketamine was used as a substrate, metabolites common with those of ketamine were identified, but the yield was approximately 3-fold higher, as judged by comparison of peak areas. This is not surprising as norketamine is considered to be the primary metabolite of ketamine, from which secondary metabolites are formed (Wieber et al., 1975).
Preliminary Detection of Phenolic and Alcoholic Metabolites by Liquid Chromatography-Mass Spectrometry. The extracted ion chromatogram for m/z 240 showed seven possible isomers, as seen in Fig. 1, but only four alcoholic isomers of hydroxynorketamine have been described in the literature (Adams et al., 1981). Furthermore, when tetradeuterated substrate was used, only four analogous peaks were visible at m/z 244 (Fig. 1, compare panels 3 and 4). Given this difference in the number of peaks, it was reasonable to consider the possibility of the formation of previously unreported phenolic metabolites, in which substitution with a hydroxyl group on the chlorobenzene ring would result in the loss of a deuterium atom, giving an [M + H]+ of m/z 243. Indeed, when an extracted ion chromatogram of m/z 243 was generated, this showed three additional peaks (Fig. 1, panel 5), with the extracted ion chromatograms of m/z 243 and m/z 244 displaying seven peaks in total with retention times contemporaneous to those observed when nondeuterated substrate was used. It should be noted that the early eluting peaks (retention times of 1.7, 2.2, and 2.4 min) correspond to alcoholic metabolites in the m/z 243 extracted ion chromatogram as a result of a trideuterated impurity, i.e., norketamine-d3 present in the tetradeuterated standard.
Hydroxyketamine metabolites were indicated by m/z 254 showing five dominant peaks. Only three peaks with the same retention time and similar intensity were observed when deuterated ketamine was used (m/z 258), indicating that these were alcoholic metabolites, and m/z 257 showed two peaks, indicating the presence of phenolic metabolites.
Evidence Supportive of the Formation of Phenolic Metabolites by pH-Dependent Extraction. To help confirm the presence of phenolic metabolites, a liquid-liquid extraction was performed with 1-chlorobutane after adjustment of the pH of the microsomal incubates. It was assumed that the primary or secondary amine was conserved, making the phenolic metabolites amphoteric. The first extraction was at pH 8.4, a pH that would be expected to be favorable for extraction of both alcoholic and phenolic metabolites into 1-chlorobutane, based on 2-, 3-, or 4-chlorophenol having a pKa of 8.5, 9.0, and 9.4, respectively, and ketamine (secondary amine) having a pKa of 6.7 and norketamine (primary amine) having a pKa of 7.5 (Cohen and Trevor, 1974). Another separate extraction was performed after adjusting the incubate to pH 12, a pH at which phenolic metabolites would be expected to have a net negative charge and therefore remain favorably partitioned in the aqueous medium. To increase analytical sensitivity, MS/MS was performed on the precursor ions [for putative hydroxyketamine (m/z 254) and its 37Cl isotope (m/z 256) and putative hydroxynorketamine (m/z 240 and 242)], and the total product ion chromatograms were recorded. Analysis of the organic extract of the incubate adjusted to pH 8.4 resulted in four of the five peaks also recorded in Fig. 1 for putative hydroxyketamine, as well as some smaller peaks (undetected in MS mode) and six of the seven peaks for putative hydroxynorketamine. Extraction at pH 12 showed only two of these peaks for hydroxyketamine and three for hydroxynorketamine, suggesting that the peaks absent from the chromatogram of the pH 12 extract were due to phenolic compounds being retained in the aqueous fraction. Readjustment of the remaining aqueous fraction from pH 12 to 8.4 followed by 1-chlorobutane extraction showed the “reappearance” of these peaks. These data further support the presence of at least two phenolic isomers of hydroxyketamine and three phenolic isomers of hydroxynorketamine. Two metabolites present in the incubate, as analyzed by injection of unextracted microsomal preparations (hydroxynorketamine 7 and hydroxyketamine 5) (Fig. 1), appeared not to be extracted into 1-chlorobutane at either pH (portion of chromatograms therefore not shown). It is likely, nevertheless, that these are hydroxylated metabolites, given the elemental composition obtained by FT-ICR-MS (see later). The lack of extraction into 1-chlorobutane infers that these metabolites are more polar, one speculative explanation being that the hydroxyl substituent is possibly adjacent to the keto function on the cyclohexane ring, favoring the formation of an enol tautomer to give a dihydroxy metabolite.
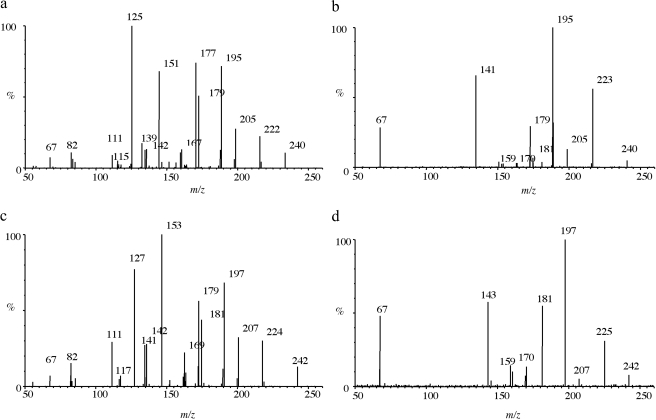
Identification of Hydroxylated Metabolites by Tandem Mass Spectrometry. MS/MS fragmentation of the metabolites by direct injection of portions of the incubates gave product spectra consistent with dehydronorketamine and hydroxynorketamine and also hydroxyketamine if ketamine was used as the substrate. The major fragment ions of monoisotopic norketamine (35Cl isotope) were m/z 207, m/z 189, m/z 179, and m/z 125, which were consistent with published data, as described by Wang et al. (2005); other ions observed were m/z 163, m/z 151, and m/z 141. Product ions for 35Cl-dehydronorketamine were m/z 205, m/z 187, m/z 177, m/z 170, m/z 142, and m/z 141. Fragmentation of 37Cl isotopes was used to help confirm the identity of the fragment ions containing 35Cl isotopes; the presence of ions with a m/z 2-unit difference in a 3:1 ratio signified a fragment containing chlorine.
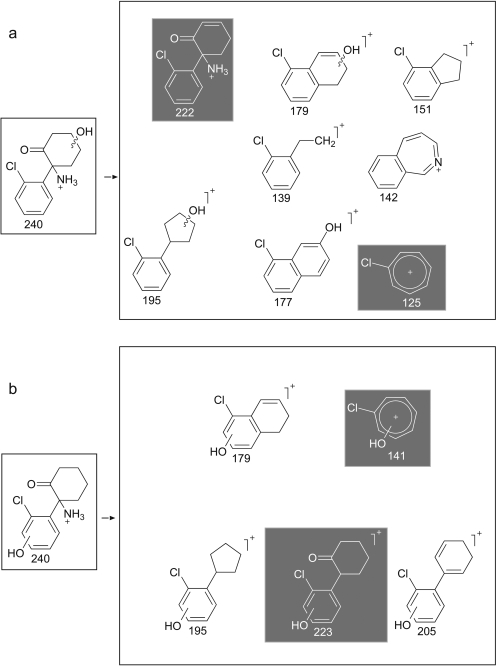
MS/MS spectra of the metabolites thought to be hydroxynorketamine fell into two classes, either displaying or not displaying a prominent product ion at m/z 141 (Fig. 2), with the possible structures of fragments of alcoholic isomers and phenolic metabolites being displayed in Fig. 3, a and b, respectively. A dominant product ion m/z 141 is consistent with a fragment of a phenolic metabolite (Fig. 3b). The absence of a phenolic group would result instead in a chloromethylbenzene fragment, and indeed an ion with an m/z 125 was prominent in the spectra not displaying a prominent m/z 141, indicating these metabolites were hydroxylated on the cyclohexanone ring rather than on the benzene ring (Fig. 3a). Fragmentation of 37Cl isotopes of the metabolites confirmed the presence of chlorine in both the m/z 125 and m/z 141 fragments (a mass shift of +2 was observed, giving m/z 127 and m/z 143). The product ion spectra for the alcoholic metabolites for which the 35Cl precursor was selected showed a weak m/z 141, but it must be emphasized that fragmentation of the 37Cl isotope showed no m/z 143, thus indicating that this m/z 141 fragment was not chlorinated and not the same structure as the prominent and diagnostic m/z 141 chlorinated fragment associated with the phenolic metabolites. Another difference was the common loss of water in alcoholic isomers to give m/z 222 from m/z 240 and m/z 179 from m/z 195 and similarly for the 37Cl isotope. This was not seen in phenolic compounds for which the loss of NH3 was much more common to give the abundant product ion of m/z 223. Other fragment ions were similar in mass to both phenolic and hydroxylated metabolites, i.e., m/z 205, m/z 195, m/z 179, and m/z 67. The same differences were observed between alcoholic and phenolic hydroxyketamine isomers, because the same ions were produced but from a different precursor ion (i.e., m/z 254). Collectively, the chromatographic and mass spectrometry data together with that gained from pH-dependent extraction are strong evidence to support the presence of phenolic as well as alcoholic metabolites. Differentiating between the phenolic isomers of hydroxyketamine and likewise hydroxynorketamine was not possible because of the commonality in fragment ions and likewise the spectra for alcoholic metabolites displayed only minor differences.
Fig. 2.
MS/MS spectra obtained from fragmenting alcoholic or phenolic hydroxynorketamine. 35Cl and 37Cl isotopes were targeted and used to help elucidate metabolite structure. a and b, typical spectra produced, respectively, for alcoholic and phenolic metabolites from 35Cl isotopes. c and d, fragmentation of the 37Cl isotopes. Prominent fragments greater than 5% of the intensity of the base peak are labeled or ions that become dominant at other collision energies.
Fig. 3.
Postulated fragments for the MS/MS spectra shown in Fig. 2. a, possible structures of fragments of alcoholic isomers of the hydroxylated metabolites. b, possible structures for phenolic metabolites. Of importance is m/z 125 (alcohols) versus m/z 141 (phenols) and m/z 222 (alcohols) compared with m/z 223 (phenols) as shown. Note that the benzyl group is drawn as a tropylium in this figure and elsewhere although no evidence of a structure has been determined (McLafferty and Winkler, 1974).
Accurate Mass Determination of Metabolites of Ketamine and Norketamines and Deuterated Analogs. FT-ICR-MS was used to obtain the accurate masses of compounds suspected of being particular metabolites and further confirmed the results obtained by ultra-performance liquid chromatography-MS/MS. In all cases, the mass accuracy for both the molecular and fragment ions agreed with the theoretical value by less than 0.5 ppm (Table 1). The product ion at m/z 141.01018 can be assumed to be the diagnostic product ion of the phenolic metabolites and the fragment with the elemental composition C7H6OCl, with a corresponding deuterated metabolite giving the expected m/z 144.02899 (C7H3D3OCl). These ions were obtained exclusively for metabolites thought to be phenolic and their deuterated equivalents. The hydroxynorketamine metabolite, HNK 7 (Fig. 1, panel 3), observed after injection of the microsomal incubate but not in the 1-chlorobutane extract, also gave an accurate mass consistent with an isomer of hydroxynorketamine, adding evidence to their proposed identity, despite this anomaly.
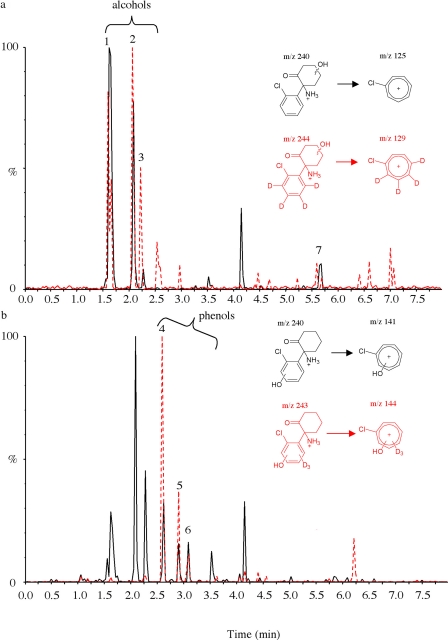
Deuterated Microsomal Metabolites as Internal Chromatographic Markers to Aid Detection of Metabolites in Humans. Phase I metabolites. Deuterated metabolites generated after microsomal incubation of deuterated ketamine or norketamine were used as an internal chromatographic marker of in vivo urinary metabolites of ketamine, after an oral dose of ketamine (50 mg) to six volunteers. Analysis of these samples, following mixed-mode SPE, established the presence of all the metabolites found to be present in microsomal incubates. Figure 4 displays the ion chromatograms for the hydroxylated metabolites, demonstrating the value of superimposing the transitions for the deuterated metabolites spiked into an elimination urine sample. Figure 4a shows the SRM transition m/z 240 → 125 superimposed with m/z 244 → 129 for alcoholic hydroxynorketamine isomers, and Fig. 4b shows the transitions m/z 240 → 141 and m/z 243 → 144 attributable to phenolic hydroxynorketamine metabolites. The same approach was used to establish the presence of urinary dehydronorketamine.
Fig. 4.
Chromatograms of a urine sample spiked with deuterated metabolites before extraction, showing isomers of hydroxynorketamine. a, superimposed chromatograms for the transitions m/z 240/125 and 244/129 for alcoholic hydroxynorketamine and hydroxynorketamine-d4 showing overlap at peaks 1 to 3 and 7. b, superimposed chromatograms for the transitions m/z 240/141 and 243/144 for phenolic hydroxynorketamine and hydroxynorketamine-d3 showing overlap at peaks 4 to 6.
Alcoholic isomers of hydroxynorketamine were detected in urine samples for 3 to 4 days, and phenolic isomers were detectable for 30 h to 2 days. Alcoholic hydroxyketamine isomers were detected for 24 to 30 h, whereas phenolic isomers of hydroxyketamine were visible for 12 to 24 h.
Phase II metabolites. Microsomal incubation of deuterated analogs of norketamine was also helpful in the identification of novel phase II metabolites in urine. After microsomal incubation of norketamine, two dominant peaks were observed in an extracted ion chromatogram (m/z 416), at retention times of 1.2 and 5.3 min; this m/z was consistent with that of protonated molecules of hydroxynorketamine glucuronide. When tetradeuterated norketamine was used as a substrate, only one significant peak was observed in the m/z 420 ion chromatogram, this being at the latter retention time of 5.3 min with the data thus supporting the conjugation of glucuronic acid to a hydroxyl group on the cyclohexanone ring. Furthermore, MS/MS fragmentation of this conjugate showed spectral characteristics similar to those of alcoholic hydroxynorketamine, with a glucuronic acid neutral loss of 176. The other peak in the extracted ion chromatogram of m/z 416 at 1.2 min indicated a glucuronide conjugated on the chlorobenzene ring, as the product ion spectrum was similar to that observed for the phase I phenolic metabolites, including the presence of the diagnostic ions m/z 223 and m/z 141. Furthermore, an intense analogous peak at m/z 419 was observed at this retention time when tetradeuterated norketamine was used as the substrate, and this was fragmented to m/z 243, a difference of 1 m/z unit from the other glucuronide, indicating hydroxylation at one of the deuterium sites and hence a conjugated phenolic metabolite.
After the optimization of collision energies of the glucuronide in microsomal incubates, SRM transitions were selected for the identification of these metabolites in volunteer urine samples. Only the alcohol-conjugated glucuronide was observed, being present for up to 30 h in samples from all six volunteers.
Discussion
We report an effective approach that integrates the use of microsomal generated metabolites and their stable isotope-labeled analogs with LC-MS/MS to aid in the identification of drug metabolites in humans. In a previous study, enzymatic synthesis was used successfully to generate novel compounds (Peters et al., 2007). The current investigation is, nonetheless, distinct in that microsomes were used to generate several deuterated metabolites for addition in their crude form to a biological matrix, in this case urine, as a valuable tool for studying drug metabolism in humans. Once metabolites are detected, MS/MS provides informative product ion spectra for identification purposes. Accurate mass determination, although not essential, is a valuable aid for confirmatory purposes, and we recommend that this option also be considered, if suitable mass spectrometry instrumentation is available.
Superimposing chromatograms of urinary and deuterated microsomal metabolites can be extremely useful, as one simply has to locate isotopic differences in chromatographic peaks, i.e., isotopic doublets, to target metabolites in urine worthy of further investigation. In this respect, our approach may be compared with previous studies in which the coadministration of drugs with their stable isotope analogs, including ketamine (Leung and Baillie, 1989), to mammals aids in the detection of metabolites in plasma or urine. It is not economically viable to coadminister the larger amounts of stable isotopes of ketamine required to perform such an investigation in humans, and there are also ethical considerations regarding coadministration of unlicensed products. The alternative microsomal approach is economical in that only a small amount (100 μg) of stable isotopes of ketamine and norketamine was required as a substrate to generate sufficient metabolic markers to aid identification of metabolites in urine after administration of 50 mg of ketamine hydrochloride to six volunteers.
Several of the metabolites of ketamine, such as norketamine, dehydronorketamine, and cyclohexanone-hydroxylated metabolites, have been reported previously in vitro (Adams et al., 1981; Woolf and Adams, 1987) and in vivo (Chang and Glazko, 1974; Wieber et al., 1975; Leung and Baillie, 1989) but not hydroxylation of the chlorobenzene moiety, with many investigations relying on derivatization and gas chromatography-mass spectrometry. The relatively recent availability of electrospray LC-MS permits the analysis of polar molecules without derivatization, and MS/MS confers increased sensitivity. This technique combined with microsome-generated deuterated analogs of metabolites facilitates detection of novel metabolites in biological matrices. Differential extraction further helped to discriminate between alcoholic and phenolic metabolites. Of interest is that Adams et al. (1981), as a small part of their elegant substantial investigation on ketamine metabolism by rat liver microsomes, used tetradeuterated ketamine as a substrate, but they comment that because there was no loss of deuterium on formation of hydroxylated metabolites the site of hydroxylation could not be aromatic. Two possible explanations for the difference in findings are that this metabolic route exists in the human but not in the rat and that the phenolic metabolites are not readily amenable to the chemical derivatization that these investigators used. As an aside, the removal of deuterium upon hydroxylation in vitro (Rettie et al., 1988) and in vivo (Hecht and Young, 1981; Walle et al., 1983) of various labeled drugs has been described to help elucidate structural characteristics in metabolic studies.
There were also differences in the alcoholic metabolites identified in the current study and those by Adams et al. (1981), who reported four hydroxynorketamine metabolites with hydroxylation sites on the cyclohexanone ring but with only three carbon atoms being involved to give C4-, C5-, and C6-hydroxyketones. Two C5-hydroxylated metabolites were therefore proposed, which were presumably diastereoisomers that can be separated on a conventional gas chromatography stationary phase. In our investigation we also observed four alcoholic metabolites for hydroxynorketamine. Logic dictates that another regioisomer of hydroxynorketamine exists, possibly a 3-hydroxyketone that was not detected in that study because derivatization was sterically hindered by the close proximity of the chlorobenzene and amine moieties. It is reasonable to consider, therefore, that the four metabolites identified and described herein represent all available carbon sites being hydroxylated on the cyclohexanone ring. We report also three alcoholic hydroxyketamine isomers, at least one more than in the previous study (Adams et al., 1981).
With respect to phase II metabolites, only one alcoholic glucuronide was observed, being present for up to 30 h in samples from all six volunteers. This metabolite was probably conjugated HNK 7 as judged by an increase in this metabolite after hydrolysis by both Escherichia coli and Helix pomatia β-glucuronidases. The lack of further glucuronide conjugates could be a result of a less favored conformational fit of other phase I hydroxylated metabolites with the active site of glucuronyl transferase. Even so, there was evidence of the presence of a phenolic conjugate in the microsomal incubate that was not detected in urine. Most likely, positive electrospray ionization of the glucuronide conjugates is not as efficient as that for the phase I metabolites, and therefore they are less likely to be detected, based on our experience with other drugs and their conjugates. With a detection window of 30 h in our administration study, it is unlikely that the targeting of intact urinary hydroxynorketamine glucuronide will be of any benefit in increasing the retrospective detection of ketamine administration for forensic purposes, as dehydronorketamine could be detected for 6 to 10 days postdose in the same volunteers (Parkin et al., 2008). Moreover, hydrolysis of urine samples collected 4 and 7 days after administration with glucuronidase from E. coli gave unremarkable results, indicating that the aglycones of hydroxylated metabolites are not likely to offer any advantage over targeting dehydronorketamine (data not shown for brevity). We also compared the data obtained after hydrolysis with glucuronidase from E. coli and H. pomatia, the latter of which has aryl sulfatase activity, and an increase (5–7-fold) in the phenolic hydroxynorketamine isomers HNK 4 and 5 was observed only with the latter in the early stage of elimination (6 and 8 h), indicating the presence of a sulfate conjugate. Other investigators reported an indication that acid-labile N-glucuronide conjugates of ketamine, norketamine, and dehydronorketamine are formed (Lin and Lua, 2004), but we found little indication to support this. Previously, glucuronides of alcoholic hydroxynorketamine were reported in a slowly migrating fraction of polar metabolites, using thin-layer chromatography, and an increase in hydroxynorketamine was seen upon hydrolysis (Chang and Glazko, 1974).
The production of several metabolites and their stable isotopes simultaneously is advantageous in laboratories that are interested in multianalyte approaches. Once metabolites of interest are identified, if there is a need, larger scale synthesis can be considered to generate enough metabolites for NMR analysis, either by chemical synthesis or by use of recombinant enzymes in various vectors including yeast strains and E. coli (Mehmood et al., 1995; Anari et al., 2000; Arnell et al., 2007; Peters et al., 2007).
When deuterium-labeled substrates are used to generate stable isotope metabolites, due consideration should be given to possible deuterium isotope effects, with greater energy being required for cleavage of a bond to deuterium than to hydrogen. In the current investigation, the deuterium was exclusively on the phenyl ring of the substrate, with aromatic hydroxylation tending to be unaffected by deuterium isotope effects. In contrast, if the deuterium had been placed on the aliphatic carbons of the cyclohexanone moiety of the substrate, the formation of alcoholic metabolites may have been more difficult because of the cytochrome P450 mechanism of aliphatic hydroxylation. All four hydrogen atoms on the phenyl ring of the stable isotopes of ketamine and norketamine were replaced with deuterium, and thus substitution to form a phenol can only result in the loss of one of the deuterium atoms. Had the phenyl ring contained fewer deuterium atoms, hydroxylation of a carbon bonded to a deuterium atom is likely to have resulted in migration of the deuterium atom to an adjacent carbon originally bonded to a hydrogen atom, referred to as the “NIH shift.” There are therefore important considerations with the use of metabolic substrates labeled at specific positions with deuterium, and the interested reader is referred to the review article by Baillie (1981) for more information on the use of stable isotopes in pharmacological research.
From our perspective, this investigation was of practical value for generating metabolites and, in particular, their stable isotope analogs for the qualitative investigation of elimination of ketamine metabolites. In addition to determining novel phenolic metabolites in vivo and performing LC-MS analysis on intact glucuronides for the first time, we revisited metabolites previously described in view of determining the relative windows of detection of urinary metabolites, which are often not analyzed for long periods of time in drug administration studies. This study helped us to determine the most useful metabolite to target in a forensic context, this being dehydronorketamine (Parkin et al., 2008), a choice supported by data showing that it has a longer plasma half-life than ketamine and norketamine in patients in intensive care units (Hijazi et al., 2003). In conclusion, the application of microsomal synthesized compounds from stable isotope substrates for use as an internal chromatographic and mass spectrometric marker is a viable approach for facilitating the identification of metabolites in vivo.
Acknowledgments
We thank Dr. Karen Homer and her colleagues at the Department of Microbiology, King's College London, for use of the FT-ICR-MS instrument, and Prof. Andrew Hutt (University of Hertfordshire) for helpful advice.
This work was supported by the Engineering and Physical Sciences Research Council [Grant Think Crime EP/C533437/1].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.026328.
ABBREVIATIONS: MS/MS, tandem mass spectrometry; HPLC, high-performance liquid chromatography; SPE, solid-phase extraction; SRM, selected reaction monitoring; MS, mass spectrometry; LC, liquid chromatography; FT, Fourier transform; ICR, ion cyclotron resonance; HNK, hydroxynorketamine.
References
- Adamowicz P and Kala M (2005) Urinary excretion rates of ketamine and norketaine following therapeutic ketamine administration: method and detection window consideration. J Anal Toxicol 29 376–382. [DOI] [PubMed] [Google Scholar]
- Adams JD Jr, Baillie TA, Trevor AJ, and Castagnoli N Jr (1981) Studies on the biotransformation of ketamine. 1–Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom 8 527–538. [DOI] [PubMed] [Google Scholar]
- Anari MR, Burton RW, Gopaul S, and Abbott FS (2000) Metabolic profiling of valproic acid by cDNA-expressed human cytochrome P450 enzymes using negative-ion chemical ionization gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 742 217–227. [DOI] [PubMed] [Google Scholar]
- Apollonio LG, Pianca DJ, Whittall IR, Kyd JM, and Maher WA (2006) A comparison of atmospheric pressure chemical ionization and electrospray ionization in testing for amphetamine-type substances and ketamine using ultra-performance liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20 2777–2780. [DOI] [PubMed] [Google Scholar]
- Arnell R, Johannisson R, Lindholm J, Fornstedt T, Ersson B, Ballagi A, and Caldwell K (2007) Biotechnological approach to the synthesis of 9α-hydroxylated steroids. Prep Biochem Biotechnol 37 309–321. [DOI] [PubMed] [Google Scholar]
- Baillie TA (1981) The use of stable isotopes in pharmacological research. Pharmacol Rev 33 81–132. [PubMed] [Google Scholar]
- Bolze S and Boulieu R (1998) HPLC determination of ketamine, norketamine, and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clin Chem 44 560–564. [PubMed] [Google Scholar]
- Chang T and Glazko AJ (1974) Biotransformation and disposition of ketamine. Int Anesthesiol Clin 12 157–177. [DOI] [PubMed] [Google Scholar]
- Cohen ML and Trevor AJ (1974) On the cerebral accumulation of ketamine and the relationship between metabolism of the drug and its pharmacological effects. J Pharmacol Exp Ther 189 351–358. [PubMed] [Google Scholar]
- Degenhardt L, Copeland J, and Dillon P (2005) Recent trends in the use of “club drugs”: an Australian review. Subst Use Misuse 40 1241–1256. [DOI] [PubMed] [Google Scholar]
- Hecht SS and Young R (1981) Metabolic α-hydroxylation of N-nitrosomorpholine and 3,3,5,5-tetradeutero-N-nitrosomorpholine in the F344 rat. Cancer Res 41 5039–5043. [PubMed] [Google Scholar]
- Hijazi Y, Bodonian C, Bolon M, Salord F, and Boulieu R (2003) Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth 90 155–160. [DOI] [PubMed] [Google Scholar]
- Hijazi Y, Bolon M, and Boulieu R (2001) Stability of ketamine and its metabolites norketamine and dehydronorketamine in human biological samples. Clin Chem 47 1713–1715. [PubMed] [Google Scholar]
- Huang MK, Liu C, Li JH, and Huang SD (2005) Quantitative detection of ketamine, norketamine, and dehydronorketamine in urine using chemical derivatization followed by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 820 165–173. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Ramsey JD, Lee T, Johnston A, and Holt DW (2005) Analysis for identification in amnesty bin samples from dance venues. Ther Drug Monit 27 793–798. [DOI] [PubMed] [Google Scholar]
- Leong HS, Tan NL, Lui CP, and Lee TK (2005) Evaluation of ketamine abuse using hair analysis: concentration trends in a Singapore population. J Anal Toxicol 29 314–318. [DOI] [PubMed] [Google Scholar]
- Leung LY and Baillie TA (1989) Studies on the biotransformation of ketamine. II–Quantitative significance of the N-demethylation pathway in rats in vivo determined by a novel stable isotope technique. Biomed Environ Mass Spectrom 18 401–404. [DOI] [PubMed] [Google Scholar]
- Lin HR and Lua AC (2004) Detection of acid-labile conjugates of ketamine and its metabolites in urine samples collected from pub participants. J Anal Toxicol 28 181–186. [DOI] [PubMed] [Google Scholar]
- Lua AC and Lin HR (2004) A rapid and sensitive ESI-MS screening procedure for ketamine and norketamine in urine samples. J Anal Toxicol 28 680–684. [DOI] [PubMed] [Google Scholar]
- McLafferty FW and Winkler J (1974) Gaseous tropylium, benzyl, tolyl, and norbornadienyl cations. J Am Chem Soc 96 5182–5189. [Google Scholar]
- Mehmood Z, Kelly DE, and Kelly SL (1995) Progesterone biotransformation by human P450 3A4 in yeast. Biotechnol Lett 17 83. [Google Scholar]
- Negrusz A, Adamowicz P, Saini BK, Webster DE, Juhascik MP, Moore CM, and Schlemmer RF (2005) Detection of ketamine and norketamine in urine of nonhuman primates after a single dose of ketamine using microplate enzyme-linked immunosorbent assay (ELISA) and NCI-GC-MS. J Anal Toxicol 29 163–168. [DOI] [PubMed] [Google Scholar]
- Parkin MC, Turfus SC, Smith NW, Halket JM, Braithwaite RA, Elliott SP, Osselton MD, Cowan DA, and Kicman AT (2008) Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 876 137–142. [DOI] [PubMed] [Google Scholar]
- Peters FT, Dragan CA, Wilde DR, Meyer MR, Zapp J, Bureik M, and Maurer HH (2007) Biotechnological synthesis of drug metabolites using human cytochrome P450 2D6 heterologously expressed in fission yeast exemplified for the designer drug metabolite 4′-hydroxymethyl-α-pyrrolidinobutyrophenone. Biochem Pharmacol 74 511–520. [DOI] [PubMed] [Google Scholar]
- Rettie AE, Boberg M, Rettenmeier AW, and Baillie TA (1988) Cytochrome P-450-catalyzed desaturation of valproic acid in vitro: species differences, induction effects, and mechanistic studies. J Biol Chem 263 13733–13738. [PubMed] [Google Scholar]
- Walle T, Wilson MJ, Walle UK, and Bai SA (1983) Stereochemical composition of propranolol metabolites in the dog using stable isotope-labeled pseudoracemates. Drug Metab Dispos 11 544–549. [PubMed] [Google Scholar]
- Wang KC, Shih TS, and Cheng SG (2005) Use of SPE and LC/TIS/MS/MS for rapid detection and quantitation of ketamine and its metabolite, norketamine, in urine. Forensic Sci Int 147 81–88. [DOI] [PubMed] [Google Scholar]
- Wieber J, Gugler R, Hengstmann JH, and Dengler HJ (1975) Pharmacokinetics of ketamine in man. Anaesthesist 24 260–263. [PubMed] [Google Scholar]
- Woolf TF and Adams JD (1987) Biotransformation of ketamine, (Z)-6-hydroxyketamine, and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica 17 839–847. [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang MH, Wang SM, Lin CC, and Liu RH (2007) Gas chromatography-mass spectrometry analysis of ketamine and its metabolites—a comparative study on the utilization of different derivatization groups. J Chromatogr A 1157 336–351. [DOI] [PubMed] [Google Scholar]
- Xiang P, Shen M, and Zhuo X (2006) Hair analysis for ketamine and its metabolites. Forensic Sci Int 162 131–134. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, and Iga T (2001) Involvement of CYP2B6 in N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 29 887–890. [PubMed] [Google Scholar]
- Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyama T, Yamamura Y, Yamada Y, and Iga T (2003) Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to heathy Japanese volunteers. Biopharm Drug Dispos 24 37–43. [DOI] [PubMed] [Google Scholar]