Abstract
The innate immune response to inhaled bacteria, such as the opportunist Pseudomonas aeruginosa, is initiated by TLR2 displayed on the apical surface of airway epithelial cells. Activation of TLR2 is accompanied by an immediate Ca2+ flux that is both necessary and sufficient to stimulate NF-κB and MAPK proinflammatory signaling to recruit and activate polymorphonuclear leukocytes in the airway. In human airway cells gap junction channels were found to provide a regulated conduit for the movement of Ca2+ from cell to cell. In response to TLR2 stimulation, by either lipid agonists or P. aeruginosa, gap junctions functioned to transiently amplify proinflammatory signaling by communicating Ca2+ fluxes from stimulated to adjacent, non-stimulated cells thus increasing epithelial CXCL8 production. P. aeruginosa stimulation also induced tyrosine phosphorylation of Connexin 43 and association with c-Src, events linked to the closure of these channels. By 4 hours post bacterial stimulation, gap junction communication was decreased indicating an autoregulatory control of the connexins. Thus, gap junction channels comprised of Connexin 43 and other connexins in airway cells provide a mechanism to coordinate and regulate the epithelial immune response even in the absence of signals from the immune system.
Keywords: Inflammation, lung, mucosa
Introduction
The epithelial cells lining the conducting airways of the respiratory tract provide a major surveillance function, initiating immune signaling to protect the lungs from inhaled bacteria. As the lower respiratory tract is normally sterile, airway cells have a low threshold for activation and are readily stimulated. The Toll-like receptors (TLR) are particularly important in recognizing bacterial components in the airway and TLR2, in conjunction with its co-receptors asialoGM1 (1, 2) and CD36 (2), provides a broadly responsive mechanism to initiate epithelial signaling in response to bacterial components as well as intact organisms. Ligation of apically displayed TLR2 initiates the canonical TLR/MyD88/NF-κB signaling cascade (3). Immediately following TLR2 activation, NF-κB and MAPK signaling promotes the expression of cytokines and chemokines, particularly CXCL8, to recruit polymorphonuclear leukocytes (PMNs) into the airway to eradicate bacteria. Even in the absence of immune cells, mucosal epithelial cells are equipped to initiate immune responses to inhaled bacteria through Ca2+ dependent signaling (4). This resulting PMN mobilization into the lung, while important in eradicating pathogens, can interfere substantially with pulmonary function. Hence, this signaling throughout the epithelium must be somehow coordinated and controlled in duration, location, and amplitude.
Adjacent airway epithelial cells are linked through a complex network of junctional proteins (5). Cell-cell communication is a fundamental property of almost all tissues; essential for the conducting tissues of the heart, muscle and nervous system but also important in coordinating the signaling properties of non-conducting tissues, including immune and mucosal cells (6, 7). Gap junctions are composed of a family of proteins that oligomerize to form channels between adjacent cells, enabling small signaling molecules, including Ca2+ and inositol triphosphate (IP3), to pass from cell to cell in a highly regulated manner. The functional unit of the gap junction is the connexin (Cx), a four pass transmembrane protein with intracellular N- and C-termini, that oligomerizes to form a hexameric channel termed a connexon. Connexons in adjacent cells associate via their extracellular loops to form gap junction channels. The gating of these channels is regulated by phosphorylation, pH, and intermolecular interactions usually involving the intracellular carboxyl terminus (8-10). Connexin 43 (Cx43), the most ubiquitously expressed connexin, has numerous carboxyl-terminal regulatory sites specifically targeted by c-Src and other kinases which function to decrease gap junction communication (8).
Gap junctions enable cells to act in a coordinated fashion and their activity has been shown to be regulated in response to proinflammatory cytokines such as TNFα (11), IL-1β (12) and IFNγ (13, 14), intact bacteria (15) and LPS (16, 17). While a number of low molecular weight molecules can move through gap junctions, Ca2+ is perhaps the best-studied second messenger that travels between cells through these channels (18). Ca2+ transients are generated at the apical surface of airway epithelial cells in the previously described signaling cascade initiated by engagement of TLR2 by microbial components (19). These Ca2+ transients could potentially travel from cell to cell activating adjacent cells not directly stimulated by adherent bacteria or bacterial products. As it has been well documented that alveolar and tracheal epithelial cells communicate Ca2+ signals through gap junctions (7, 20, 21), we postulated that airway cells could similarly transmit TLR2 mediated proinflammatory signals through these channels. In this report we demonstrate that gap junctions are involved in the initial spread of Ca2+ fluxes in the mucosal epithelium that are generated as a consequence of TLR2–dependent signaling and that this signaling is subsequently regulated by the ability of TLR2 to induce changes in the phosphorylation state of Cx43.
Materials and Methods
Reagents
Pam3Cys-Ser-Lys4 (P3C) was purchased from EMC Microcollections. Calcein-AM, Vybrant DiI, Thapsigargin, and Fluo-3/AM were purchased from Molecular Probes. Apyrase, carbenoxolone, 18α-glycyrrhetinic acid, pluronic acid, monoclonal anti-β-actin antibody and polyclonal anti-Cx43 antibody (for immunoprecipitation) were purchased from Sigma-Aldrich. Mouse anti-phosphotyrosine clone 4G10 was purchased from Upstate Biotechnology. HRP-conjugated goat anti-ECS antibody was from Bethyl Laboratories and used to detect the FLAG moiety. A mouse anti-connexin 43 antibody was purchased from BD Biosciences and used for western blotting. The activated c-Src (Tyr416) antibody was from Cell Signaling. Connexin 43 (H-150), caveolin-1 (N-20), TLR2 (H-175) and c-Src (B-12 and SC-18 (for immunoprecipitation)) antibodies were from Santa Cruz Biotechnology. Unless otherwise noted, additional reagents were obtained from Invitrogen. The pcDNA 3.1 and NF-κB luciferase reporter plasmids were gifts from J-D. Li (University of Rochester).
Cell culture and bacteria
1HAEo- human airway epithelial cell lines (D. Gruenert, Pacific Medical Center Research Institute, San Francisco, California) (48) were grown as previously described (4, 22). Airway cells were transfected using FuGENE 6 (Roche) according to the manufacturers instructions. 48 hours after transfection with pcDNA3.1 constructs, cells were grown under neomycin selection. Experiments using these cell lines were performed after cells had been grown under selection for at least 2 weeks. 1HAEo- cells expressing Flag-TLR2 have been previously described (19). P. aeruginosa PAO1 was grown on Luria-Bertani agar plates, re-suspended in 1HAEo- complete media or MEM and heat killed for 1 hour at 60 °C.
Mouse infections
C57BL/6 mice (7-10 days old) were intranasally inoculated with 108 cfu P. aeruginosa PAO1. The mice were administered 18α-glycyrrhetinic acid (5 mg/kg in DMSO/PBS) or vehicle by intraperitoneal injection 12 hours prior, 2 hours prior, and immediately following bacterial inoculation. After 4 hours of infection, mice were euthanized with pentobarbital. Lungs were collected and cell suspensions were subjected to red cell lysis, blocking of nonspecific binding by incubation with 10% normal mouse serum and mouse Fc block (Clone 2.4G2, BD Biosciences), and staining with FITC conjugated anti-Ly6G/Ly6C (Clone RB6-8C5, BD Biosciences) and PE-conjugated anti-CD45 (Clone 30-F11, Caltag) or appropriate isotype controls. Cells were gated on their forward scatter/side scatter properties and analyzed for expression of both CD45 and Gr-1 using a BD FACSCalibur equipped with CellQuest software. Analysis of FACS data was performed using WinMDI software. Animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Columbia University.
Reverse transcriptase polymerase chain reaction
1HAEo- cells were grown to confluence and stimulated with 108 cfu/mL heat-killed P. aeruginosa. RNA was prepared using the High Pure RNA Isolation Kit (Roche) according to the manufacturer's instructions. 1 μg of total RNA was used as template to generate cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). 2 μL of cDNA was used as template in PCR using the following primers: Cx26 (5′-ACTCCACCAGCATTGGAAAG-3′ and 5′-TGGGAGATGGGGAAGTAGTG-3′, amplicon length: 172 bp), Cx32 (5′-TCCCTGCAGCTCATCCTAGT-3′ and 5′-CCCTGAGATGTGGACCTTGT-3′, amplicon length: 156 bp), Cx43 (5′-ATGAGCAGTCTGCCTTTCGT-3′ and 5′-TCTGCTTCAAGTGCATGTCC-3′, amplicon length: 226 bp), and β-Actin (5′-TCCTCCCTGGAGAAGAGCTAC-3′ and 5′-TAAAGCCATGCCAATCTCATC-3′, amplicon length: 512 bp).
Quantification of intercellular communication
A co-culture assay to monitor gap junction communication was performed as previously described (23). Subconfluent monolayers of 1HAEo- cells were trypsinized and loaded with Calcein-AM or Vybrant DiI as indicated by the manufacturer (Molecular Probes). Calcein-AM is cleaved by cytosolic esterases and becomes exclusively gap junction permeable. Vybrant DiI associates with cell membranes and does not transfer to unlabeled cells. The two cell populations were mixed in the ratio of 10:1 (Vybrant DiI:Calcein-AM) and plated to ensure confluence. After 2 hours 108 cfu/mL P. aeruginosa PAO1 were added to selected wells. Monolayers were visualized by confocal microscopy or trypsinized, resuspended in PBS and analyzed on a Becton Dickinson FACSCalibur using CellQuest (BD Biosciences) and WinMDI software. Gap junction communication was calculated as the total number of red cells staining positive for Calcein for 4-6 replicate samples; data is representative of at least three independent experiments.
Connexin 43 constructs
Full length connexin 43 cDNA was amplified out of 1HAEo-RNA and cloned into the HinDIII/BamH1 sites of pCDNA3.1. To block c-Src phosphorylation of Cx43, a tyrosine to phenylalanine mutation was created at Y247 using the QuikChange Site-Directed mutagenesis kit (Stratagene). A Cx43 truncation mutant, which removes the regulatory sites present on the carboxyl terminus, was generated by introducing a premature stop codon at alanine 257. Construct integrity was confirmed by direct sequencing of the plasmid. These constructs were then used to generate stable cell lines as described above.
TLR2 small interfering RNA (siRNA)
1HAEo- cells were infected with both pRS-TLR2-1 and pRS-TLR2-2 or a pRS-scramble retrovirus for 18 hours in the presence of 1 μg/mL polybrene (Sigma-Aldrich) and selected on puromycin as previously described (19, 24). The pBabe-puro-enhanced GFP plasmid was used to monitor the efficiency of transfection to 293T cells and infection to airway cells. Knockdown of TLR2 and lack of off target effects of the retroviral infection were verified by western blotting.
Ca2+ imaging
1HAEo- cells were grown to 95% confluence in coverglass chamberslides and loaded for 45 minutes at room temperature with 2 μM Fluo-3/AM in the presence of 0.02% pluronic acid in MEM. Cells were washed with PBS and incubated at room temperature for 1 hour in MEM. Fluo-3/AM fluorescence images were collected using a Zeiss LSM 510 META scanning confocal microscope and analyzed using the ImageJ program. For some experiments, TLR2 siRNA expressing cells, labeled with Vybrant DiI, were mixed in excess with unlabeled cells expressing the pRS scrambled plasmid and allowed to reach >90% confluence before Fluo-3/AM labeling. Images are shown are representative of at least three independent experiments.
CXCL8 ELISA
1HAEo-cells were grown to confluence on 96-well plates and weaned from serum overnight. Cells were pre-incubated with inhibitors for 30 minutes, stimulated with 108 cfu/mL P. aeruginosa PAO1, 15 μg/mL P3C, or 1 μM thapsigargin for 60 min in the presence or absence of inhibitor, and CXCL8 was measured in the supernatants by enzyme linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Pharmigen). For kinetic studies the cells were incubated for the indicated times with P3C, washed with PBS to remove agonist, and incubated for 3 hours in fresh MEM before supernatant collection. Data for each condition were normalized to total protein and plotted as the fold increase over unstimulated control or plotted as the percent of stimulated untreated control or for sextuplicate samples and are representative of at least three independent experiments.
Preparation of triton soluble and insoluble fractions and immunoprecipitations
1HAEo-cells were grown in six well dishes to confluence and stimulated with 108 cfu/mL heat killed P. aeruginosa for the indicated times. After stimulation, cells were lysed in ice-cold PBS containing 1% Triton X-100, Complete Mini protease inhibitor (Roche), 1 mM sodium orthovanadate, 2 mM phenylmethylsufonyl fluoride and 100 mM sodium fluoride for 30 minutes. Cells lysates were centrifuged for 10 minutes at 13,000g and the supernatants were collected as the triton soluble fractions. The triton insoluble pellets were resuspended by sonication in RIPA buffer (1% NP-40, Complete Mini protease inhibitor (Roche), 0.25 mM sodium deoxycholate, 1 mM sodium orthovanadate, 2 mM phenylmethylsufonyl fluoride and 100 mM sodium fluoride). Protein concentration was determined with the Bio-Rad Protein Assay and equal amounts of protein separated by SDS-PAGE. Densitometry was performed using a Molecular Dynamics Model 345 densitometer. Scanner control SI was used to capture images and IQMac v1.2 was used for analysis.
Immunoprecipitations and western blotting were performed as previously described (25).
NF-κB luciferase assay
1HAEo- cells stably expressing Cx43 wild type and mutant constructs were grown on 24-well plates to 50% confluence and transiently transfected with a NF-κB-luciferase reporter plasmid using Fugene 6 (Roche). After 24 hours, cells were weaned from serum for 16 hours and then stimulated with 108 cfu/mL heat-killed PAO1 in MEM for 1 hour followed by a 3 hour incubation in MEM alone. Lysis and luciferase assays were performed using the reagents and protocol for the dual luciferase reporter assay system (Promega) and analyzed with a luminescence plate reader. Luciferase activity was standardized by protein concentration; data were plotted as fold increase over unstimulated for quadruplicate samples and are representative of at least three independent experiments.
Statistical analysis
Statistical significance between groups was evaluated by Student's t-test using GraphPad Instat version 3.0 (GraphPad), with the exception of mouse experiments which utilized the Mann-Whitney test. Differences between groups were considered significant at p < 0.05.
Results
Expression of connexins in airway epithelial cells
To establish how connexins might be linked to innate immune signaling in airway cells, we first examined the effects of bacterial stimulation on the expression of the major connexins in airway cells. Constitutive expression of Cx26 and Cx43 was suggested by RT-PCR over an 8 hour period following bacterial exposure, with substantially less Cx32 observed (Fig. 1A). The distribution of the connexins following bacterial stimulation was also examined as both TLR2 and Cx43 have been previously localized to lipid raft or triton insoluble cell fractions in association with a caveolin-1 scaffold (26, 27). Cx43 was found almost exclusively in the triton insoluble fraction (Fig. 1B). In addition to Cx43, TLR2 and proteins known to interact with Cx43, namely Caveolin-1, Zona occludens-1 (ZO-1) and c-Src (28, 29), were also abundant in the fraction enriched for cellular components expected to be organized in lipid rafts (Fig. 1B).
Figure 1. Connexin expression in airway cells.
(A) Connexins 26, 32, 43 and β-actin were identified in 1HAEo- cells exposed to P. aeruginosa for the indicated times by reverse transcriptase PCR; β–actin is shown as a control. (B) Cx43, TLR2, and known Cx43 interacting partners: ZO-1, c-Src, and Caveolin-1 (Cav-1) were detected in triton soluble and insoluble fractions of P. aeruginosa exposed 1HAEo- lysates by immunoblotting (WB). Images are representative of at least three independent experiments.
Ca2+ transients move through gap junctions in airway cells
To determine if TLR2 initiated Ca2+ fluxes are communicated through gap junctions, experiments were performed with cells expressing TLR2 siRNA, unable to generate Ca2+ fluxes in response to the TLR2 agonist P3C, and control monolayers expressing scrambled siRNA, which retain functional receptors. Ca2+ fluxes were readily visualized in airway epithelial cells expressing scrambled siRNA, initiated within seconds by the TLR2 agonist P3C (Fig. 2A); whereas the cells expressing TLR2 siRNA were incapable of responding to this agonist, but responded to thapsigargin (Fig. 2B). By labeling the TLR2 siRNA cells with Vybrant DiI (red) and co-culturing them with unlabeled cells that are competent to respond to P3C, the induced Ca2+ wave was observed to spread into adjacent TLR2 siRNA cells (Fig. 2C and supplementary Fig. 1). This Ca2+ wave could be communicated either through gap junctions, as we predicted, or by paracrine signaling in which secreted ATP from the stimulated cell activates purinergic receptors on adjacent epithelial cells causing Ca2+ release (30). To test for this possibility, the epithelial cells were pretreated with either a gap channel blocker, 18α-glycyrrhetinic acid (10 μM) (Fig. 2D) or with apyrase (50 U/mL), which hydrolyzes extracellular ATP (Fig. 2E) and changes in Ca2+ monitored. Ca2+ fluxes were not observed in the TLR2 siRNA cells in the presence of the gap junction blocker, whereas apyrase had no effect, indicating that spread of the Ca2+ transients from cells stimulated with a TLR2 agonist was mediated by gap junctions.
Figure 2. Ca2+ fluxes move through gap junctions in airway cells.
In the left panels-representative images of Ca2+ fluxes (green) imaged in Fluo-3/AM loaded 1HAEo- cells in response to P3C (P) or thapsigargin (T); in the right panels-timecourse of calcium fluxes for (A) cells expressing scrambled siRNA; (B) cells expressing TLR2 siRNA; (C) cells expressing TLR2 siRNA, labeled with Vybrant DiI (red) and co-cultured in excess of unlabeled scrambled siRNA expressing cells (untreated). The blue arrows indicate a TLR2 siRNA expressing Vybrant DiI labeled recipient cell adjacent to an unlabeled donor cell; (D) cells cultured as in (C) treated with 10 μM 18α-glycyrrhetinic acid (AGA); (E) cells cultured as in (C) treated with 50 U/mL apyrase. Images are representative of at least three independent experiments.
Regulation of inflammatory signaling through gap junctions
TLR2 initiated Ca2+ fluxes rapidly stimulate NF-κB and MAPKs to induce expression of cytokines and chemokines such as CXCL8 (2). We investigated the timecourse of CXCL8 secretion in 1HAEo-cells in response to P3C stimulation (Fig. 3A). Consistent with our earlier findings regarding MAPK and NF-κB activation in response to P. aeruginosa (31), we observed maximal CXCL8 secretion after one hour of exposure to P3C that rapidly decreases, suggesting regulation of this inflammatory response.
Figure 3. Gap junctions contribute to inflammatory signaling.
(A) Timecourse of CXCL8 secretion by 1HAEo- cells in response to stimulation with P3C as detected by ELISA. The data is represented as fold increase over unstimulated (*p <0.001). (B) CXCL8 secretion by 1HAEo- cells after 1 hour exposure to Pam3Cys (open bars, *p <0.05) or thapsigargin (black bars,**p <0.01) in the presence of the gap junction inhibitor 18α-glycyrrhetinic acid (AGA). The data is represented as fold increase over unstimulated. (C) CXCL8 secretion by 1HAEo-cells after 1 hour exposure to P. aeruginosa in the presence of the gap junction inhibitors 18α-glycyrrhetinic acid (AGA) or carbenoxolone (Carb) or the nucleotidase apyrase (APY). The data is represented as percentage of the CXCL8 secreted by stimulated untreated cells (*p<0.05,**p<0.01, NS - not significant). ELISA data is from sextuplicate samples and three independent experiments with significance determined by Student's t-test. (D) PMN recruitment into mouse lungs during P. aeruginosa infection as determined by flow cytometry. Neonates were treated with vehicle (DMSO in PBS) or 18α-glycyrrhetinic acid (AGA) and intranasally inoculated with PBS or P. aeruginosa. After 4 hours, the lungs were collected and lung cell suspensions were stained with PE-CD45 and FITC-Ly6G/Ly6C (Gr-1). PMNs are represented as % Ly6C/Ly6G cells+ of the total CD45 cells (*p < 0.05 by Mann-Whitney test; bars indicate median values).
As the Ca2+ transient generated by TLR2 ligation is itself sufficient to activate CXCL8 secretion (4, 19), we sought to investigate if these Ca2+ fluxes could elicit proinflammatory responses in adjacent unstimulated cells utilizing gap junction communication. To test this hypothesis, 1HAEo- cells were pre-treated with the gap junction blocker 18α-glycyrrhetinic acid and stimulated with either P3C or thapsigargin for 1 hour, and CXCL8 secretion measured (Fig. 3B). As predicted, blockade of gap junction communication between these cells reduced this chemokine response. Additionally, we found that the same was true when 1HAEo- cells were stimulated with the physiologically relevant P. aeruginosa; CXCL8 secretion was inhibited by the gap junction blockers 18α-glycyrrhetinic acid and carbenoxolone (Fig. 3C). To address the possibility that secreted ATP could elicit Ca2+ fluxes in adjacent cells via purinergic receptors we performed this experiment in the presence of the nucleotidase apyrase. Inhibition of purinergic signaling did not block CXCL8 secretion in response to P. aeruginosa. Taken together, these results suggest that gap junction communication, but not purinergic signaling, contributes to the CXCL8 response to this inhaled pathogen.
We wished to further verify the involvement of gap junction communication in the inflammatory response to an inhaled pathogen using a well documented neonatal model of P. aeruginosa infection (2). Mice infected with 108 cfu of P. aeruginosa PAO1 exhibit a substantial influx of neutrophils into their lung by 4 hours after infection. This response was significantly (p<0.05, as compared to vehicle treated PAO1 infected mice) inhibited by pretreatment of these mice with the gap junction inhibitor 18α-glycyrrhetinic acid (Fig. 3D), which has been previously shown not to diminish neutrophil chemotaxis by itself (32).
Epithelial cells exhibit repeated waves of Ca2+ transients in response to bacterial ligands (4); thus, open gap junctions could facilitate spread of proinflammatory signals throughout the airway epithelium. However, gap junction communication is known to be regulated through intermolecular interactions and phosphorylation (8, 33), and is likely to be subject to similar regulatory mechanisms in airway cells as part of the TLR2 cascade. Gap junctions were open in airway cells in the absence of bacterial stimulation (Fig. 4) and immediately following P3C application (Fig. 2), but by 4 hours post stimulation movement of the gap junction permeable dye calcein from cell to cell was significantly inhibited (Fig. 4 A, B), suggesting that an autoregulatory cascade activates gap junction closure following the initial response to TLR2 activation, consistent with the kinetics observed for CXCL8 secretion (Fig 3A).
Figure 4. Intercellular communication is inhibited at 4 hours following P. aeruginosa exposure.
(A) Communication between 1HAEo- cells was measured by quantifying the transfer of the gap junction permeable, green fluorescent dye Calcein-AM to Vybrant DiI labeled cells (red) by flow cytometry and plotted as the number of double labeled cells (*p < 0.001, Student's t-test). Data is average of quadruplicate samples and is representative of at least three experiments. (B) Cells treated as in (A) were imaged by confocal microscopy. Images are representative of at least three independent experiments.
TLR2 dependent signaling induces phosphorylation of Cx43
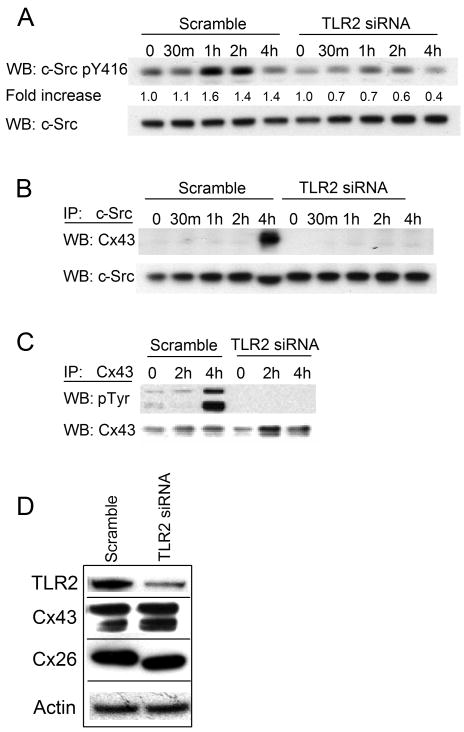
In many types of cells Cx43 activity is regulated by c-Src phosphorylation (11, 34, 35). To determine if c-Src is similarly involved in the TLR2 signaling cascade, we first monitored c-Src activation in cells expressing TLR2 following exposure to bacteria. By 1 hour post stimulation there was an increase in c-Src phosphorylation at tyrosine 416, a site associated with kinase activation (36), whereas cells expressing TLR2 siRNA did not show activation of c-Src upon exposure to bacteria (fold increase of 1.6 vs. 0.7) (Fig. 5A). TLR2 RNA interference was verified in these samples by western blotting and no off target effects were observed for Cx43, Cx26, or β–actin (Fig. 5D).
Figure 5. TLR2 signaling mediates phosphorylation of Cx43.
(A) Triton insoluble lysates of P. aeruginosa stimulated 1HAEo- cells expressing scrambled or TLR2 siRNA were immunoblotted for active (pY416) and total c-Src. Densitometry was performed on pY416 bands and normalized to total c-Src. Data is presented as the fold increase of pY416 over unstimulated cells. (B) c-Src immunoprecipitates (IP) from cell lysates were probed for Cx43 or c-Src by immunoblotting (WB). (C) Cx43 immunoprecipitates (IP) were probed for phosphotyrosine (pTyr) or Cx43. (D) Lysates from Scramble and TLR2 siRNA expressing cells were subjected to western blotting for TLR2, Cx43, Cx26, and β–actin are shown as a control. Data is representative of three independent experiments.
We next examined whether bacteria stimulate an interaction between c-Src and Cx43 which has been shown to decrease gap junction communication (28, 29). c-Src was immunoprecipitated from lysates of airway cells following bacterial exposure and screened for association with Cx43. At 4 hours post bacterial stimulation c-Src and Cx43 were found to be associated, but only in the cells capable of TLR2 signaling (Fig. 5B). There was no co-immunoprecipitation of c-Src and Cx43 in cells expressing TLR2 siRNA. Note that this 4 hour time point post stimulation correlates with the diminished CXCL8 secretion observed by ELISA (Fig. 3A) and cell-cell communication directly observed by confocal imaging (Fig. 4B). In addition, Cx43 was immunoprecipitated from these lysates and shown to be tyrosine phosphorylated at 4 hours post bacterial exposure correlating with decreased intercellular communication at this timepoint, a response that was also dependent on TLR2 (Fig. 5C).
Cx43 phosphorylaton limits proinflammatory signaling
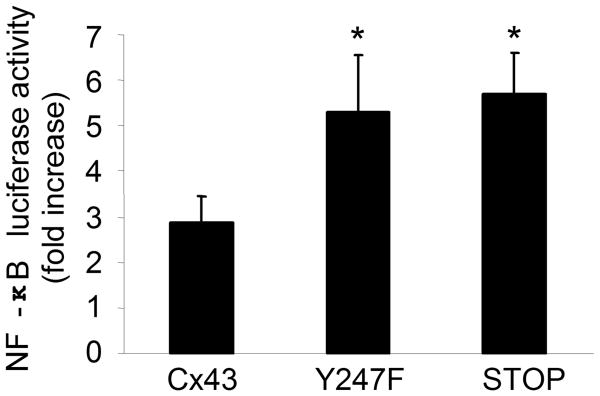
Experiments were done to establish if the state of the Cx43 channel is directly involved in the amplification of the epithelial proinflammatory response. The activation of NF-κB was measured in airway cells expressing Cx43 with a mutation in a c-Src phosphorylation site (Y247F) or a carboxyl terminal truncation at alanine 257 (STOP); these mutants are unable to close Cx43 channels in response to c-Src (37-39). Airway cells expressing either the Y247F or the STOP mutation had 2-2.5 fold more NF-κB activation at 4 hours in response to bacteria than cells transfected with wild type Cx43 (Fig. 6, p < 0.01). Thus the status of the Cx43 channel is directly involved in amplifying proinflammatory signals initiated in response to bacteria.
Figure 6. NF-κB activity is increased in cells expressing Cx43 mutants.
NF-κB activity was measured using a luciferase reporter assay in 1HAEo- cells stably expressing WT Cx43, Cx43 mutated at a c-Src phosphorylation site (Y247F), or a Cx43 c-terminal truncation mutant (STOP). The cells were transiently transfected with a NF-κB reporter construct, exposed to P. aeruginosa and luminescence measured. The data are represented as the fold increase in NF-κB activity measured at 4 hours after stimulation over that of unstimulated cells (*p < 0.01 compared to cells expressing WT Cx43, Student's t-test). Data is representative of three independent experiments.
Discussion
The importance of the respiratory mucosa in initiating the innate immune response to inhaled pathogens and instructing subsequent adaptive immune responses is becoming increasingly apparent. It appears now that part of this role requires gap junction intercellular communication to coordinate proinflammatory responses to pathogens through Ca2+ signaling. At least three connexins are expressed in airway epithelial cells and contribute to cell-cell communication (40, 41). We focused on Cx43 because of its established role in inflammation. Cx43 was found in a triton insoluble fraction of airway cells along with c-Src and TLR2 and was involved in the spread of proinflammatory Ca2+ fluxes generated as a consequence of TLR2 signaling.
TLR2 is a broadly responsive pattern recognition receptor, critically important in host defense (3). TLR2 ligation results in the immediate and transient increase in intracellular Ca2+ that is required for the appropriate activation of NF-κB and subsequent secretion of CXCL8. While the generation of Ca2+ fluxes as a consequence of TLR2 activation is well documented (19, 42), the contribution of gap junctions to the spread of these Ca2+ fluxes in airway cells and to host defense is less well understood. At least one consequence of the Ca2+ fluxes activated by TLR2 is the amplification of proinflammatory responses to bacteria, by activating adjacent cells through gap junctions.
Increased amounts of intracellular Ca2+ have been associated with the hyperinflammatory state and endogenous activation of NF-κB in airway epithelial cells from CF patients (43, 44). While this has been ascribed to elevated stores of ER Ca2+ due to repeated bacterial stimulation (45), it may also affect the status of gap junction communication. Chanson and co-workers suggested that c-Src-associated gap junction regulation was defective in CF cells (11), although a specific mechanism that links c-Src and defective CFTR activity was not proposed. There are high levels of proinflammatory cytokines in CF airways, particularly TNFα (46) and ample evidence that links TNFα, IL-1β and IFN-γ with decreased gap junction communication (47). Connexin expression in various types of immune cells, monocytes, macrophages, Langerhans cells, migroglia and dendritic cells (6, 48, 49) has been documented and contributes to effective immune signaling. In CF, elevated levels of TNFα are present, but apparently fail to activate gap junction closure (11). Our data along with that of Chanson (11) and Ribeiro (45, 50) suggest that persistently elevated Ca2+ in CF cells may be linked to the failure of c-Src gating of the channel. Recent work has demonstrated that increases in intracellular Ca2+ are themselves responsible for decreasing gap junction communication and dependent on calmodulin, although the levels of intracellular Ca2+ observed in that particular epithelial cell type (300 to 700nM) (51) were much higher than has been observed in airway epithelial cells in response to bacteria (100 nM) (4).
Cx43 channels are gated in airway cells through c-Src phosphorylation of the carboxyl termini. While alterations in Cx43 protein levels have been observed in other inflammatory situations (52, 53), our work and that of others shows that changes in intercellular communication can occur without modulating Cx43 expression but rather through alterations in the phosphorylation state of Cx43 (11, 54). This response was not an immediate consequence of TLR2 ligation, but was somewhat delayed and detected at 4 hours following stimulation. Several independent experiments were consistent with this delayed kinetics: the co-immunoprecipitation of Cx43 and c-Src and the demonstration of Cx43 phosphorylation at 4 hours leading to the decrease in intercellular communication seen at the same timepoint. Exactly what signals the recruitment of c-Src to Cx43 is unclear but suggests that there are additional components in the auto-regulatory cascade. The overall effect of this gating, however, enables the epithelium to immediately respond to bacterial infection but limits the extent of signaling, shutting off gap junction communication at a time when professional phagocytes should already be recruited and active.
The participation of gap junctions in mucosal defense appears to vary depending upon both the nature of the pathogen and the specific epithelial barrier that is activated. In the experiments presented we used an extracellular pathogen, P. aeruginosa, that activates airway cells through recognition by TLR2 (19). P. aeruginosa activation of TLR2/Ca2+ transients and NF-κB can be reproduced by superficial stimuli such as the TLR2 agonist P3C or by antibody to a TLR2 co-receptor asialoGM1 (2). The intracellular pathogen Shigella, which targets gastrointestinal epithelial cells, similarly activates Ca2+ fluxes, which amplify the host inflammatory response. However, Shigella exploits open gap junctions to facilitate its own cell to cell dissemination (13). Whether this process is specific to the gut epithelium or to Shigella is not established, but indicates that cell-cell communication in mucosal cells is an inherent component of the host response to mucosal infection. In contrast to the airway epithelium, TLRs are not accessible on exposed surfaces of gut mucosal cells, preventing consistent activation by commensal flora (55). Thus it is not surprising that Ca2+ fluxes of sufficient amplitude to travel through gap junctions in the gut epithelium are only generated in response to invasive, as opposed to superficial infection.
The association between innate immune signaling initiated by TLR2 and gap junction communication provides a previously unrecognized mechanism to account for the coordinated behavior of a mucosal surface in response to perceived infection. The components of this signaling cascade, the generation of Ca2+ transients and regulation through c-Src association and tyrosine phosphorylation of Cx43 gap junctions, are analogous to that described in detail in other tissues. However, key regulatory components that trigger channel gating remain to be identified, and may be important in diseases of excessive airway inflammation, such as cystic fibrosis. The association between TLR2 activation, generation of Ca2+ fluxes and Cx43 gating provides a potentially useful target for the pharmacological inhibition of excessive mucosal inflammation.
Supplementary Material
References
- 1.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 2.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa Flagella Activate Airway Epithelial Cells through asialoGM1 and Toll-Like Receptor 2 as well as Toll-Like Receptor 5. Am J Respir Cell Mol Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic Fibrosis Pathogens Activate Ca2+-dependent Mitogen-activated Protein Kinase Signaling Pathways in Airway Epithelial Cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 5.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of Airway Tight Junctions by Proinflammatory Cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oviedo-Orta E, Howard Evans W. Gap junctions and connexin-mediated communication in the immune system. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1662:102–112. doi: 10.1016/j.bbamem.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 8.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Herve JC, Bourmeyster N, Sarrouilhe D. Diversity in protein-protein interactions of connexins: emerging roles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Chanson M, Berclaz PY, Scerri I, Dudez T, Wernke-Dollries K, Pizurki L, Pavirani A, Fiedler MA, Suter S. Regulation of Gap Junctional Communication by a Pro-Inflammatory Cytokine in Cystic Fibrosis Transmembrane Conductance Regulator-Expressing but Not Cystic Fibrosis Airway Cells. Am J Pathol. 2001;158:1775–1784. doi: 10.1016/S0002-9440(10)64133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John GR, Scemes E, Suadicani SO, Liu JSH, Charles PC, Lee SC, Spray DC, Brosnan CF. IL-1β differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. PNAS. 1999;96:11613–11618. doi: 10.1073/pnas.96.20.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Nhieu GT, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 14.Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-α Plus IFN-γ Induce Connexin43 Expression and Formation of Gap Junctions Between Human Monocytes/Macrophages That Enhance Physiological Responses. J Immunol. 2003;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- 15.Fujita T, Ashikaga A, Shiba H, Uchida Y, Hirono C, Iwata T, Takeda K, Kishimoto A, Hirata R, Kawaguchi H, Shiba Y, Kurihara H. Regulation of IL-8 by Irsogladine maleate is involved in abolishment of Actinobacillus actinomycetemcomitans-induced reduction of gap-junctional intercellular communication. Cytokine. 2006;34:271–277. doi: 10.1016/j.cyto.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Jara PI, Boric MP, Saez JC. Leukocytes Express Connexin 43 After Activation with Lipopolysaccharide and Appear to Form Gap Junctions with Endothelial Cells After Ischemia-Reperfusion. PNAS. 1995;92:7011–7015. doi: 10.1073/pnas.92.15.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh TH, Hsu WC, Chen YS, Hsu CJ, Lee SY. Lipopolysaccharide decreases connexin 43 expression on nasal epithelial cells in vitro. Acta Oto-Laryngologica. 2005;125:1091–1096. doi: 10.1080/00016480510037906. [DOI] [PubMed] [Google Scholar]
- 18.Charles AC, Naus CC, Zhu D, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun J, Prince A. Activation of Ca2+-Dependent Signaling by TLR2. J Immunol. 2006;177:1330–1337. doi: 10.4049/jimmunol.177.2.1330. [DOI] [PubMed] [Google Scholar]
- 20.Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291:L596–601. doi: 10.1152/ajplung.00036.2006. [DOI] [PubMed] [Google Scholar]
- 21.Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium. 1998;23:1–9. doi: 10.1016/s0143-4160(98)90069-0. [DOI] [PubMed] [Google Scholar]
- 22.Cozens AL, Yezzi MJ, Yamaya M, Steiger D, Wagner JA, Garber SS, Chin L, Simon EM, Cutting GR, Gardner P, et al. A transformed human epithelial cell line that retains tight junctions post crisis. In Vitro Cell Dev Biol. 1992;28A:735–744. doi: 10.1007/BF02631062. [DOI] [PubMed] [Google Scholar]
- 23.Czyz J, Irmer U, Schulz G, Mindermann A, Hulser DF. Gap-Junctional Coupling Measured by Flow Cytometry. Experimental Cell Research. 2000;255:40–46. doi: 10.1006/excr.1999.4760. [DOI] [PubMed] [Google Scholar]
- 24.Brummelkamp TR, Bernards R, Agami R. A System for Stable Expression of Short Interfering RNAs in Mammalian Cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 25.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. The EMBO journal. 2007;26:701–709. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin Family Members Target to Lipid Raft Domains and Interact with Caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 27.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giepmans BNG, Moolenaar WH. The gap junction protein connexin 43 interacts with the second PDZ domain of the zona occludens-1 protein. Current Biology. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 29.Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src Regulates the Interaction between Connexin-43 and ZO-1 in Cardiac Myocytes. J Biol Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 30.Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993;106:995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- 31.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-κB by Adherent Pseudomonas aeruginosa in Normal and Cystic Fibrosis Respiratory Epithelial Cells. J Clin Invest. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scerri I, Tabary O, Dudez T, Jacquot J, Foglia B, Suter S, Chanson M. Gap Junctional Communication Does not Contribute to the Interaction Between Neutrophils and Airway Epithelial Cells. Cell Communication & Adhesion. 2006;13:1–12. doi: 10.1080/15419060600631250. [DOI] [PubMed] [Google Scholar]
- 33.Moreno AP. Connexin phosphorylation as a regulatory event linked to channel gating. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2005;1711:164–171. doi: 10.1016/j.bbamem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Kanemitsu MY, Loo LWM, Simon S, Lau AF, Eckhart W. Tyrosine Phosphorylation of Connexin 43 by v-Src Is Mediated by SH2 and SH3 Domain Interactions. J Biol Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Hertzberg EL, Spray DC. Regulation of Connexin43-Protein Binding in Astrocytes in Response to Chemical Ischemia/Hypoxia. J Biol Chem. 2005;280:7941–7948. doi: 10.1074/jbc.M410548200. [DOI] [PubMed] [Google Scholar]
- 36.Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987;49:65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 37.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural Changes in the Carboxyl Terminus of the Gap Junction Protein Connexin 43 Indicates Signaling between Binding Domains for c-Src and Zonula Occludens-1. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 38.Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284:C511–520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154:815–828. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiszniewski L, Sanz J, Scerri I, Gasparotto E, Dudez T, Lacroix JS, Suter S, Gallati S, Chanson M. Functional expression of connexin 30 and connexin 31 in the polarized human airway epithelium. Differentiation. 2007;75:382–392. doi: 10.1111/j.1432-0436.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.Isakson B, Olsen CE, Boitano S. Laminin-332 alters connexin profile, dye coupling and intercellular Ca2+ waves in ciliated tracheal epithelial cells. Respiratory Research. 2006;7 doi: 10.1186/1465-9921-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 43.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol. 2001;281:L71–78. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- 44.Tabary O, Zahm JM, Hinnrasky J, Couetil JP, Cornillet P, Guenounou M, Gaillard D, Puchelle E, Jacquot J. Selective Up-Regulation of Chemokine IL-8 Expression in Cystic Fibrosis Bronchial Gland Cells in Vivo and in Vitro. Am J Pathol. 1998;153:921–930. doi: 10.1016/S0002-9440(10)65633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro CMP, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic Airway Infection/Inflammation Induces a [Ca2+]i-dependent Hyperinflammatory Response in Human Cystic Fibrosis Airway Epithelia. J Biol Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 46.Heeckeren Av, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive Inflammatory Response of Cystic Fibrosis Mice to Bronchopulmonary Infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chanson M, Derouette JP, Roth I, Foglia B, Scerri I, Dudez T, Kwak BR. Gap junctional communication in tissue inflammation and repair. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2005;1711:197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Matsue H, Yao J, Matsue K, Nagasaka A, Sugiyama H, Aoki R, Kitamura M, Shimada S. Gap Junction-Mediated Intercellular Communication between Dendritic Cells (DCs) Is Required for Effective Activation of DCs. J Immunol. 2006;176:181–190. doi: 10.4049/jimmunol.176.1.181. [DOI] [PubMed] [Google Scholar]
- 49.Oviedo-orta E, Hoy T, Evans WH. Intercellular communication in the immune system: differential expression of connexin 40 and 43, and perturbation of gap junction channel functions in peripheral blood and tonsil human lymphocyte subpopulations. Immunology. 2000;99:578–590. doi: 10.1046/j.1365-2567.2000.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro CMP, Paradiso AM, Livraghi A, Boucher RC. The Mitochondrial Barriers Segregate Agonist-induced Calcium-dependent Functions in Human Airway Epithelia. J Gen Physiol. 2003;122:377–387. doi: 10.1085/jgp.200308893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lurtz MM, Louis CF. Intracellular calcium regulation of connexin 43. Am J Physiol Cell Physiol. 2007;293:C1806–1813. doi: 10.1152/ajpcell.00630.2006. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Cobo M, Gingalewski C, De Maio A. Expression of the connexin 43 gene is increased in the kidneys and the lungs of rats injected with bacterial lipopolysaccharide. Shock. 1998;10:97–102. doi: 10.1097/00024382-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Yeh TH, Hsu WC, Chen YS, Hsu CJ, Lee SY. Lipopolysaccharide decreases connexin 43 expression on nasal epithelial cells in vitro. Acta Oto-Laryngologica. 2005;125:1091–1096. doi: 10.1080/00016480510037906. [DOI] [PubMed] [Google Scholar]
- 54.Giepmans BNG, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin 43: role in the regulation of cell-cell communication. J Biol Chem. 2000 doi: 10.1074/jbc.M005847200. M005847200. [DOI] [PubMed] [Google Scholar]
- 55.Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.