Abstract
Objectives
Leukocyte migration through venular walls is a fundamental event during inflammation, but many aspects of this response, including the mechanisms associated with leukocyte migration through the vascular basement membrane (BM) in vivo, are poorly understood. Here we investigated and compared the means by which neutrophils and monocytes migrate through the venular BM. Specifically, as we have previously reported on the existence of neutrophil permissive sites (termed matrix protein low expression regions; LERs) within the venular BM, we have now investigated the role of these sites in monocyte transmigration in vivo.
Methods and Results
Analysis of CCL2-stimulated mouse cremaster muscles by immunofluorescent staining and confocal microscopy demonstrated that both neutrophils and monocytes use LERs for penetrating venular walls, but independent and distinct mechanisms are used by the 2 cell types. Collectively, (1) neutrophil but not monocyte transmigration led to enlargement of LERs, (2) monocytes showed a greater extent of deformability in migrating through the venular BM, and (3) only extravasated neutrophils were associated with the carriage of laminin fragments.
Conclusions
The findings provide novel insights into mechanisms of leukocyte transmigration by presenting the first in vivo evidence for distinct modes used by neutrophils and monocytes in penetrating the vascular BM.
Keywords: monocytes, leukocyte transmigration, inflammation, vascular basementmembrane, leukocyte shape-change
Migration of leukocytes from the circulation into the surrounding tissue plays a key role in host defense but can also contribute to the pathogenesis of inflammatory disorders.1 This response is mediated by a cascade of complex molecular and cellular events that have collectively been termed and investigated as the leukocyte adhesion cascade.2 There is a growing understanding of the mechanisms that mediate leukocyte responses within the vascular compartment, but less is known about the subsequent steps that mediate and regulate leukocyte migration through venular walls.2 The principal reason for the relatively slow progress in this aspect of the field is the complex nature of the vessel wall that cannot be accurately modeled and studied in vitro. Specifically, the venular wall is composed of 2 cellular components (endothelial cells and pericytes) and a noncellular specialized extracellular matrix barrier that surrounds blood vessels, the vascular basement membrane (BM). Endothelial cells (ECs) act as the first barrier for emigrating leukocytes, and although there is evidence for leukocyte transendothelial cell migration to occur via paracellular and transcellular routes,3-5 the former is the principal mode of crossing this barrier.2 In contrast to ECs, pericytes present a discontinuous cellular layer within venular walls,6 and there is in vivo evidence for leukocyte penetration of the pericyte sheath through gaps between nearby cells7 but also via a transcellular route.5 Pericytes are embedded within the vascular BM and together with ECs contribute to the generation of this structure. The vascular BM is a tightly packed network of extracellular matrix proteins, primarily collagen IV and laminins (laminin-8/10), interconnected by other glycoproteins such as perlecan and nidogens.8 The vascular BM provides a distinct and formidable barrier to emigrating leukocytes, but the mechanisms by which it is breached at sites of inflammation remains poorly understood.
Leukocytes have the ability to degrade and to interact with components of the venular BM via their range of proteases and specific integrins (eg, β1 integrins), and there is much in vitro evidence suggesting the involvement of such molecular interactions in leukocyte migration through the BM.9 Although the role of leukocyte proteases in this response remains contentious,10 there are now a number of in vivo studies to support the idea of a role for proteases in leukocyte penetration of the BM.7,11 Despite these findings, there is no in vivo evidence for long-term damage or compromising of the vascular wall at sites of inflammation, suggesting that the potential role of a proteolytic event is to facilitate leukocyte transmigration as mediated by other mechanisms, possibly governed by the nature of the vascular wall itself. In this context we have previously reported on the existence of regions of low matrix protein deposition (termed low expression regions or LERs) in the vascular BM of postcapillary venules of unstimulated mouse cremaster muscle.7 These regions were found in the networks of certain venular BM components (laminins and collagen IV) where they exhibited ≈<60% deposition of protein as compared to the average level found in the venular wall. LERs were on average ≈9 μm2 in area and were directly aligned with gaps between adjacent pericytes. Importantly, these sites acted as “gates” for infiltrating neutrophils and were transiently remodeled during neutrophil transmigration. To extend these significant findings we have now investigated the ability of monocytes, as compared to neutrophils, in penetrating the vascular BM via LERs and have found that although both these leukocyte subtypes preferentially use LERs for breaching the vascular BM, they use different and independent mechanisms. Briefly, the results indicate that although neutrophil migration leads to enlargement of LERs, monocyte migration occurs in the absence of BM remodeling apparently through the greater ability of this cell type to exhibit shape-change and deformability. Collectively, the findings of this study provide the first in vivo evidence for distinct mechanisms used by different leukocyte subtypes in penetrating the vascular BM.
Materials and Methods
To elucidate mechanisms of neutrophil and monocyte migration through the vascular BM, cremasteric muscles of CX3CR1eGFP/+ mice expressing endogenously fluorescent monocytes12 were stimulated with CCL2, LTB4 or LPS. At the end of the in vivo test-period, tissues were collected, fixed and immunostained for components of the vessel wall (eg, the vascular BM) and neutrophils, as previously described.7 Tissues were subsequently analyzed by confocal microscopy and 3D reconstructions of vessels were performed using 3D imaging software to both localize the position of the leukocytes relative to the BM and to characterize the profile of venular BM (eg, the size of LERs).7 A full description of the methods used is available as part of the supplemental materials (available online at http://atvb.ahajournals.org).
Results
Monocyte Migration Induced by CCL2 Occurs Through the Vascular BM LERs but Does Not Induce Their Remodeling
We have previously reported on the ability of neutrophils to use permissive regions characterized by low levels of laminin-10 and collagen IV (termed LERs) within the BM of mouse cremasteric venules in penetrating the vascular wall.7 To extend these findings to other leukocyte subtypes we have now investigated the role and regulation of expression of LERs in monocyte transmigration. For this purpose we studied leukocyte transmigration as elicited by topically applied CCL2 using the mouse strain CX3CR1eGFP/+ that exhibit endogenously eGFP-labeled monocytes.12 After 1 to 4 hours, tissues were removed and whole mount immunostained with antibodies against MRP14 (used as a neutrophil marker) and a laminin-α5 chain (Lmα5, marker for the venular BM) that enabled analysis of neutrophil and monocyte interactions with the venular BM. Of interest, topical application of CCL2 induced the transmigration of both neutrophils and monocytes (Figure 1A) in a time-dependent manner with a greater number of neutrophils accumulating within the first 2 hour postapplication of CCL2 (Figure 1B). The number of transmigrated neutrophils and monocytes were however almost identical at the 4-hour time-point. This noted differences in the kinetics of neutrophil and monocyte migration at the earlier time-points appeared to be a reflection of the number of these leukocyte subtypes found in circulating blood (ie, 13.7±1.1% and 4.5±0.8% for neutrophils and inflammatory monocytes, respectively). When corrected for this monocyte/neutrophil ratio, transmigration of these cells were identical in the first 2 hours postapplication of CCL2, with monocyte transmigration being greater at the 4-hour time-point (Figure 1B, inset). Furthermore, although neutrophils were found at the vessel wall in clusters (“hot spot”; indicated by circles in Figure 1A), transmigrating monocytes appeared to be randomly positioned. Analysis of the relative intensity of the eGFP signal showed that >92% of the transmigrated monocytes exhibited the characteristics of the inflammatory subpopulation (ie, Gr1+CCR2+CX3CR1low).13
Figure 1.
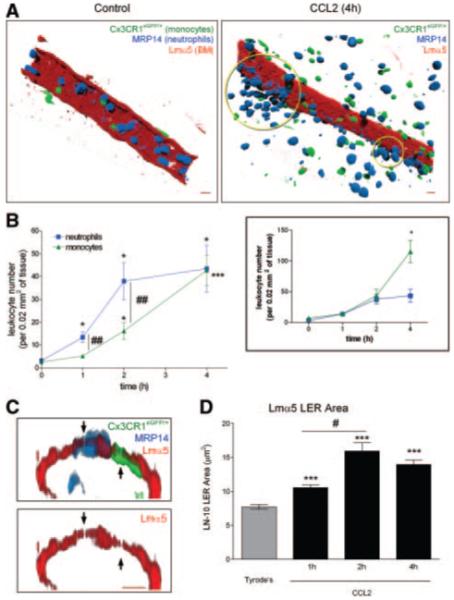
CCL2-induced monocyte and neutrophil transmigration through LERs and BM remodeling. A, Images of control and CCL2-stimulated cremasteric venules. B, Time course of monocyte and neutrophils transmigration. C, Venular cross-sections with leukocytes migrating through Lmα5 LERs. D, Time course of the remodeling of the LER area. (Please see the supplemental materials for details).
Investigation of venular cross-sections demonstrated that transmigrating neutrophils and monocytes can penetrate the venular BM by migrating through LERs (Figure 1C). CCL2 also induced a time-dependent remodeling of the Lmα5 LERs (Figure 1D), a response that was directly associated with the kinetics of neutrophil but not monocyte migration. Indeed, when animals were treated with an antineutrophil-depleting antibody before CCL2-stimulation, neutrophil transmigration was completely inhibited but the monocyte numbers in blood (not shown) or in the tissue postapplication of CCL2 (Figure 2A) were unaltered. In contrast, the remodeling of Lmα5 LERs in CCL2-stimulated tissues was completely abolished in neutrophil-depleted animals, but this effect did not alter the ability of monocytes to use LERs for penetrating the BM (Figure 2B). Collectively these results demonstrate that in the present CCL2-driven reaction, Lmα5 LER remodeling is strictly neutrophil-dependant and that monocytes can penetrate the vascular BM independently of neutrophils and without the need to remodel the BM.
Figure 2.
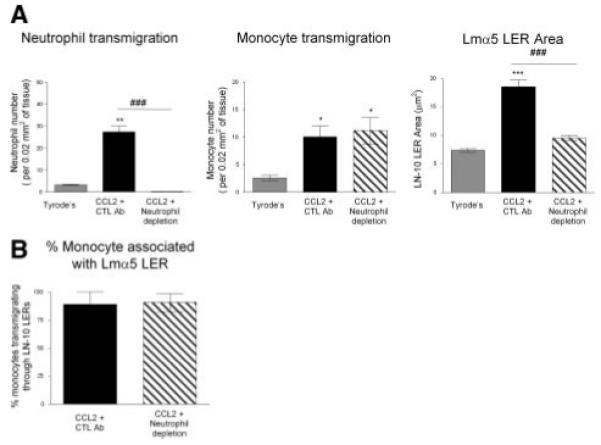
CCL2-induced neutrophil-dependent LER remodeling. A, Effect of neutrophil depletion on leukocyte transmigration response and Lmα5 LERs remodeling. B, Percentage of transmigrating monocytes associated with a BM LER. (Please see the supplemental materials for details).
Monocytes Transmigrate Through LERs via Formation of Membrane Protrusions and Deformation of Their Cell Body In Vivo
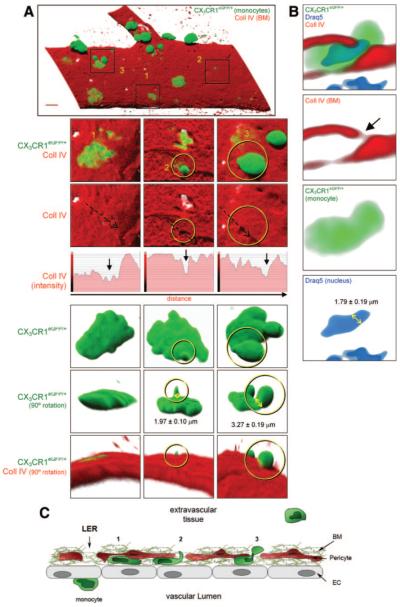
As monocyte transmigration through the BM did not lead to enlargement of LERs, we sought to further investigate the mechanism by which these cells penetrate the venular BM by studying the morphology of transmigrating monocytes. Using the methods detailed above, observation of 3D reconstructed images of venules from CCL2-stimulated tissues identified monocytes at multiple stages of their emigration through the vascular BM (Figure 3A and supplemental Video I), ie, flattened under/within the BM (position 1 in Figure 3A and 3C), showing small “investigating” protrusions within the BM and directed toward the extravascular space (position 2 in Figure 3A and 3C) and finally, exhibiting larger (“body”) protrusions at a more advanced stage of their migration through the BM (>1/3 of the cell body outside the BM; position 3 in Figure 3A and 3C). Analysis of the protrusions indicated that investigating protrusions were of an average diameter of 1.97±0.10 μm and height of 4.78±0.41 μm which increased to protrusions of an average diameter of 3.27±0.19 μm at a more advanced stage of their emigration, ie, when almost fully through the BM. Of importance, almost all monocytes observed migrating through the BM were associated with an LER, the size of these regions generally being smaller than LERs remodeled postneutrophil transmigration (Figure 3A, middle panels; results not shown). Our results also indicated the ability of monocyte nuclei to squeeze through LERs (Figure 3B) with a diameter of 1.79±0.19 μm. Together these findings indicate that the preferred mechanism for monocytes to penetrate the vascular BM involves “squeezing” through small vulnerable regions, such as LERs.
Figure 3.
Monocyte protrusion formation while penetrating LERs. A, Monocytes embedded within the BM and exhibiting at least 3 distinct morphological shapes after CCL2-stimulation. B, Venular cross-section showing a monocyte squeezing both its body and nucleus. C, Schematic diagram of the different stages of monocyte migration. (Please see the supplemental materials for details).
Neutrophil Migration Through Venular BM In Vivo Is Also Associated With Formation of Protrusions but to a Lesser Extent to That Observed With Monocytes
To directly compare the morphological changes exhibited by monocytes and neutrophils, neutrophil shape-change was analyzed in multiple inflammatory reactions, ie, as induced by CCL2, LTB4, and LPS. As found with monocytes (and using the techniques detailed above), analysis of CCL2-stimulated tissues by confocal microscopy showed neutrophils in different stages of their emigration through the vascular BM. Specifically, neutrophils were seen flattened under/within the BM (arrow in Figure 4A) and also exhibiting protrusions (circle, Figure 4A). The profile of protrusions exhibited by neutrophils penetrating the vascular BM was however different from that seen with monocytes. Specifically, the early “investigating” protrusions exhibited by neutrophils had a diameter of 2.93±0.27 μm (32.7% bigger than that observed with monocytes, P<0.01), whereas later-stage cell “body” protrusions showed a diameter of 3.97±0.34 μm (17.6% bigger than that observed with monocytes, P<0.05). Furthermore, a significantly greater percentage of monocytes within the vascular BM showed protrusions (53.7±7.2% and 19.2±10.3%, monocytes and neutrophils, respectively, P<0.01).
Figure 4.
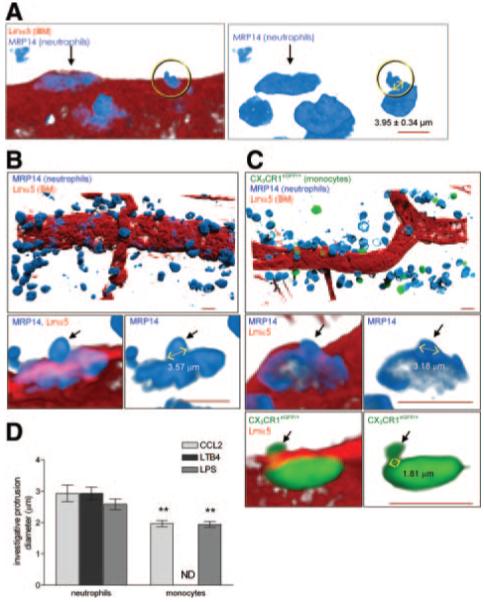
Neutrophil protrusion formation during venular BM penetration. A, Morphology of 2 neutrophils migrating through the BM after CCL2 stimulation. B and C, Neutrophil and monocyte infiltration and their morphology during transmigration through the BM after LTB4 (B) or LPS (C) stimulation. D, Diameter of “invasive” protrusions. (Please see the supplemental materials for details).
Remarkably similar results were obtained when leukocyte migration through cremasteric venules was analyzed in tissues stimulated by 2 other stimuli, LTB4 and LPS. LTB4, a highly potent neutrophil chemoattractant, induced a large neutrophil migration response (Figure 4B), but within the in vivo time period investigated (2 hours) did not cause monocyte infiltration (not shown). In LPS-stimulated tissues (6 hours) both neutrophil and monocyte migration were noted (Figure 4C). These reactions were both associated with enlargement of LERs (not shown) and also formation of cellular protrusions during leukocyte migration through the BM (Figure 4B and 4C, bottom panels), in line with the findings in CCL2-stimulated tissues. Detailed analysis of the characteristics of cell protrusions in these 3 different inflammatory reactions demonstrated that although both leukocyte subtypes exhibited “investigative” and “body” protrusions, neutrophil investigative protrusions were consistently larger in diameter than that observed for monocytes (Figure 4D). At the more advanced stage of their migration through the BM, neutrophils also showed a trend toward forming larger body protrusions than that noted for monocytes (data not shown). Finally, similarly to the CCL2-induced reaction, in LPS-stimulated tissues a significantly greater percentage of monocytes penetrating the vascular BM showed protrusions (76.6±6.0% and 47.7±8.1%, for monocytes and neutrophils, respectively, P<0.01) and in the LTB4-induced reaction, only 32.4±7.3% of neutrophils exhibited protrusions. The formation of protrusions by both leukocyte subtypes during their emigration through the BM in vivo was confirmed in an in vitro transwell assay using inhibitors of actin G polymerization (latrunculin B) and of myosin-II contraction (blebbistatin) (supplemental Result I). Collectively the results indicate that although neutrophils can also exhibit protrusions at the level of the vascular BM in vivo, their shape and frequency is significantly different from that observed with monocytes.
Transmigrated Neutrophils but not Monocytes Are Laminin-Positive In Vivo
Because in CCL2-stimulated tissues, the remodeling of LERs in the vascular BM is entirely neutrophil-dependent (Figure 2A), we sought to investigate the potential mechanisms for this response. As previously described with IL-1β,7 analysis of CCL2-stimulated cremaster muscles indicated that a large percentage of transmigrated neutrophils were Lmα5-positive (87.0±3.4% at 2 hours post-stimulation). In contrast, no laminin-positive transmigrated monocytes were detected in tissues stimulated with CCL2 (Figure 5A). These results suggested the involvement of a neutrophil-specific proteolytic event, and based on our previous findings,7,11 the potential role of neutrophil elastase in this reaction was investigated. Intravenous pretreatment of animals with a specific inhibitor of neutrophil elastase (NE), ONO-5046, led to a significant inhibition of CCL2-induced neutrophil migration and LER remodeling (71% and 54% reduction, respectively) but had no effect on monocyte infiltration (Figure 5B). Furthermore, in ONO-5046-treated mice, a greater number of neutrophils were detected at the level of the vascular BM as compared to vehicle-treated controls (5.2±1.1 cells versus 1.8±0.4 cells/200 μm vessel segment, respectively; P<0.01). The inhibitory effect of ONO-5046 on neutrophil migration (but not monocyte migration) through small permissive regions in vivo was confirmed in an in vitro transwell essay (supplemental Result II). Of interest, an inhibitor of MMP-2/MMP-9 had no effect on CCL2-induced neutrophil or monocyte transmigration in vivo but significantly suppressed neutrophil migration in TNFα-stimulated tissues (supplemental Result III), suggesting that the role of specific proteases in neutrophil transmigration in vivo is governed by the inflammatory stimulus. Collectively the present findings suggest that neutrophil, but not monocyte, migration through the vascular BM involves a protease-dependent carriage of BM components.
Figure 5.

Neutrophil but not monocyte transmigration is associated with the carriage of Lmα5 on the emigrated cells (A), and both neutrophil migration and LER remodeling are suppressed by a neutrophil elastase inhibitor (B). C, Schematic diagram of the different stages of monocyte and neutrophil migration. (Please see the supplemental materials for details).
Discussion
The difficulties associated with creating physiologically relevant in vitro models of venular walls have been instrumental in the relatively slow progress in our understanding of the mechanisms of leukocyte migration through this complex structure. This is particularly relevant with respect to the mechanisms by which leukocytes migrate through the vascular basement membrane, a specialized and vital matrix protein component of vessel wall.14 To enable us to study the process of leukocyte migration through the vascular BM in vivo, we have developed an experimental approach that allows 3D analysis of the vascular BM in intact whole mounted tissues as investigated by immunofluorescent staining and confocal microscopy.7,11 Using this approach we identified for the first time regions within the BM of mouse cremasteric venules where the expression of certain matrix proteins was lower than the average venular level, sites that have been termed low expression regions (LERs). Of importance, these sites appeared to act as “gates” for emigrating neutrophils and were remodeled by transmigrating neutrophils in response to IL-1β.7 To extend these significant findings we have now investigated the involvement of these regions in transmigration of monocytes as compared to neutrophils.
To investigate the potential role of LERs in monocyte transmigration, leukocyte migration in CCL2-stimulated mouse cremasteric venules was analyzed. CCL2 is a potent chemoattractant for inflammatory monocytes, cells that express high levels of the principal CCL2 receptor CCR2 on their cell surface.15 Of interest, in the murine system, topically applied CCL2 induced a time-dependent transmigration of both monocytes and neutrophils, a finding that is in line with the reported expression of CCR2 on murine neutrophils16 (data not shown). Analysis of the vascular BM in CCL2-stimulated cremaster muscles indicated a time-dependent remodeling of LERs. Furthermore, as found with neutrophils, monocytes used LERs for emigrating through venular BM. As a number of studies have suggested that neutrophils can pave the way for subsequent monocyte migration,17 the ability of CCL2 to induce both neutrophil and monocyte migration enabled us to investigate whether neutrophil-mediated remodeling of the BM facilitated monocyte infiltration. Although depleting the mice of their circulating neutrophils had no effect on monocyte infiltration, it totally inhibited the remodeling of the BM noted in CCL2-stimulated tissues. Under these conditions the percentage of monocytes that transmigrated through LERs was unaltered, suggesting that monocyte migration through permissive regions within the vascular BM does not involve remodeling of these sites and can occur independently of neutrophils, a finding which is in accordance with other reports.18 The neutrophil-independent migration of monocytes was also supported by the fact that transmigrating monocytes were found randomly positioned within the vessel wall and often associated with small LERs (ie, similar size to LERs that had not been remodeled). In contrast, neutrophils were often found at the vessel wall in clusters, transmigrating at specific sites (“hot-spots”) that were distinct from regions of monocyte emigration. Collectively the profile and kinetics of the present results demonstrate that monocyte migration can occur independently of neutrophils and vice versa, but they do not rule out the possibility that under certain inflammatory conditions monocyte migration may be facilitated by neutrophils or that neutrophil migration may be aided through monocyte emigration.
To gain further insight into the mechanism by which monocytes penetrate the vascular BM, detailed analysis of monocyte morphology during their emigration through LERs was performed. CCL2-elicited transmigrating monocytes were seen to exhibit small protrusions within the BM at an early stage of their emigration through the vascular BM. Similar results were noted in LPS-stimulated tissues. Because of similarities in their shape/size and localization, these protrusions could be related to the membrane invaginations and invasive protrusions described previously during leukocyte migration through endothelial cells3,4 but also during cell motility at the interface with matrix proteins.19-21 The observed early expression of membrane protrusions at the level of the BM could therefore be a mechanism by which monocytes detect permissive regions within the BM such as LERs. Whether these protrusions act as a means of presenting adhesive or proteolytic molecules to the BM requires further investigations. However, the fact that monocyte migration is not associated with remodeling of the BM and was not suppressed by an MMP-2/MMP-9 inhibitor argues against a proteolytic event in this response.
Neutrophils are well known for their ability to squeeze through narrow regions (eg, capillaries) and in our studies exhibited significant protrusions and shape-change at the level of the vascular BM in vivo in multiple inflammatory reactions (CCL2, LPS, and LTB4). The frequency and profile of neutrophil protrusions was however significantly different to that observed for monocytes. Specifically, in all reactions investigated, percentage of neutrophils showing protrusions was on average ≈2-fold less than that noted for monocytes and neutrophils characteristically showed investigative protrusions with significantly bigger diameters. The apparently less invasive profile of neutrophil shape change at the level of the vascular BM suggests that neutrophils may use complementary mechanisms to facilitate their migration through this barrier. In this context, transmigrated neutrophils but not monocytes were found to be laminin-positive in the extravascular tissue, indicating that neutrophil but not monocyte transmigration involves proteolytic cleavage or carriage of BM-derived laminin fragments. Indeed in line with our previous findings where neutrophil migration in response to IL-1β and LTB4 was suppressed by a specific neutrophil elastase (NE) inhibitor,7,22 CCL2-induced neutrophil emigration also appeared to be NE-dependent. In addition, pretreatment of mice with an NE inhibitor suppressed CCL2-induced remodeling of LERs without effecting monocyte infiltration. Taken together our data suggest that although monocyte migration through the vascular BM can occur through the ability of these cells to squeeze through small permissive sites (ie, LERs), neutrophil migration through the BM is facilitated by neutrophil-derived NE, thus overriding a need for neutrophils to develop multiple or smaller invasive protrusions (Figure 5C).
In conclusion, the present study demonstrates small permissive regions within the vascular BM termed LERs are used as the preferred sites by both emigrating neutrophils and monocytes in penetrating the venular wall. However, although neutrophil migration leads to remodeling of these sites, monocyte migration is strongly governed by the deformability of these cells. Indeed, monocytes exhibited significantly more invasive morphological changes in vivo suggesting that the principal mechanism by which they migrate through the vascular BM is by “squeezing” through small permissive sites. The findings shed much light on the mechanisms of leukocyte migration through the venular BM and provide the first line of in vivo evidence for distinct modes used by different leukocyte subtypes in penetrating this critical vascular structure.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by generous funds from The Wellcome Trust (to S.N.; Ref: 081172/Z/06/Z).
Footnotes
Disclosures None.
References
- 1.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–805. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 5.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 7.Wang S, Voisin M-B, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1539. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 9.Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 10.Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci. 2005;118:2067–2076. doi: 10.1242/jcs.02340. [DOI] [PubMed] [Google Scholar]
- 12.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1997–2008. doi: 10.1161/01.ATV.0000142812.03840.6f. [DOI] [PubMed] [Google Scholar]
- 16.Reichel CA, Khandoga A, Anders HJ, Schlondorff D, Luckow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol. 2006;79:114–122. doi: 10.1189/jlb.0605337. [DOI] [PubMed] [Google Scholar]
- 17.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 19.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 20.Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol. 2006;85:151–157. doi: 10.1016/j.ejcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Friedl P, Weigelin B. The biology of cell locomotion within three-dimensional extracellular matrix. Nat Immunol. 2008;9:960–969. [Google Scholar]
- 22.Young RE, Voisin M-B, Wang S, Dangerfield JP, Nourshargh S. Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice. Br J Pharmacol. 2007;151:628–637. doi: 10.1038/sj.bjp.0707267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.