Nanomechanical systems that are designed to trap and release molecules from pores in response to a stimulus are currently the subject of intense investigation.[1–23] Such systems have potential applications for precise drug delivery. A photo-activated material, for example, could release a drug under external control at a specific time and location for phototherapy. Nanomaterials suitable for this type of operation must consist of both an appropriate container and a photoactivated moving component.
Mesoporous silica made by sol-gel methodology[24–26] is a very useful container for molecules because the pores of both particles[24,27] and films[25] are accessible to liquids and gases. The pores in the silica materials are formed by templating agents, usually surfactants, that are removed after the formation of the structure is completed.[24,25] Diffusion of molecules driven by concentration gradients, including biomolecules, into and out of the pores has been demonstrated.[28] Methods have been developed to make tubular pores whose inner diameters can be varied between about 1 and 10 nm.[29] The pore walls and silica frameworks can readily be derivatized with a large variety of molecules.[30–32] Mesoporous silica nanoparticles with an average diameter of about 200 nm can enter cells and have been used as gene transfection reagents, cell markers, and carriers of molecules such as drugs and proteins.[5,33–39]
Photoactivated moving parts based on the photoisomerization of azobenzene derivatives have been used in conjunction with mesoporous silica.[9–14] The dynamics of the motion in pores of azobenzene derivatives, with substituents ranging from hydrogen atoms to dendrimers, has been studied.[40] The decrease in the size of trans to cis isomers of azobenzene molecules attached to pore interiors has been used to regulate the transport of molecules through pores to electrodes.[13] Of most relevance to this Communication, the back and forth wagging motion has been demonstrated to act as a molecular impeller that regulates the release of molecules from the pores of silica nanoparticles under “remote control” upon photoexcitation.[9,14] Azobenzene-driven release, unlike that regulated by many other nanomachines, can occur in aqueous environments.
A second category of molecular machines, called nanovalves, has been made by attaching various caps and molecular moving parts to the pore entrances, to control the entrance and egress of molecules into and from the pores. Examples of caps include coumarin dimers,[7–9] CdS[5] or gold nanoparticles,[6] polymeric gels,[1–3] and gold membranes.[4] Valves based on moving parts that respond to external stimuli have been demonstrated including trapping and releasing of molecules by pH,[17] redox,[16] and competitive binding.[15] A completely reversible nanovalve that can be opened and closed at will has been used to control release of molecules from ≈2 nm pores of mesoporous silica nanoparticles.[16] Examples of non-valved light-controlled release include photosensitive liposomes[41–44] and liposomes containing gold particles heated by photo-excitation,[45] but only one type of valve actuated by light has been reported.[18] Currently, the majority of these valves (including the light-driven valve) only function in organic solvents because the supramolecular recognition and binding motifs are disrupted by highly polar and hydrogen-bonding solvents such as water.
Herein we report the use of nanoimpeller-controlled mesostructured silica nanoparticles to deliver and release anticancer drugs into living cells upon external command. Using light-activated mesostructured silica (LAMS) nanoparticles, luminescent dyes and anticancer drugs are only released inside of cancer cells that are illuminated at the specific wavelengths that activate the impellers. The quantity of molecules released is governed by the light intensity and the irradiation time. Human cancer cells (a pancreatic cancer cell line, PANC-1 and a colon cancer cell line, SW480) were exposed to suspensions of the particles and the particles were taken up by the cells. Confocal microscopy imaging of cells containing the particles loaded with the membrane-impermeable dye, propidium iodide (PI), shows that the PI is released from the particles only when the impellers are photoexcited (≈0.1Wcm−2), resulting in staining of the nuclei. The anticancer drug camptothecin (CPT) was also loaded into and released from the particles inside the cells under light excitation, and apoptosis was induced. Intracellular release of molecules is sensitively controlled by the light intensity, irradiation time, and wavelength, and the anticancer drug delivery inside of cells is regulated under external control.
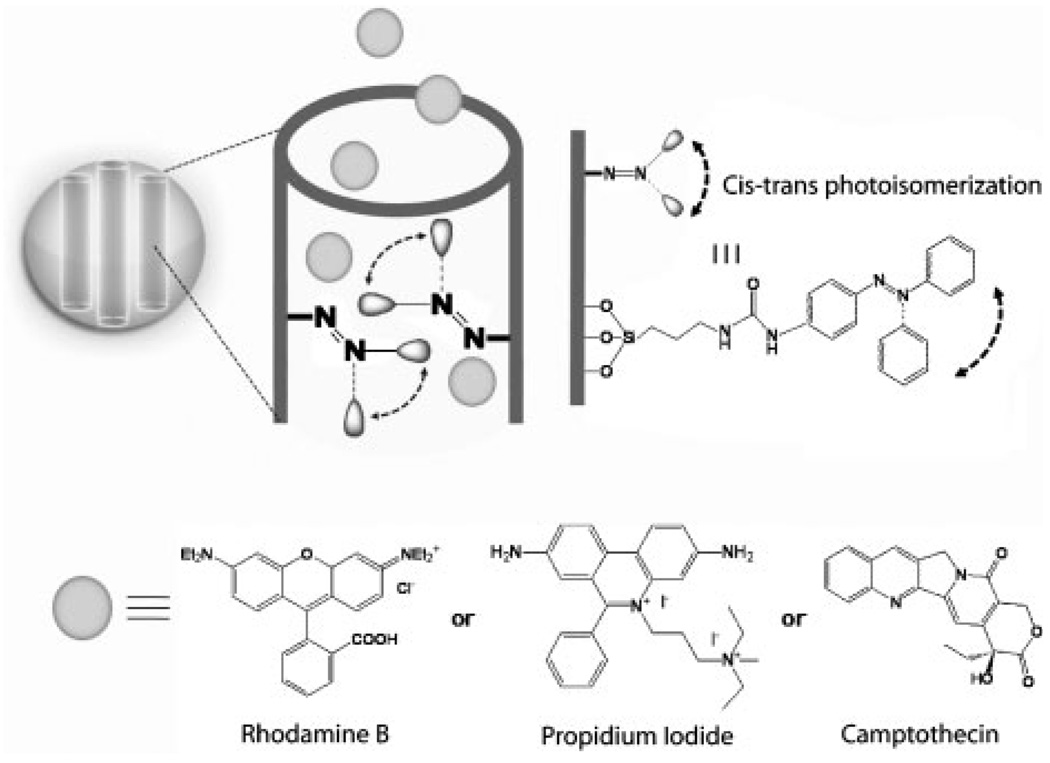
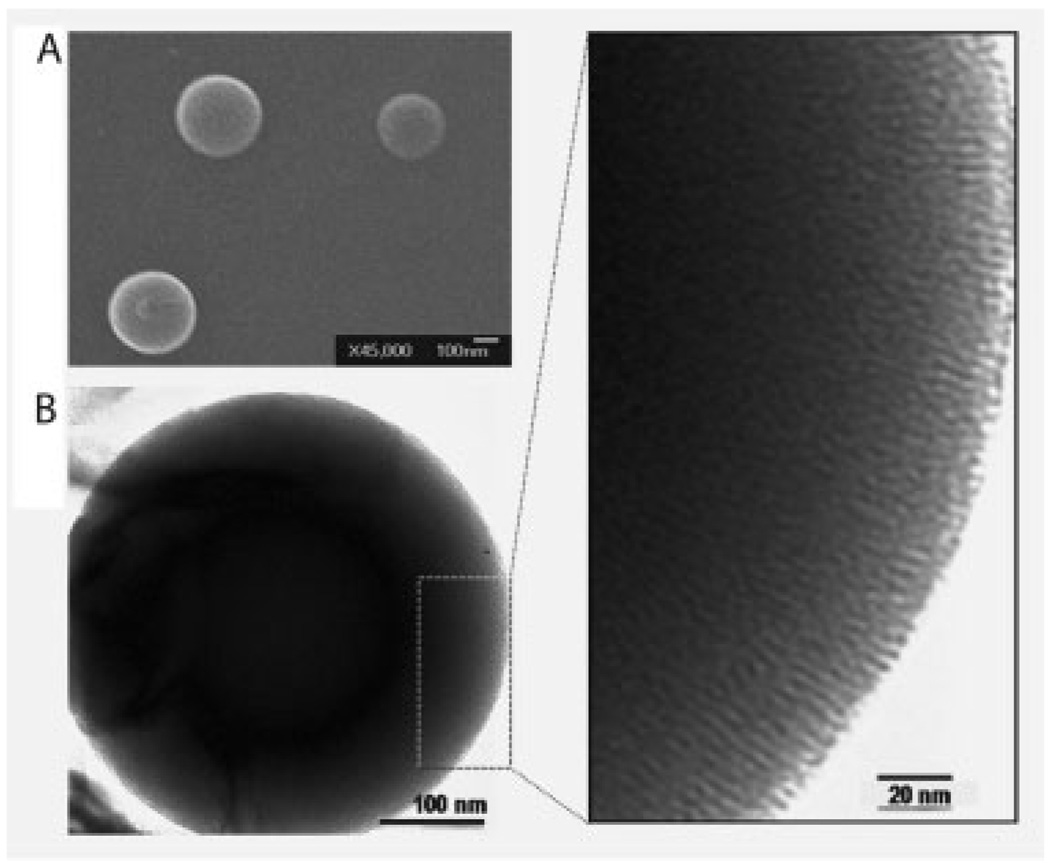
The LAMS particles functionalized with azobenzene molecules were synthesized using modifications of reported processes.[12,14] In the resulting particles, azobenzene moieties were positioned in the pore interiors with one end attached to the pore walls and the other end free to undergo photoisomerization (Scheme 1). The morphology of the spherical particles with ordered arrays of the pores was proven by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Figure 1). The X-ray diffraction pattern exhibited a strong Bragg peak indexed as {100} at 2θ=2.43 °, corresponding to a d-spacing of ≈3.6 nm. Analysis of the nitrogen sorption isotherm of the particles taken at 77 K indicated the Barrett–Joyner–Halenda (BJH) average pore diameter of 1.9±0.1 nm, Brunauer–Emmett–Teller (BET) surface area of 621.19 m2 g−1, and total pore volume of 0.248 cm3 g−1. It was calculated from UV-vis spectroscopy that the silica particles contain about 2.4 wt% of the azobenzene derivatives.
Scheme 1.
Designed pore interiors of the light-activated mesostructured silica (LAMS) nanoparticles functionalized with azobenzene derivatives. Continuous illumination at 413 nm causes a constant trans–cis photoisomerization about the N=N bond causing dynamic wagging motion of the azobenzene derivatives and results in the release of the molecules through and out of the mesopores.
Figure 1.
Characterization of the surfactant-extracted LAMS particles. A) Scanning electron microscopy (SEM) and B) transmission electron microscopy (TEM) images of the particles. Right: magnified portion of the TEM image.
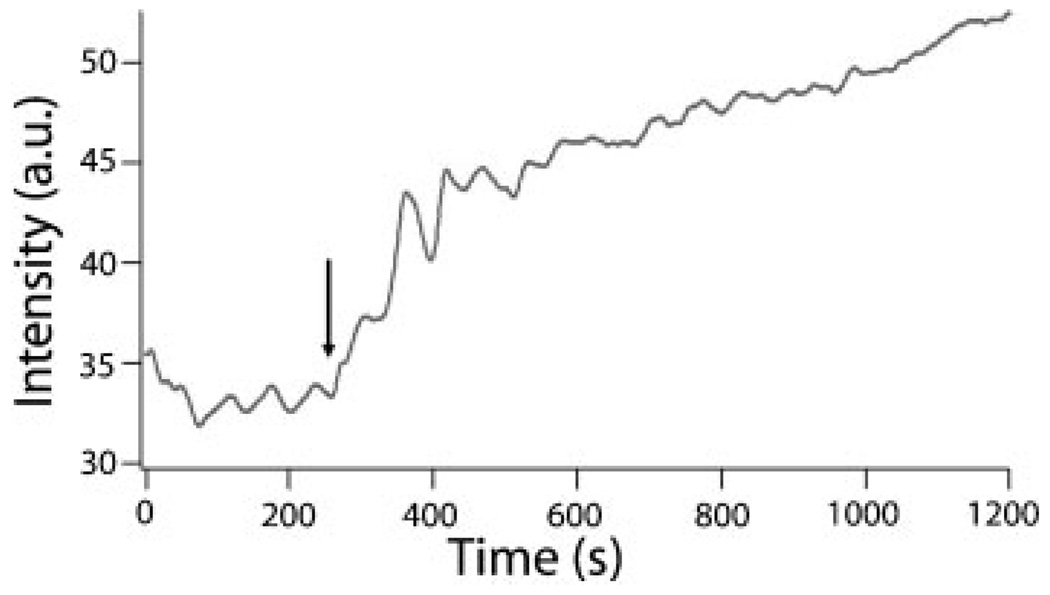
Controlled expulsion of the pore contents into solution was monitored by luminescence spectroscopy.[14] Hydrophilic Rhodamine B was chosen as a probe dye to verify that the moving parts are able to trap and release the probe molecules in an aqueous environment. The fluorescence emission spectrum of the Rhodamine B probe molecules that were released from the particles into water was recorded at one second intervals. The intensities at the emission maximum (λ≈575 nm) as a function of time are plotted in Figure 2. The impellers in the nanopores trap the probe molecules in the dark and promptly release them in response to the light excitation.
Figure 2.
Time-dependent release of Rhodamine B dye from the photoexcited particles into water. The arrow indicates the time at which the azobenzene activation light was turned on.
Based on the successful operation of the impeller in water, in vitro studies were carried out on two human cancer cell lines (PANC-1 and SW480). To detect the photoresponsive behavior of the impellers inside of cells, a membrane-impermeable dye, PI was chosen as the fluorescent probe molecule and loaded into the particles following the same procedure as that used for the Rhodamine B loading. The cells were cultured overnight on a Lab-Tek chamber slide system (Nalge Nunc International). After 3 h of incubation in the dark with a 10 µgmL−1 homogeneous suspension of PI-loaded LAMS containing ≈0.24µgmL−1 of the azobenzene machines, the cells were irradiated at 413 nm, a wavelength at which both cis and trans azobenzene isomers have almost the same extinction coefficient. The cells were exposed to three different excitation fluences (≈0.01, 0.1, 0.2 W cm−2) with exposure times ranging from 0 to 5 min. As a control, the cells were also exposed to 676 nm, a wavelength at which azobenzene does not absorb, at the same light intensities for the same amounts of time as in the release experiments. After irradiation, the cells were again incubated in the dark for 10 min to allow the released PI to stain the nuclei of the cells, and then examined by confocal microscopy (λex=337 nm; Carl Zeiss LSM 310 laser scanning confocal microscope).
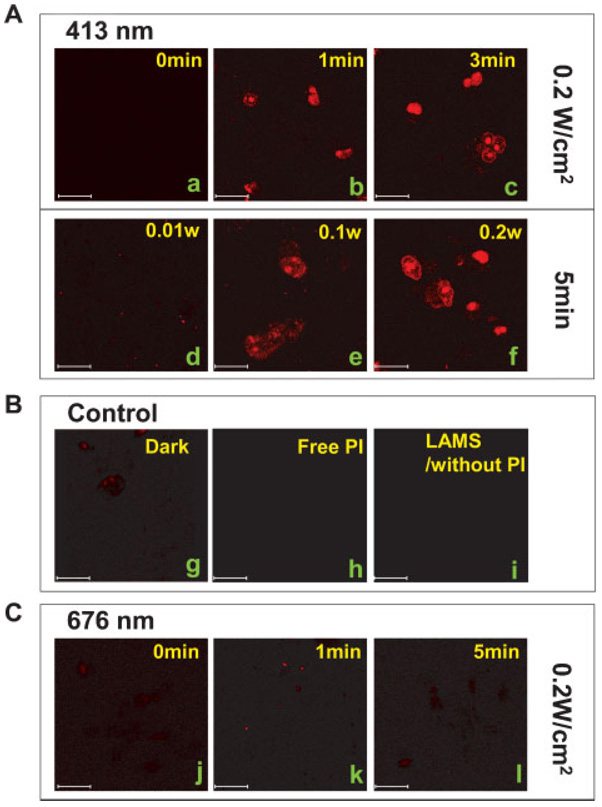
Confocal fluorescence images of the PANC-1 cells showed that only after the photoactivation of the azobenzene impellers was the PI released from the LAMS, resulting in staining of the cell nuclei (Figure 3). When the cells were irradiated for 5 min with 413 nm light of ≈0.2 W cm−2 beam intensity, the nuclei were stained red, but negligible staining of the nuclei was observed in the cells kept in the dark. For cells excited with a decreased intensity, ≈0.1 W cm−2, the nuclei were stained to a lighter red, and no staining was observed from ≈0.01 W cm−2 irradiation, which did not activate the impellers enough to enable them to release much PI (Figure 3A, d–f). When exposed to different excitation times of up to 5min under constant fluence of≈0.2Wcm−2 at 413 nm, the nuclei were stained increasingly redder with increasing activation time (Figure 3A, a–c,f)), verifying that the amount of PI released is directly related to the total number of photons absorbed. The cells were not stained when the LAMS were irradiated at 676 nm (≈0.2 Wcm−2) because that wavelength is not absorbed by the impellers (Figure 3C). These results prove that the impeller operation can be regulated by the light intensity, excitation time, and specific wavelength, and that these controllable factors directly affect the amount of the pores’ contents that is released. When cells were incubated with free PI that were not loaded into the particles, cell staining did not occur (Figure 3B, h), proving that the free PI molecules cannot enter the cells. The staining of the nuclei is thus caused only by the PI that is carried into the cells by the LAMS and released from the particles when they are photoexcited.
Figure 3.
Confocal microscopy images of the photocontrolled staining of the nuclei of PANC-1 cancer cells. Plasma-membrane-impermeable propidium iodide (PI) molecules were loaded in the pores of LAMS and the dye-loaded particles were incubated with the cells for 3 h in the dark. The cells were then exposed to the activation beam for 1 to −5 min. After further incubation in the dark for 10 min, the cells were examined with confocal microscopy (λex=337 nm) A) Cells incubated with the PI-loaded LAMS and illuminated for 0 (a), 1 (b), 3 (c), or 5 min (f) under a constant ≈0.2 W cm−2, 413 nm light or with different light intensities (≈0.01 (d) or ≈0.1 W cm−2 (e) for 5 min with a 413 nm light). B) PANC-1 cells incubated with the PI-loaded LAMS (g), free PI molecules (h), or empty LAMS (i) were kept in the dark and exposed to a 413 nm light. C) Cells incubated with the PI-loaded LAMS were illuminated with ≈0.2 W cm−2, 676 nm light for 0 (j), 1 (k), or 5 min (l). Scale bar: 30 µm.
Similar results were obtained in experiments using colon cancer cells SW480 (Supporting Information). Staining of the nuclei was caused by illuminating the LAMS with ≈0.2Wcm−2, 413 nm light. The LAMS particles function controllably in multiple cell types.
To test the ability of the LAMS to transport and then controllably release drug molecules inside cancer cells, the particles were loaded with the anticancer drug camptothecin (CPT).A10 µg mL−1 homogeneous suspension of the drug-loaded particles was added to the cancer cells. After 3 h of incubation in the dark, the cells were irradiated with ≈0.1 W cm−2, 413 nm light for various excitation times (0 to 10 min). The power density of ≈0.1 W cm−2 was chosen for this experiment based on the PI cell staining results. For the confocal cell imaging measurements, the irradiated cells were again incubated for 48 h in the dark and then stained with a 1:1 mixture solution of PI and Hoechst 33342 dye to investigate the cell death. As control experiments, cells incubated with empty LAMS particles and cells without any treatment were exposed to the excitation light.
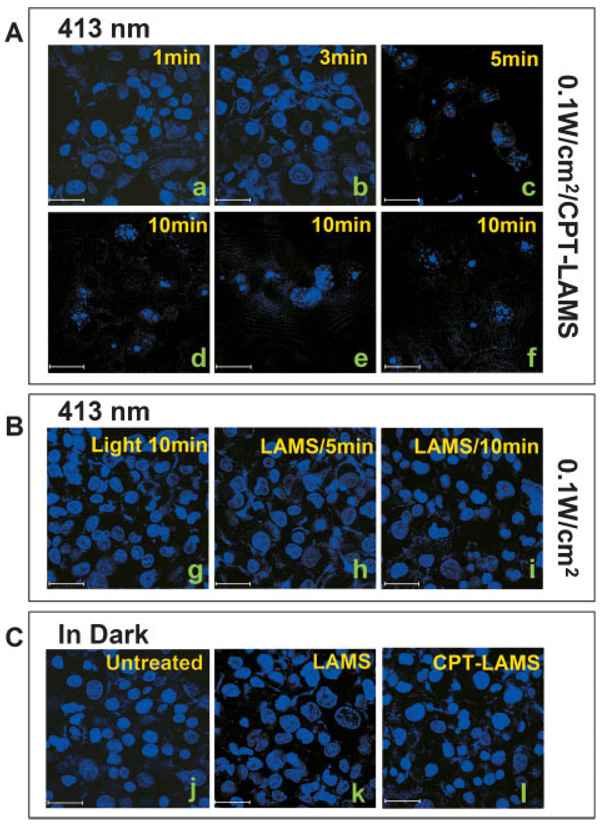
Cell death was induced under photocontrol. In the absence of light excitation, the CPT remained in the particles and the cells were not damaged (Figure 4C, l). Illumination, however, promptly expelled the CPT from the particles, causing cancer cell apoptosis that is demonstrated by nuclear fragmentation and chromatin condensation[46–48] (Figure 4A). The cell nuclei all fluoresced blue from the Hoechst 33342 dye while no red fluorescent cell nuclei stained by the PI dye were detected, confirming that the cell death did not result from cellular membrane damage but from apoptosis by the released CPT inside of the cells. The cells containing empty LAMS particles (no CPT) that were exposed to the excitation beam for 10 min did not undergo cell death, indicating that the LAMS particles are biocompatible with the living cells (Figure 4B, h and i). The ≈0.1 W cm−2, 413 nm activation light beam did not affect the cell survival (Figure 4B, g). CPT suspended in PBS was not taken up by the cells due to its insolubility and thus did not kill the cells. These observations demonstrate that cancer cell apoptosis is caused only by the CPT released from the LAMS particles inside cells under external photocontrol.
Figure 4.
Light-triggered delivery of the anticancer drug camptothecin (CPT) inside PANC-1 cancer cells to induce apoptosis. CPT molecules were loaded into the pores of the LAMS and a homogeneous suspension of the CPT-loaded particles (10 µg mL−1) was added to the cells that were incubated in Lab-Tek chamber slides for 3 h in dark. The cells were then irradiated under ≈0.1 W cm−2, 413 nm light for 1 to 10 min, again incubated in the dark for 48 h, and double-stained with propidium iodide/Hoechst 33342 solution (1:1). A) CPT-loaded particles were incubated with cancer cells and illuminated for 1 (a), 3 (b), 5 (c), or 10 min (d–f). B) As a control, cells (with no particles) were exposed to the light for 10 min (g), and cells including the CPT-unloaded LAMS were exposed for 5 (h) or 10 min (i). C) Untreated cells (j), cells incubated with CPT-unloaded (k) or -loaded (l) LAMS were kept in the dark for 48 h. Scale bar: 30 µm.
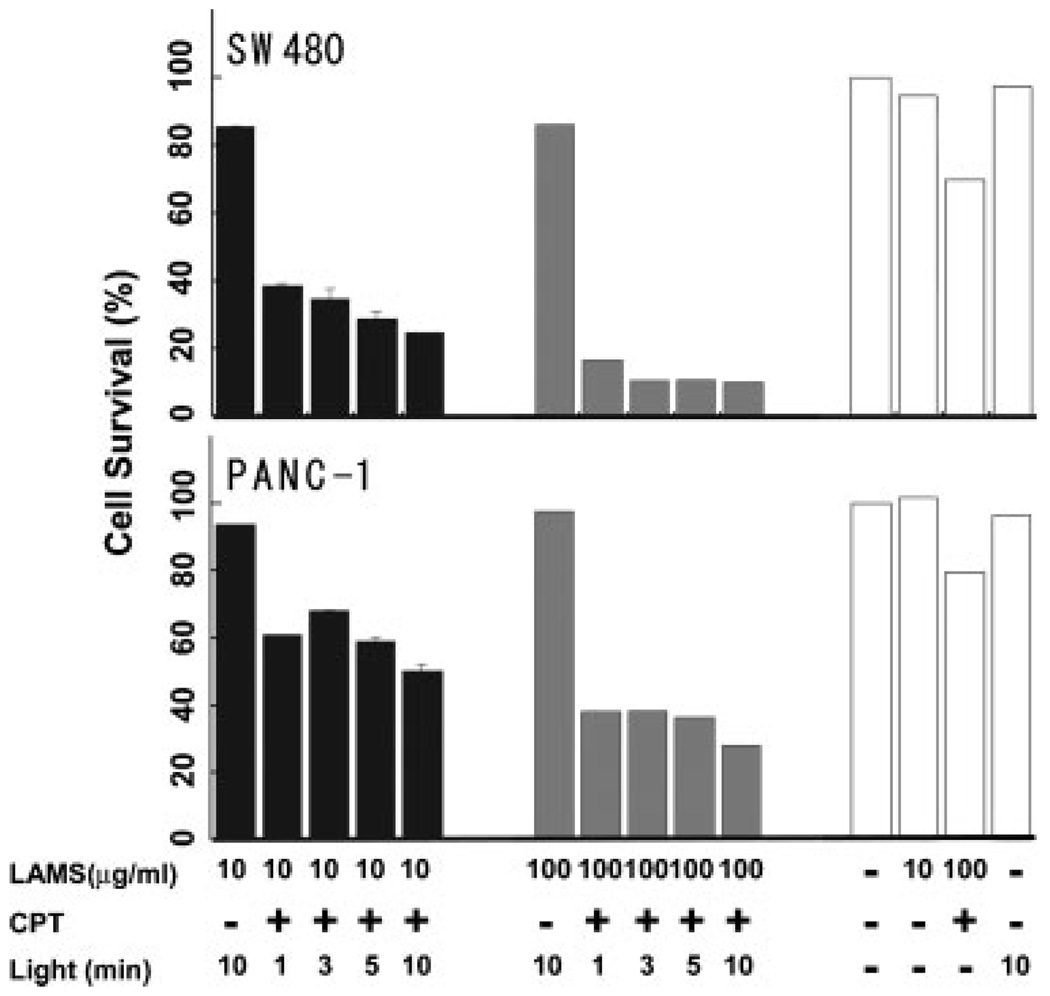
To further confirm that cell death was caused by the cytotoxicity of the CPT expelled from the particles, quantitative measurements of cell viability were made for another set of the same samples (10 µg mL−1 particles incubated with cells) placed in 96-well plates. After incubation with LAMS with and without CPT loading and illumination with ≈0.1W cm−2, 413 nm, the cells were kept in the incubator for an additional 72 h. The number of surviving cells was then counted using a cell counting kit from Dojindo Molecular Technologies, Inc.[39] The result showed that the cell death induced by CPT only occurred under light illumination, and that the cell death rate increases with longer cell illumination time, which is consistent with the cell morphologic observations. The surviving cells decreased to about half after 10 min of photoexcitation of the impellers (Figure 5). At a higher concentration (100 µg mL−1) of the particles, cell survival decreased more dramatically; only ≈40% of the PANC-1 cells and ≈14% of the SW480 survived the released CPT after 10 min of light excitation (Figure 5).
Figure 5.
In vitro cytotoxicity assay. 5000 PANC-1 or SW480 cancer cells were incubated with different concentrations of CPT-loaded or unloaded particles in 96-well cell-culture plates. After incubation for 72 h following the light excitation, the numbers of surviving cells were counted using the cell counting kit. The viability is shown as the percentage of the viable cell number in treated wells compared to untreated wells. All experiments were performed in triplicate, and the results are shown as the mean ± SD. Here, LAMS refers to cells treated with 10 or 100 µgmL−1 LAMS. CPT refers to whether the CPT was loaded (+) or absent (−) in the LAMS. Light refers to whether the cells were exposed to blue light (wavelength 413 nm) for 0, 1, 3, 5, or 10 min, followed by incubation for 72 h.
In summary, we demonstrated that the biocompatible nanoimpeller-based delivery system regulates the release of molecules from nanoparticles inside of living cells. This nanoimpeller system may open a new avenue for drug delivery under external control at a specific time and location for phototherapy. Manipulation of the machine is achieved by remote control by varying both the intensity of the light and the time that the particles are irradiated at the specific wavelengths where the azobenzene impellers absorb. The CPT loading (≈0.6 wt%) in the LAMS was higher than that for underivatized mesostructured silica,[39] possibly because of the hydrophobic molecular interactions between azobenzene moieties and CPT. When excited at 413 nm, the continuous photoisomerization of the azobenzene acts as an impeller and expels CPT out of the pores. The light intensity needed to activate the impellers, ≈0.1 W cm−2 at 413 nm, does not damage the cells. The action of the LAMS is monitored by release of PI and the consequent staining of the cell nuclei, and by the release of CPT that induces apoptosis. The delivery and release capability of light-activated mesostructured silica particles containing molecular impellers is the first step towards a novel platform for the next generation of nanotherapeutics with both spatial and temporal external control.
Experimental Section
Synthesis of Light-Activated Mesostructured Silica Nanoparticles
The chemicals for the particle synthesis were purchased from Sigma–Aldrich. The bifunctional modification strategy[31,32] was used to incorporate 4-phenylazoaniline (4-PAA) into the interiors of the particle pores. Organosilane molecules containing azobenzene moieties were first generated via coupling reaction of 4-PAA (0.142 g) with isocyanatopropylethoxysilane (ICPES) (0.71 mL) linker in ethanol (5 mL) under N2 for 4 h. In another flask, the templating agent dodecyltrimethylammonium bromide (DTAB) (1 g), NaOH (2 m, 3.5 mL), and deionized H2O (480 mL) were stirred for 30 min at 80 °C. To this surfactant solution, tetraethylorthosilicate (TEOS) (4.67 g) and the ethanol solution containing the azobenzene machines were slowly added and vigorously stirred. After 2 h the particles were filtered and washed with MeOH. The surfactant was extracted by stirring 1 g of the particles in MeOH (100 mL) with concentrated HCl solution (1 mL) for 6 h at 60 °C.
Dye Loading Procedure
The probe molecules, Rhodamine B or propidium iodide, are loaded into the mesopores by soaking and stirring ≈20 mg of the particles in aqueous solution of the dye (1 mm) at room temperature for 12 h. The suspensions of particles in aqueous dye solution were then centrifuged for ≈10 min, and the supernatant was decanted. The particles were suspended again in deionized water and sonicated for at least 10 min. This step was repeated at least twice to thoroughly remove the dyes adsorbed onto the particle surface. The particles were then dried at room temperature.
Anticancer Drug Loading Procedure
A solution of dimethylsulfoxide (DMSO) (0.6 mL) containing CPT molecules (1 mg) was prepared, and LAMS particles (10 mg) were added. After stirring the suspension for 24 h, the mixture was centrifuged for 10 min and the supernatant solution removed. The CPT-loaded LAMS particles were then dried under vacuum. To determine the amount of CPT molecules loaded in the LAMS, the drug-loaded LAMS were suspended and sonicated with DMSO (4 mL), placed in a quartz cuvette as in the release experiment, and irradiated by ≈0.2 W cm−2, 413 nm light for 10 min. The DMSO suspension of the particles was then centrifuged and the UV-vis absorption spectrum of the supernatant solution containing the released CPT molecules was measured. The concentration of CPT calculated from the absorbance was ≈0.09 mm. To confirm that most of the loaded CPT molecules were released from the particles, the supernatant taken out for the absorbance measurement was placed back into the cuvette with the centrifuged particles, excited for 50 min, and the absorbance measurement was repeated. It was determined that about 0.12 mg of CPT molecules was loaded into 20 mg of the particles.
Spectroscopic Setup for Controlled Release Experiments
The Rhodamine B-loaded particles were carefully placed on the bottom of a cuvette filled with deionized H2O. The liquid above powder was monitored continuously by a 10 mW, 530 nm probe beam. The LAMS powder was activated with a 10 mW, 457 nm excitation beam. Both the cis and trans azobenzene isomers absorb at that wavelength with a conversion quantum yield of about 0.4 for trans to cis and 0.6 for cis to trans.[40] The release profiles are obtained by plotting the luminescence intensity at the emission maximum as a function of time.
Cell Culture
PANC-1 and SW480 Cells were obtained from the American-type culture collection and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) and Leibovitz’s L-15 medium (Cellgro), respectively, supplemented with 10% fetal calf serum (Sigma, MO), 2% L-glutamine, 1% penicillin, and 1% streptomycin stock solutions with regular passage.
Cell Death Assay
Cell death was examined by using the propidium iodide and Hoechst 33342 double-staining method. The cells incubated on a Lab-Tek chamber slide system were stained with propidium iodide/Hoechst 33342 (1:1) for 5 min after treatment with CPT-loaded LAMS or free LAMS particles followed by light irradiation, and then examined with fluorescence microscopy. The cell survival assay was performed by using the cell-counting kit from Dojindo Molecular Technologies, Inc. Cancer cells were seeded in 96-well plates (5000 cells per well) and incubated in fresh culture medium at 37 °C in a 5% CO2, 95% air atmosphere for 24 h. After incubation with LAMS with and without CPT loaded and illumination with ≈0.1 W cm−2, 413 nm light, the cells were kept in the incubator for an additional 72 h. The cells were then washed with PBS and incubated in DMEM with 10% WST-8 solution for another 2 h. The absorbance of each well was measured at 450 nm with a plate reader. Since the absorbance is proportional to the number of viable cells in the medium, the viable cell number was determined by using a previously prepared calibration curve (Dojindo Co.).
Statistical Analysis
All results are expressed as mean values ± the standard deviation (SD). Statistical comparisons were made by using Student’s t-test after analysis of variance. The results were considered to be significantly different at a P value <0.05.
Acknowledgments
We thank Monty Liong and Eunwoo Choi for useful discussions and TEM measurement. This work was made possible by grants from the National Science Foundation (CHE 0507929 and DMR 0346601) to J. I. Z., NIH grant CA 32737 to F. T., and the University of California (UC) Lead Campus for Nanotoxicology Training and Research Program, funded by the UC TSR&TP to E. C.
Footnotes
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
Contributor Information
Jie Lu, Department of Microbiology, Immunology and Molecular Genetics, California NanoSystems Institute, Jonsson Comprehensive, Cancer Center, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095 (USA)
Eunshil Choi, Department of Chemistry and Biochemistry, California NanoSystems Institute, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095 (USA).
Fuyuhiko Tamanoi, Email: fuyut@microbio.ucla.edu, Department of Microbiology, Immunology and Molecular Genetics, California NanoSystems Institute, Jonsson Comprehensive, Cancer Center, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095 (USA).
Jeffrey I. Zink, Email: zink@chem.ucla.edu, Department of Chemistry and Biochemistry, California NanoSystems Institute, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095 (USA).
References
- 1.Fu Q, Rao GVR, Ista LK, Wu Y, Andrzejewski BP, Sklar LA, Ward TL, Lopez GP. Adv. Mater. 2003;15:1262. [Google Scholar]
- 2.Kwon IC, Bae YH, Kim SW. Nature. 1991;354:291. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 3.Rudzinski WE, Chipuk T, Dave AM, Kumbar SG, Aminabhavi TM. J. Appl. Polym. Sci. 2003;87:394. [Google Scholar]
- 4.Santini JT, Cima MJ, Langer R. Nature. 1999;397:335. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 5.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. J. Am. Chem. Soc. 2003;125:4451. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 6.Torney F, Trewyn BG, Lin VSY, Wang K. Nat. Nanotechnol. 2007;2:295. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 7.Mal NK, Fujiwara M, Tanaka Y. Nature. 2003;421:350. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 8.Mal NK, Fujiwara M, Tanaka Y, Taguchi T, Matsukata M. Chem. Mater. 2003;15:3385. [Google Scholar]
- 9.Zhu Y, Fujiwara M. Angew. Chem. Int. Ed. 2007;46:2241. doi: 10.1002/anie.200604850. [DOI] [PubMed] [Google Scholar]
- 10.Alvaro M, Benitez M, Das D, Garcia H, Peris E. Chem. Mater. 2005;17:4958. [Google Scholar]
- 11.Liu N, Yu K, Smarsly B, Dunphy DR, Jiang YB, Brinker CJ. J. Am. Chem. Soc. 2002;124:14540. doi: 10.1021/ja027991w. [DOI] [PubMed] [Google Scholar]
- 12.Liu N, Chen Z, Dunphy DR, Jiang YB, Assink RA, Brinker CJ. Angew. Chem. Int. Ed. 2003;42:1731. doi: 10.1002/anie.200250189. [DOI] [PubMed] [Google Scholar]
- 13.Liu NG, Dunphy DR, Atanassov P, Bunge SD, Chen Z, Lopez GP, Boyle TJ, Brinker CJ. Nano Lett. 2004;4:551. [Google Scholar]
- 14.Angelos S, Choi E, Vogtle F, DeCola L, Zink JI. J. Phys. Chem. C. 2007;111:6589. [Google Scholar]
- 15.Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Chem. Mater. 2006;18:5919. [Google Scholar]
- 16.Nguyen TD, Tseng HR, Celestre PC, Flood AH, Liu Y, Stoddart JF, Zink JI. Proc. Natl. Acad. Sci. USA. 2005;102:10029. doi: 10.1073/pnas.0504109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TD, Leung KCF, Liong M, Pentecost CD, Stoddart JF, Zink JI. Org. Lett. 2006;8:3363. doi: 10.1021/ol0612509. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TD, Leung KCF, Liong M, Liu Y, Stoddart JF, Zink JI. Adv. Funct. Mater. 2007;17:2101. [Google Scholar]
- 19.Nguyen TD, Liu Y, Saha S, Leung KCF, Stoddart JF, Zink JI. J. Am. Chem. Soc. 2007;129:626. doi: 10.1021/ja065485r. [DOI] [PubMed] [Google Scholar]
- 20.Saha S, Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Adv. Funct. Mater. 2007;17:685. [Google Scholar]
- 21.Fernandez-Pacheco R, Arruebo M, Marquina C, Ibarra R, Arbiol J, Santamaria J. Nanotechnology. 2006;17:1188. doi: 10.1088/0957-4484/17/16/011. [DOI] [PubMed] [Google Scholar]
- 22.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. J. Am. Chem. Soc. 2003;125:7860. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 23.Angelos S, Johansson E, Stoddart JF, Zink JI. Adv. Funct. Mater. 2007;17:2261. [Google Scholar]
- 24.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Nature. 1992;359:710. [Google Scholar]
- 25.Lu Y, Ganguli R, Drewien CA, Anderson MT, Brinker CJ, Gong W, Guo Y, Soyez H, Dunn B, Huang MH, Zink JI. Nature. 1997;389:364. [Google Scholar]
- 26.Huang MH, Dunn BS, Soyez H, Zink JI. Langmuir. 1998;14:7331. [Google Scholar]
- 27.Huh S, Wiench JW, Yoo JC, Pruski M, Lin VSY. Chem. Mater. 2003;15:4247. [Google Scholar]
- 28.Han YJ, Stucky GD, Butler A. J. Am. Chem. Soc. 1999;121:9897. [Google Scholar]
- 29.Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenkert JL. J. Am. Chem. Soc. 1992;114:10834. [Google Scholar]
- 30.Hernandez R, Franville AC, Minoofar P, Dunn B, Zink JI. J. Am. Chem. Soc. 2001;123:1248. doi: 10.1021/ja003634e. [DOI] [PubMed] [Google Scholar]
- 31.Minoofar PN, Dunn BS, Zink JI. J. Am. Chem. Soc. 2005;127:2656. doi: 10.1021/ja045185e. [DOI] [PubMed] [Google Scholar]
- 32.Minoofar PN, Hernandez R, Chia S, Dunn B, Zink JI, Franville AC. J. Am. Chem. Soc. 2002;124:14388. doi: 10.1021/ja020817n. [DOI] [PubMed] [Google Scholar]
- 33.Lin YS, Tsai CP, Huang HY, Kuo CT, Hung Y, Huang DM, Chen YC, Mou CY. Chem. Mater. 2005;17:4570. [Google Scholar]
- 34.Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY. J. Am. Chem. Soc. 2004;126:13216. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- 35.Slowing II, Trewyn BG, Lin VS. J. Am. Chem. Soc. 2007;129:8845. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 36.Arruebo M, Galan M, Navascues N, Tellez C, Marquina C, Ibarra MR, Santamaria J. Chem. Mater. 2006;18:1911. [Google Scholar]
- 37.Weh K, Noack M, Hoffmann K, Schroder KP, Caro J. Micro-porous Mesoporous Mater. 2002;54:15. [Google Scholar]
- 38.Besson E, Mehdi A, Lerner DA, Reye C, Corriu RJP. J. Mater. Chem. 2005;15:803. [Google Scholar]
- 39.Lu J, Liong M, Zink JI, Tamanoi F. Small. 2007;3:1341. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 40.Sierocki P, Maas H, Dragut P, Richardt G, Vogtle F, Cola LD, Brouwer FA, Zink JI. J. Phys. Chem. B. 2006;110:24390. doi: 10.1021/jp0641334. [DOI] [PubMed] [Google Scholar]
- 41.Schneider R, Tirand L, Frochot C, Vanderesse R, Thomas N, Gravier J, Guillemin F, Barberi-Heyob M. Anti-Cancer Agents Med. Chem. 2006;6:469. doi: 10.2174/187152006778226503. [DOI] [PubMed] [Google Scholar]
- 42.Ebrahim S, Peyman GA, Lee PJ. Surv. Ophthalmol. 2005;50:167. doi: 10.1016/j.survophthal.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Spratt T, Bondurant B, O’Brien DF. Biochim. Biophys. Acta. 2003;1611:35. doi: 10.1016/s0005-2736(02)00602-8. [DOI] [PubMed] [Google Scholar]
- 44.Bondurant B, Mueller A, O’Brien DF. Biochim. Biophys. Acta. 2001;1511:113. doi: 10.1016/s0005-2736(00)00388-6. [DOI] [PubMed] [Google Scholar]
- 45.Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. J. Controlled Release. 2007;122:86. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Cancer Res. 1996;56:1713. [PubMed] [Google Scholar]
- 47.Belloc F, Dumain P, Boisseau MR, Jalloustre C, Reiffers F, Bernard P, Lacombe F. Cytometry. 1994;17:59. doi: 10.1002/cyto.990170108. [DOI] [PubMed] [Google Scholar]
- 48.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry. 1997;27:1. [PubMed] [Google Scholar]