Abstract
Objectives
Antioxidant depletion is common in critically ill patients. This study was designed to determine the effects of PN, with or without glutamine (Gln) supplementation, on systemic antioxidant status in adult patients after major surgery who required parenteral nutrition (PN) in the surgical intensive care unit (SICU) setting.
Methods
Fifty-nine SICU patients who required PN following pancreatic surgery or cardiac, vascular or colonic (non-pancreatic) surgery were randomized in a double-blind study to receive standard PN (Gln-free) or Gln-supplemented PN (Gln-PN) in which Gln was provided as alanyl-Gln dipeptide. Conventional PN vitamin and mineral doses were administered to all subjects. Plasma concentrations of the antioxidant glutathione (GSH) and the anti-oxidant nutrients α-tocopherol, vitamin C and zinc were determined at baseline (initiation of study PN) and again after 7 days of study PN. Data were analyzed for the total study cohort and within the pancreatic surgery and non-pancreatic (cardiac, vascular and colonic) surgery patient subgroups.
Results
Mean plasma antioxidant concentrations were within or slightly below the normal ranges at baseline. However, a high percentage of patients demonstrated below normal baseline plasma concentrations of GSH (59%), vitamin C (59%) and zinc (68%), respectively. A lower percentage of patients exhibited below normal plasma α-tocopherol levels (21%). Study PN significantly improved plasma zinc levels in the entire study group and each surgical subgroup. Gln-PN significantly improved the change in plasma reduced GSH from baseline to day 7 in the non-pancreatic surgery patients (PN: −0.27 µM vs Gln-PN: +0.26 µM; p<0.03).
Conclusions
Low plasma levels of key antioxidants were common in this group of SICU patients despite administration of PN containing conventional micronutrients. Compared to standard PN, Gln-supplemented PN improved plasma GSH levels in SICU patients after cardiac, vascular or colonic operations.
Keywords: α-tocopherol, critical illness, glutathione, parenteral nutrition, vitamin C, zinc
Critically ill surgical patients requiring prolonged stays in surgical intensive care units (SICU) are at high risk for the development of nosocomial infection, sepsis syndrome, multiple organ failure, and related systemic inflammatory responses [1–2]. These individuals are typically extremely catabolic and exhibit compromised immune function, factors which are intimately linked to complex interactions of antioxidants, thiol-disulfide redox regulation and zinc homeostasis. Oxidative stress is common in critical illness as a result of the generation of oxygen- and nitrogen-derived free radicals and disruption of redox signaling and antioxidant control systems, including the decline in key antioxidant compounds [1–5]. The consequences of oxidative stress include protein oxidation, lipid peroxidation and DNA damage, which may contribute to cellular and organ dysfunction in SICU patients [2]. Enteral tube feedings and parenteral nutrition (PN) support is routinely provided to prevent micronutrient depletion, attenuate body protein catabolism and improve immune responses in this clinical setting.
Vitamin C, vitamin E (α-tocopherol) and glutathione (GSH) the major low-molecular weight intracellular thiol antioxidant, are major mediators of host defense as direct free radical scavengers [3, 6]. GSH is inter-related with the major antioxidant nutrients vitamin C and α-tocopherol as it maintains these nutrients in their reduced (functional) state [7]. Low plasma concentrations of vitamin C and α-tocopherol and whole blood GSH have been variously reported in catabolic and critically ill patients [1–2, 6] and supplementation of these have been suggested to maintain redox homeostasis in critical illness [6]. We previously reported low plasma GSH in catabolic patients following high dose chemo-radiotherapy for bone marrow transplantation [7]. Low circulating antioxidant levels during severe illness likely reflects increased utilization coupled with inadequate exogenous provision (via diet or nutritional support) relative to needs. In the case of the GSH, a possible decline in precursor amino acids (cysteine, glycine and glutamate) for synthesis of this tripeptide may occur. Plasma GSH is largely derived from the liver and skeletal muscle which releases GSH in proportion to tissue concentrations [4, 8]. Thus, plasma GSH levels may reflect systemic oxidative stress [9]. Zinc is also important in the antioxidant defense system as a component in enzymes involved in free radical detoxification [10, 11] and in stabilization of thiol pools [12]. Previous studies have shown that zinc depletion is associated with increased oxidative stress in trauma patients [13]. In some studies, supplementation of zinc attenuated indices of oxidative stress, improved immune function and/or attenuated cytokine production [9, 14]. Thus, maintenance of optimal redox status and host defenses in catabolic conditions is interrelated with vitamin C, α-tocopherol and zinc nutriture and the levels of GSH and other antioxidants [1,4,6].
Unfortunately, the effects of conventional PN as a method to improve antioxidant nutrient levels in critically ill patients unable to be adequately fed by the enteral route have been little studied. Limited available data suggests that conventional PN does not maintain vitamin E and GSH redox status in catabolic patients undergoing bone marrow transplantation or after operation [7, 15], but it is unknown whether current levels of supplementation are adequate to maintain GSH and other antioxidants in the SICU setting [15].
Gln may be a conditionally essential amino acid during critical illness when its utilization exceeds endogenous production [16]. Gln is a precursor for GSH synthesis (possibly via glutamate), and parenteral Gln supplementation restores plasma and tissue GSH status in catabolic animal models, where it is also associated with increased resistance to infection [16–18]. The current study was designed to test the hypothesis that Gln-supplemented PN would improve plasma GSH concentrations and concomitantly increase key plasma antioxidant nutrient concentrations in critically ill SICU patients. We also sought to determine the effects of PN providing conventional doses of vitamins and minerals on these indices. Our specific aims were to determine: 1) whether key plasma antioxidant levels are low in PN-requiring SICU patients; 2) whether conventional PN improves these values from baseline over a 7-day period; and 3) whether alanyl-Gln dipeptide supplementation further upregulates plasma GSH, vitamin C, α-tocopherol and zinc levels in this clinical setting.
Methods
Human Subjects
The data for this paper were derived from a randomized, double-blinded clinical study conducted in the SICUs at Emory University Hospital in Atlanta, GA. The main study was designed to test whether administration of Gln-PN decreased nosocomial infection in selected SICU patients requiring PN, and preliminary clinical data of the study are reported elsewhere [19]. Adult patients (aged 18 to 79 years) who were expected to require at least seven days of PN support following pancreatic, cardiac, vascular, or colonic surgeries were eligible. Patients with any of the following situations were excluded from enrollment: (1) active malignancy, (2) acute or uncontrolled infection, (3) significant hepatic dysfunction defined as serum total bilirubin > 4.0 mg/dL or transaminases > 5-fold of normal values, (4) severe renal dysfunction defined as serum creatinine concentrations > 3-fold of normal values or acute renal failure requiring dialysis. Subjects who did not receive study PN for at least 5 days were excluded from further analysis per pre hoc criteria. The APACHE II score of illness severity at baseline (initial day of study PN) was calculated for each subject using standard methods. The study was approved by the Institutional Review Board (IRB) of Emory University.
Subjects were block-randomized on the basis of surgical type to receive either alanyl-GLN dipeptide-supplemented PN (Gln-PN) or conventional, Gln-free PN (control-PN). Study PN was given as isocaloric and isonitrogenous solutions infused continuously over 24 hours via a central venous catheter. Patients in the Gln-PN group received 0.5 g/kg/day of alanyl-Gln (Dipeptiven®, Fresenius AG, Bad Homburg, Germany) added to 1.0 gram/kg/day of a Gln-free amino acid solution (15% Clinisol® Baxter, Deerfield, IL). Control patients were given Clinisol® at a goal initial PN amino acid dose of 1.5 g/kg/day. The total caloric intake goal for all patients was 1.3 × basal energy expenditure (calculated by the Harris-Benedict equation) from PN plus any enteral intake. Initial non-amino acid PN calories were provided as 70% as dextrose and 30% as lipid emulsion (20% Intralipid® Kabi, Alameda, CA). All subjects received 10 mL M.V.I.-12® (Mayne Pharma, Wilmington, NC) and 1 mL Multitrace-5® Concentrate (American Regent, Inc., Shirley, NY) daily with PN, providing 200 mg vitamin C, 10 mg α-tocopherol and 5 mg zinc/day. As a component of the standard nutrition support protocol, the same amount of vitamins and trace elements were provided in PN of study patients who received PN before study entry. Patients were followed daily clinically by the investigators and were transitioned to enteral nutrition without Gln supplementation as indicated. Plasma concentrations of vitamin C, α-tocopherol, zinc and GSH were determined at baseline (initial day of study PN) and after 7 days of study PN (i.e. on study day 8). Blood was collected in trace element-free plasma collection tubes.
Determination of plasma vitamin C, α-tocopherol and zinc concentrations
Plasma concentrations of vitamin C and α-tocopherol were determined by high performance liquid chromatography (HPLC) at the USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, as previously described [7, 19–21]. Briefly, the dehydroascorbic acid plasma concentration was reduced with homocysteine and mixed with 0.50 mol perchloric acid/L. Vitamin C was separated using a Waters HPLC apparatus with a model 710B auto sampler and a Bioanalytical Systems LC4B electrochemical detector with amperometric detection (Bioanalytical Systems, West Lafayette, IN). Plasma α-tocopherol was measured via reversed-phase HPLC consisting of a 510 pump, a 710B WISP autosampler, and a 490 multiwavelength detector set to 292 nm (Waters, Milford, MA). The lipid fraction of the plasma was extracted with hexane and dissolved in methanol and ethyl ether. The α-tocopherol content was determined by the peak height ratio method [21]. Plasma zinc concentrations were determined with a Perkin-Elmer 5000 atomic absorption spectrometer (Roche Molecular Systems, Inc, Branchburg, NJ) [7].
Determination of plasma GSH concentration
Reduced GSH concentration was determined via HPLC as previously described [7, 22]. Briefly, 3 mL of blood was collected in a heparinized syringe from the central venous line and 1.5 mL was immediately transferred to a microcentrifuge tube containing preservation buffer. After centrifugation (12,000 g for 30 s), a 200 µL supernatant was transferred to a tube containing ice-cold 200 µL 10% perchloric acid, 0.2 M boric acid and 10 mM γ-glutamyl-glutamate. Samples were then derivatized with iodoacetic acid and dansyl chloride. Plasma GSH concentrations were determined by HPLC on a 3-aminopropyl column (Waters, Milford, MA) [7, 22].
Statistical analysis
Data were analyzed using SPSS statistical software (Chicago, IL). Results are reported as mean ± SE. Unpaired student’s t-tests were used to compare the means of antioxidant concentrations in control-PN and Gln-PN groups and between pancreatic surgery and nonpancreatic surgery cohorts. The absolute values and change in plasma antioxidant concentrations from baseline to day 7 were examined using paired student’s t-tests. Chi-square analysis was used to compare the proportional changes of subjects with below normal blood antioxidant concentrations. The level of statistical significance was set at p < 0.05.
Results
Sixty-three patients who met entry criteria were initially enrolled; 31 patients were randomized to control-PN and 32 to Gln-PN. Four subjects (2 control-PN and 2 Gln-PN) did not receive the required 5 days of study PN. One control-PN subject died on Day 4 with multiple organ failure associated with bacteremic sepsis; a second control-PN subject was discharged to a nursing home facility on Day 4. One Gln-PN subject was discharged to home on Day 4 and a 2nd Gln-PN subject had PN was discontinued on day 2. Thus, data on 29 control-PN and 30 GLN-PN subjects comprised the final study group (n=59). Demographic and clinical characteristics were similar between control-PN and Gln-PN groups (Table 1).
Table 1.
Demographic and clinical characteristics of study subjects
| Control-PN (n=29) |
Gln-PN (n=30) |
||
|---|---|---|---|
| Gender | Male | 21 | 19 |
| Female | 8 | 11 | |
| Diagnosis | Pancreatic surgery | 17 | 15 |
| Cardiac surgery | 5 | 6 | |
| Vascular surgery | 5 | 6 | |
| Colonic surgery | 2 | 3 | |
| Age (yrs) (mean ± SEM) | 57±3 | 56±3 | |
| Hospital stay prior to study entry | 12±2 | 10±1 | |
| Days from surgery to study entry | 3.5±0.4 | 4.2±0.6 | |
| PN days prior to study entry | 4±1 | 6±2 | |
| APACHE II score | 13.1±1.2 | 13.4±1.4 | |
Data are shown as absolute numbers or as group mean ± SE. All comparisons are not significantly different between groups.
Table 2 shows antioxidant concentrations at baseline and after seven days of study PN in the total study group. At baseline, the mean plasma concentrations of reduced GSH, vitamin C, α-tocopherol and zinc were within or slightly below the normal ranges for each compound. In the entire study group, administration of study PN, which contained identical conventional multivitamin and mineral doses (please see Methods), did not alter plasma GSH, but significantly increased plasma concentrations of vitamin C, α-tocopherol and zinc. However, as shown in Table 3, at baseline more than 50% of patients demonstrated below-normal plasma concentrations of GSH, vitamin C and zinc, while low levels of α-tocopherol were less frequent (21%). The percentage of study subjects with below-normal levels of GSH and vitamin C were unchanged after 7 days of study PN, while the percentage of subjects demonstrating low plasma levels of α-tocopherol decreased (not significantly). In contrast, the percentage of patients with below-normal plasma levels of zinc was decreased significantly with one week of study PN, from 68% to 27% (Table 3).
Table 2.
Plasma antioxidant concentrations at baseline and after seven days of PN in the total study group
| Baseline | Day 7 | |
|---|---|---|
| GSH (1.50–2.50 µM) |
1.67±0.13 | 1.62±0.13 |
| Vitamin C (0.40–2.20 mg/dL) |
0.40±0.06 | 0.73±0.12* |
| α-tocopherol (500–1800 µg/dL) |
708±40 | 894±52* |
| Zinc (70–130 µg/dL) |
66±4 | 90±4.2* |
Data shown as mean ± SE.
P<0.05 Day 7 versus baseline values.
Table 3.
Percentage of subjects with below-normal plasma antioxidant concentrations at baseline and after seven days of PN in the total study group
| Baseline (%) | Day 7 (%) | |
|---|---|---|
| GSH | 59 | 46 |
| Vitamin C | 59 | 47 |
| α-tocopherol | 21 | 11 |
| Zinc | 68 | 27* |
P<0.05, baseline by Chi-square test day 7 versus baseline.
Patients in the non-pancreatic surgery subgroup had a significantly higher level of illness severity at baseline (APACHE II score = 14.8±1.4) compared to the pancreatic surgery patients (APACHE II score = 9.8 ±1.1; P<0.05). Because indices of oxidative stress and plasma redox are positively associated with illness severity [5], data were analyzed based on the type of surgery, i.e. pancreatic surgery versus non-pancreatic surgery (cardiac, vascular and colonic surgery). At baseline, plasma GSH levels were significantly higher and zinc levels significantly lower, respectively, in the non-pancreatic surgery patients compared to the pancreatic surgery patients (Table 4). Baseline plasma vitamin C and α-tocopherol levels were similar between non-pancreatic and pancreatic surgery patients.
Table 4.
Plasma antioxidant concentrations at baseline and after seven days of PN in the cardiovascular/colonic and pancreatic surgical cohorts.
| Baseline | Day 7 | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-pancreatic surgery | Pancreatic surgery | Non-pancreatic surgery | Pancreatic surgery | |||||
| Metabolite | Mean ± SE | % below normal range | Mean ± SE | % below normal range | Mean ± SE | % below normal range | Mean ± SE | % below normal range |
| GSH (1.50–2.50 µM) | 1.70±0.18 | 50 | 1.17±0.12† | 68 | 1.75±0.16 | 40 | 1.27±0.18 | 57 |
| Vitamin C (0.40–2.20 mg/dL) | 0.41±0.09 | 71 | 0.45±0.06 | 46 | 0.82±0.21* | 48 | 0.60±0.10 | 45 |
| α-tocopherol (500–1800 µg/dL) | 703±59 | 24 | 713±54 | 17 | 921±88* | 13 | 863±53* | 5 |
| Zinc (70–130 µg/dL) a | 58±4 | 75 | 75±6† | 61 | 83±5* | 36* | 99±6* | 15* |
Data shown as mean ± SE.
P<0.05 non-pancreatic versus pancreatic surgery subgroups;
P<0.05 Day 7 versus baseline values.
After 7 days of study PN, plasma vitamin C, α-tocopherol and zinc levels in the nonpancreatic surgery group were significantly increased compared to baseline values. In the pancreatic surgery cohort, α-tocopherol and zinc levels were similarly increased compared to baseline after 7 days of PN and vitamin C levels tended to increase (NS) (Table 4). The percentage of patients in each surgical cohort with below-normal plasma zinc levels significantly decreased with study PN (Table 4). Mean plasma GSH concentrations were not altered after 7 days of study PN in either the pancreatic surgery or the non-pancreatic surgery cohorts.
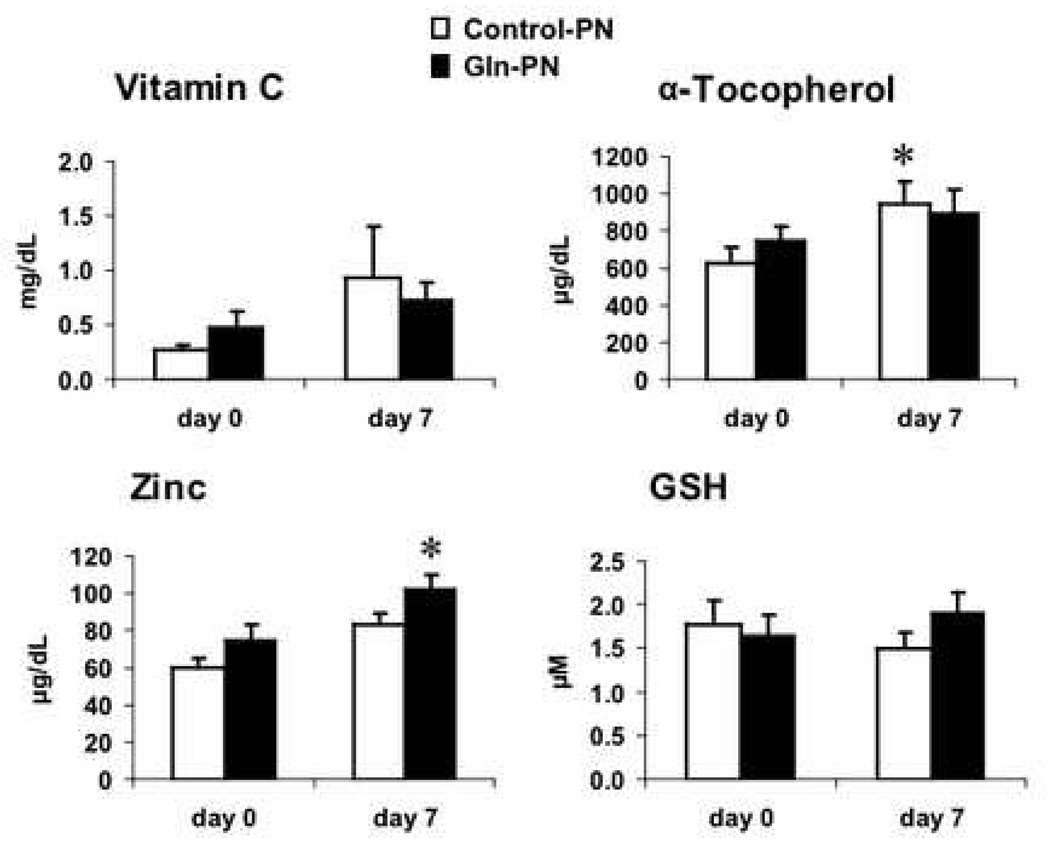
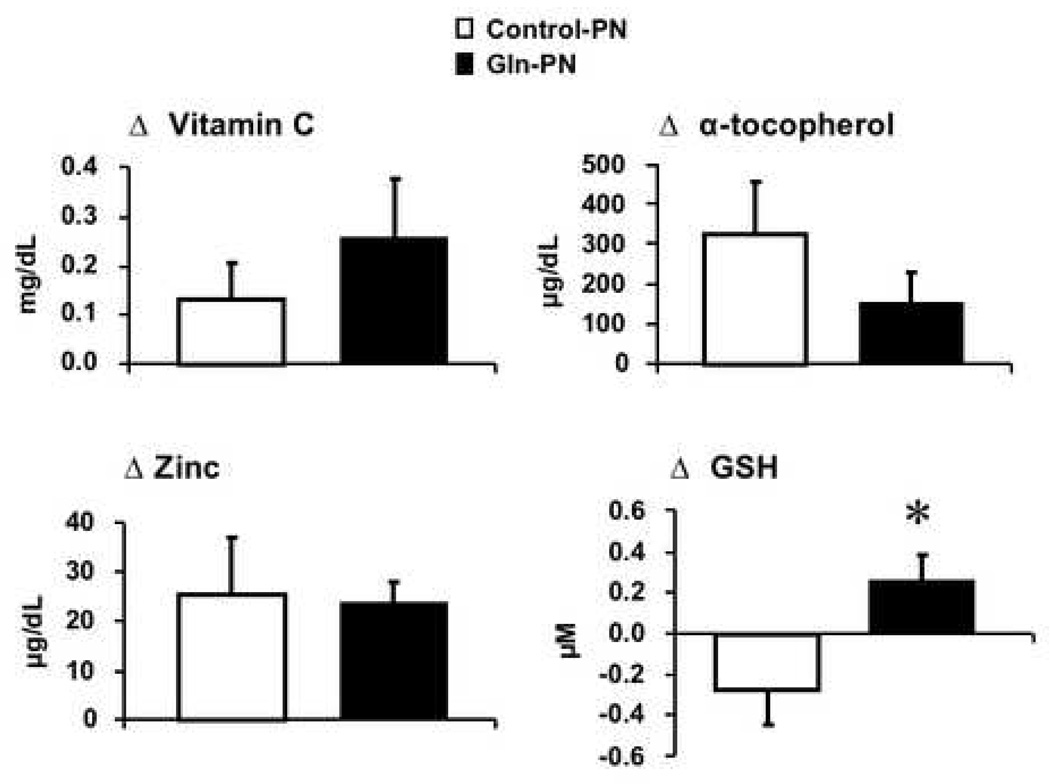
The effect of Gln-PN on plasma antioxidant levels was examined in the entire study group and the two surgical subgroups. The APACHE II scores were similar between patients receiving control-PN and Gln PN in entire study group (control PN: 13.1 ± 1.5 versus Gln-PN: 12.6± 1.2, p-value = NS) and pancreatic surgery subgroup (control PN: 9.4 ± 1.5 versus Gln-PN: 11.1 ± 1.5, p-value = NS) and non-pancreatic surgery subgroup (control PN: 17.7 ± 2.1 versus Gln-PN: 14.1 ± 1.9, p-value = NS). Plasma levels of vitamin C, α-tocopherol, zinc and GSH were similar between Control-PN and Gln-PN subjects at baseline and day 7 in the entire study group and the pancreatic surgery subgroup; their changes over the 7-day treatment period were also similar between patients receiving Control-PN and Gln-PN. Plasma GSH levels were low and unaffected by Gln-PN in either the entire study group and in pancreatic surgery subgroups (data not shown). Figure 1 shows the effects of Gln-PN in non-pancreatic surgery cohort. In non-pancreatic surgery patients, plasma α-tocopherol levels were significantly increased in the control-PN patients (baseline: 628 ± 87 versus day 7; 953 ± 121 µµg/dL, p=0.038) and zinc levels were significantly improved in the Gln-PN group (day 0: 60.5 ± 4.9 vs day 7: 84.0± 5.8 µg/dL, p=0.001). In addition, as shown in Figure 2, in this non-pancreatic surgery cohort, changes of plasma concentrations of vitamin C, α-tocopherol and zinc were similar between control-PN and Gln-PN groups (vitamin C: conrol-PN 0.13± 0.07 vs Gln-PN 0.25 ±0.12 mg/dL, α-tocopherol:324.3 ± 133 vs 151.4 ± 80.0 µg/dL; zinc: 25.1 ± 11.8 vs 23.5 ± 4.65 µg/dL). On the contrary, the change in plasma GSH concentrations was significantly increased with Gln-PN compared to thechange (decrease) observed with control-PN in the non-pancreatic surgery patients (control-PN - 0.27 ± 0.17 µM versus Gln-PN: + 0.26 ± 0.13 µM; p=0.026) (Figure 1).
Figure 1.
Absolute plasma antioxidant concentrations in non-pancreatic surgical patients at baseline and after receiving control-PN or Gln-PN for 7 days. Plasma concentrations of Vitamin C, α-tocopherol, zinc and GSH were similar between the two study groups at baseline and day 7. However, compared with baseline (day 0) values, α-tocopherol levels were significantly improved with control-PN at 7 days (* p<0.04, day 0 versus day 7) and zinc levels were significantly improved with Gln-PN (*p<0.002, day 0 versus day 7). No differences in absolute plasma vitamin C or GSH values occurred from baseline to day 7 in either PN group.
Figure 2.
Change from baseline to day 7 in plasma antioxidant concentrations in non-pancreatic surgical patients comparing effects of control-PN and Gln-PN. The change in plasma vitamin C, α-tocopherol and zinc was similar between PN groups. However, change from baseline in plasma GSH concentrations (increase) was significantly different in individuals receiving Gln-PN compared to change from baseline (decrease) with control-PN. * p<0.03 control-PN versus Gln-PN.
Discussion
Our study shows that a large proportion of SICU patients exhibit below-normal plasma levels of vitamin C, GSH, and zinc after common major operations in which PN was required due to intestinal failure. The high incidence of vitamin C, GSH and zinc depletion is consistent with previous findings in other groups of critically ill patients [6, 7, 13, 23–30]. The percentage of patients with low plasma α-tocopherol concentrations was lower than the percentage of patients with low vitamin C, reduced GSH and zinc levels. Possible mechanisms for the depletion of these antioxidant nutrient compounds include inadequate intake in the perioperative period, increased antioxidant consumption to scavenge free radicals, increased urinary losses and/or cellular redistribution as an acute phase response [23–25, 28–30]. EDTA-containing sedatives commonly used in the ICU may increase urinary zinc secretion, which may lead to zinc depletion [31].
One of our goals was to assess the effects of conventional PN on these antioxidants in these severely catabolic patients. Our PN formulations provided vitamin C at 200 mg/day, α-tocopherol at 10 mg/day and zinc at 5 mg/day to all study subjects. However, plasma concentrations of vitamin C remained low in approximately one-half of patients after PN was administered for 7 days (Table 2, Table 3 and Table 4). This finding suggests that the amount of vitamin C provided in standard PN multivitamin preparations is not adequate to normalize plasma vitamin C levels in these types of critically ill surgical patients.
Nathens et al gave a large group of predominately trauma patients either placebo or large doses of parenteral vitamin C (3000 mg/day) plus enteral α-tocopherol (3000 IU/day) for up to 28 days after SICU admission [24]. Most patients received enteral tube feedings throughout the study. Mean serum ascorbate levels in the study patients were at the lower limit of normal at entry (similar to our study cohort) and remained essentially unchanged in the placebo-treated patients. Subjects receiving vitamin C supplementation achieved normal serum levels of vitamin C within 1 day of therapy, which were maintained at the upper range of normal, without evidence of toxicity, for the duration of the study [24]. Long et al [25] examined the effectiveness of PN supplemented with varied amounts of ascorbic acid for 2 days in 14 severely injured patients. They found that plasma ascorbic acid concentrations were not changed when ascorbic acid was given at 300 mg/day and only increased to low normal levels when it was given at a dose of 1000 mg/day [25]. Therefore, additional studies are needed to define the metabolic and clinical efficacy of larger doses of vitamin C supplementation than are routinely administered in critically ill surgical patients as a method to support anti-oxidant processes.
Although plasma α-tocopherol depletion was not as frequent in our study as was depletion of vitamin C, 21% of our entire study group exhibited values below the normal limit at entry, and 11% remained low after 7 days of PN. We did observe an increase in mean plasma α-tocopherol levels and a tendency for fewer subjects to exhibit below-normal plasma levels after 7 days of PN (Table 2 and Table 3). It is possible that low α-tocopherol levels in these individuals reflected a response to oxidative stress associated with the primary operation. Limited data suggests that soybean oil-based intravenous lipid emulsions, as used in the PN mix of our patients, may increase vitamin E requirements, possibly due to utilization to quench peroxidized lipids present in lipid emulsion [7, 32]. We previously found that plasma α-tocopherol progressively declined in bone marrow transplant recipients receiving conventional PN with soybean oil-based lipids compared to α-tocopherol levels in clinically matched subjects receiving only micronutrients and a very low amount of lipid emulsion [7]. In the study of Nathens et al [24], baseline serum α-tocopherol levels were similar to our baseline plasma values. In that study, control subjects demonstrated a gradual increase in α-tocopherol values over time, but were only significantly increased in the α-tocopherol-supplemented group (3,000 IU/day, as q8 hr divided doses of 1000 IU given enterally). This may reflect the decrease in oxidative stress as patients recovered from surgery and its complications. As with vitamin C, additional trials seem indicated to assess the metabolic, redox and clinical effects of larger doses of vitamin E supplementation in critically ill surgical patients.
Our conventional PN trace element preparation provided 5 mg of zinc/day and significantly improved plasma zinc concentrations and decreased the proportion of subjects with below normal zinc levels after 7 days. In a previous study conducted in patients after coronary bypass surgery, plasma zinc levels remained low two months after surgery when zinc was not supplemented [33]. Therefore, we conclude that our short-term regimen of zinc supplementation at 5 mg/day was effective in terms of normalizing plasma zinc levels in our SICU patients. Some of this effect may be due to clinical improvement of the subjects over time.
“In a previous study in patients with multiple organ failure, both total and reduced whole blood GSH was depleted, although plasma total GSH was elevated [1]. We found that plasma GSH (reduced GSH) was below our laboratory normal range in 59% of study subjects at entry, similar to our previous findings in catabolic bone marrow transplantation patients [7]. We did not measure whole blood GSH in the present study, but such data would have been of interest. Differences in plasma GSH between the previous study and our data may reflect differences in clinical characteristics of the patients in the two studies. GSH is a major intracellular antioxidant thiol and its concentration is much higher in cells than in the plasma. Whole blood GSH principally measures GSH in erythrocytes; it changes in response to the balance of its synthesis and loss and is correlated to oxidative stress. On the other hand, plasma GSH is largely derived from the liver (and also muscle and other extrahepatic tissues) [8], which release GSH in proportion to tissue concentration, and thus can be considered a reflection of systemic antioxidant capacity”.
Our study suggests that disease severity is not the only determinant of plasma GSH concentration in catabolic, hospitalized adults. Although patients in the non-pancreatic surgery cohort had higher APACHE II scores than those in the pancreatic surgery cohort, plasma GSH levels were significantly lower in pancreatic surgery patients, consistent with the GSH depletion induced by pancreatitis as previously shown by Schoenberg et al [34].
Previous studies indicate that Gln-supplemented PN is associated with decreased protein catabolism and decreased nosocomial infections in hospital patients, the latter effect was consistent with a potential role in upregulating immune functions [16, 19, 35]. Gln is a precursor molecule for GSH synthesis in translational models [16–18] and Gln-supplemented PN significantly attenuated GSH depletion in skeletal muscle of post-surgical patients [26]. GSH is required to maintain vitamins C and E, while zinc is known to stabilize thiol pools, and plays an important role in immune functions [12]. Therefore, we tested whether Gln supplementation would improve the antioxidants vitamins C and E, Gln and the antioxidant-associated mineral zinc. We did not find any advantage of Gln-PN over Gln-free control-PN in maintaining or improving patients’ antioxidant status in the total study group, as GSH, vitamin C, α-tocopherol and zinc concentrations were similarly improved in both Gln-PN and control groups over time. Gln-PN also did not improve concentrations of GSH, vitamin C, α-tocopherol or zinc in the pancreatic surgery subgroup. In the non-pancreatic surgical subgroup, who exhibited higher APACHE II scores, Gln-PN did not alter the change from baseline in plasma concentrations of vitamin C, α-tocopherol or zinc over time. However, in this cohort, the change from baseline in plasma GSH concentrations was significantly increased with Gln-PN compared to the decrease observed with control-PN (Figure 2).
Limitations of this study include the relatively small number of patients studied and the lack of selenium determinations. Selenium participates in antioxidant defense as an essential component of glutathione peroxidases, which catalyzes the reduction of hydroxyperoxides by GSH. Decreased selenium levels were observed in trauma patients [6, 11] and patients receiving coronary bypass surgery [33]. As demonstrated by Berger et al, low serum selenium concentrations were normalized after 7 days of selenium supplementation (62 mg/day) in critically ill trauma patients [36]. In a recent multi-center clinical trial of patients with severe systemic inflammatory response syndrome, sepsis, and/or septic shock, supplementation of intravenous sodium-selenite (1000 µg/day) for 14 days significantly improved whole blood selenium concentrations and decreased the 28-day mortality rate [37].
In conclusion, our findings confirm that depletion of major antioxidants in the bloodstream is common in critically ill SICU patients after major cardiac, vascular, colonic and pancreatic operations. Despite the administration of standard PN containing conventional amounts of micronutrients, vitamin C and GSH levels remained low in a significant proportion of patients after 7 days of PN. Thus, increasing the amount of vitamin C and GSH substrates in PN should be tested as an approach to normalize plasma levels and potentially clinical outcomes. Given the improvement in plasma GSH with Gln-supplemented PN in the cardiac, vascular and colonic surgery subgroup, intravenous Gln represents an approach to improve plasma GSH concentrations in selected SICU patients that should be further investigated.
Acknowledgements
Supported by Emory General Clinical Research Center (GCRC) grant M01 RR00039, a grant from Fresenius-Kabi (TRZ), and USDA Agricultural Research Service Cooperative Agreement 58-1950-4-40 (JBB). The authors thank the nursing staff of the Emory University Hospital Surgical Intensive Care Units and Ewald Schlotzer, Ph.D., of Fresenius-Kabi for their support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flaring UB, Rooyackers OE, Hebert C, Bratel T, Hammarqvist F, Wernerman J. Temporal changes in whole-blood and plasma glutathione in ICU patients with multiple organ failure. Intensive Care Med. 2005;31:1072–1078. doi: 10.1007/s00134-005-2687-0. [DOI] [PubMed] [Google Scholar]
- 2.Goodyear-Bruch C, Pierce JD. Oxidative stress in critically ill patients. Am J Crit Care. 2002;11:543–551. [PubMed] [Google Scholar]
- 3.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 5.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Plasma redox status relates to severity in critically ill patients. Crit Care Med. 2000;28:1812–1814. doi: 10.1097/00003246-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients. a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 7.Jonas CR, Puckett AB, Jones DP, Griffith DP, Szeszycki EE, Bergman GF, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72:181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 8.Kretzschmar M, Pfeifer U, Machnik G, Klinger W. Glutathione homeostasis and turnover in the totally hepatectomized rat: evidence for a high glutathione export capacity of extrahepatic tissues. Exp Toxicol Pathol. 1992;44:273–281. doi: 10.1016/S0940-2993(11)80244-7. [DOI] [PubMed] [Google Scholar]
- 9.Samiec PS, Drews-Botsch C, Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 10.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L-O, Kroncke K-D, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr. 2003;133 doi: 10.1093/jn/133.5.1448S. 1448S–1451. [DOI] [PubMed] [Google Scholar]
- 12.Moriarty-Craige SE, Ha KN, Sternberg P, Jr, Lynn M, Bressler S, Gensler G, Jones DP. Effects of long-term zinc supplementation on plasma thiol metabolites and redox status in patients with age-related macular degeneration. Am J Ophthalmol. 2007;143:206–211. doi: 10.1016/j.ajo.2006.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger MM, Cavadini C, Chiolero R, Dirren H. Copper, selenium, and zinc status and balances after major trauma. J Trauma. 1996;40:103–109. doi: 10.1097/00005373-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130 doi: 10.1093/jn/130.5.1399S. 1399S–1406. [DOI] [PubMed] [Google Scholar]
- 15.Baines M, Shenkin A. Lack of effectiveness of short-term intravenous micronutrient nutrition in restoring plasma antioxidant status after surgery. Clin Nutr. 2002;21:145–150. doi: 10.1054/clnu.2001.0524. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler TR, Szeszycki EE, Estivariz CF, Puckett AB, Leader LM. Glutamine: from basic science to clinical applications. Nutrition. 1996;12:S68–S70. doi: 10.1016/s0899-9007(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 17.Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114–119. doi: 10.1097/00000658-199202000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oehler R, Roth E. Regulative capacity of glutamine. Curr Opin Clin Nutr Metab Care. 2003;6:277–282. doi: 10.1097/01.mco.0000068962.34812.ac. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler TR, Fernandez-Estivariz C, Griffith DP, Szeszycki EE, Bazargan N, Luo M, et al. Parenteral nutrition supplemented with alanyl-glutamine dipeptide decreases infectious morbidity and improves organ function in critically ill post-operative patients: Results of a double-blind, randomized, controlled study. JPEN J Parenter Enteral Nutr. 2004;28:S11–S12. abst. [Google Scholar]
- 20.Behrens WA, Madere R. A highly sensitive high-performance liquid chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological fluids, and foods. Anal Biochem. 1987;165:102–107. doi: 10.1016/0003-2697(87)90206-5. [DOI] [PubMed] [Google Scholar]
- 21.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 22.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 23.Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. 1996;63(5):760–765. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 24.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. 2003;109:144–148. doi: 10.1016/s0022-4804(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 26.Flaring UB, Rooyackers OE, Wernerman J, Hammarqvist F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci. 2003;104:275–282. doi: 10.1042/CS20020198. [DOI] [PubMed] [Google Scholar]
- 27.Wernerman J, Luo JL, Hammarqvist F. Glutathione status in critically-ill patients: possibility of modulation by antioxidants. Proc Nutr Soc. 1999;58:677–680. doi: 10.1017/s0029665199000889. [DOI] [PubMed] [Google Scholar]
- 28.Boosalis MG, Solem LD, Cerra FB, Konstantinides F, Ahrenholz DH, McCall JT, et al. Increased urinary zinc excretion after thermal injury. J Lab Clin Med. 1991;118:538–545. [PubMed] [Google Scholar]
- 29.Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646–651. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 2004;35:163–168. doi: 10.1161/01.STR.0000105391.62306.2E. [DOI] [PubMed] [Google Scholar]
- 31.Higgins TL, Murray M, Kett DH, Fulda G, Kramer KM, Gelmont D, et al. Trace element homeostasis during continuous sedation with propofol containing EDTA versus other sedatives in critically ill patients. Intensive Care Med. 2000;26:S413–S421. doi: 10.1007/pl00003785. [DOI] [PubMed] [Google Scholar]
- 32.Kharb S, Ghalaut VS, Ghalaut PS. Alpha tocopherol concentration in serum of critically ill patients. J Assoc Physicians India. 1999;47:400–402. [PubMed] [Google Scholar]
- 33.Antila H, Salo M, Nanto V, Irjala K, Brenner R, Vapaavuori M. Serum iron, zinc, copper, selenium, and bromide concentrations after coronary bypass operation. JPEN. 1990;14:85–89. doi: 10.1177/014860719001400185. [DOI] [PubMed] [Google Scholar]
- 34.Schoenberg MH, Birk D, Beger HG. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62:1306S–1314S. doi: 10.1093/ajcn/62.6.1306S. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, Bye R, et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med. 1992;116:821–828. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]
- 36.Berger MM, Baines M, Chiolero RL, Wardle CA, Cayeux C, Shenkin A. Influence of early trace element and vitamin E supplements on antioxidant status after major trauma: a controlled trial. Nutrition Research. 2001;21:41–54. [Google Scholar]
- 37.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]