Abstract
The chemokine stromal cell-derived factor 1α (SDF-1α, CXCL12) and its receptor CXCR4 are implicated as key mediators of hematopoietic stem cell retention, cancer metastasis, and HIV infection. Their role in myocardial infarction is not as well defined. The non-invasive in vivo quantitation of CXCR4 expression is central to understanding its importance in these diverse processes as well in the cardiac response to injury.
Methods
Using high specific activity [99mTc-MAS3]-NHS prepared by solid-phase pre-loading, recombinant SDF-1α was radiolabeled under aprotic conditions and purified by gel-filtration chromatography (GFC). Radiotracer stability and transmetallation under harsh conditions were quantified by GFC. Affinity, specificity, and Bmax were quantified, with adenoviral-expressed CXCR4 on non-expressing cells and endogenous receptor on rat neonatal cardiomyocytes, using a high-throughput live-cell binding assay. Blood half-life, biodistribution, and clearance of intravenously injected [99mTc-MAS3]-SDF-1α were quantified in Sprague-Dawley rats before and after experimentally induced myocardial infarction.
Results
[99mTc-MAS3]-SDF-1α could be prepared in 2 hr total with a specific activity of 8.0 × 107 MBq/mmol (2,166 Ci/mmol) and a radiochemical purity >98%. Degradation of the radiotracer after boiling for 5 min, with and without 1 mM dithiothreitol (DTT), and transmetallation in 100% serum at 37°C for 4 hr, were negligible. [99mTc-MAS3]-SDF-1α exhibits high specificity for CXCR4 on the surface of living rat neonatal cardiomyocytes, with an affinity of 2.7 ± 0.9 nM and a Bmax of 4.8 × 104 binding sites per cell. After intravenous injection, 99mTc-labeled SDF-1α displays a blood half-life of 25.8 ± 4.6 min, rapid renal clearance with only 26.2 ± 6.1% ID remaining in the carcass at 2 hr, consistently low uptake in most organs (<0.1% ID/g), and no evidence of blood-brain barrier penetration. In the setting of myocardial infarction, CXCR4 expression levels in the myocardium increased over 5-fold, as quantified using [99mTc-MAS3]-SDF-1α and confirmed using confocal immunofluorescence.
Conclusion
We describe a 99mTc-labeled SDF-1α radiotracer that can be used as a sensitive and specific probe for CXCR4 expression in vivo, and demonstrate that this radiotracer is able to quantify changes in CXCR4 expression under different physiologic and pathologic states. Taken together, CXCR4 levels should now be quantifiable in vivo in a variety of animal model systems of human diseases.
Keywords: SDF-1α, CXCR4, Chemokines, SPECT Radiotracers, Myocardial Infarction
INTRODUCTION
Chemokines are a family of structurally related glycoproteins, ranging in size from 6 to 14 kDa, with potent leukocyte activation and/or chemotactic activity. They fall into 4 classes, CC, CXC, C, and CX3C, based on the position of the first two conserved cysteine residues in the mature protein (1). Stromal-derived factor-1 (SDF-1, CXCL12) belongs to the CXC class of chemokines based on its amino acid motif.
SDF-1α and its receptor CXCR4 are an important chemokine/receptor pair, which play a crucial role in numerous biological processes including hematopoiesis, vasculogenesis, neuronal development, and immune cell trafficking (2–4). They also have been implicated in various pathological conditions such as cancer (5,6), infection with the human immunodeficiency virus (HIV; (7)), and various inflammatory conditions (8,9).
Recently, there has been increased focus on the role of chemokines in cardiovascular development (10) and in adult cardiac disease such as acute coronary syndromes (11) and heart failure (12). Chemokines and their receptors are present and functional on the myocardium and on vascular smooth muscle cells (13), and are capable of mediating important biological processes in a manner that is independent of inflammatory cells (14). Both SDF-1α and its receptor CXCR4 are essential for cardiogenesis (15), and CXCR4 is expressed on early hematopoietic stem cells (HSC) and endothelial progenitor cells (4). SDF-1α even appears to modulate myocyte contractility in rodent model systems (12). Recently, several studies have demonstrated that circulating chemokine levels, including that of SDF-1α, are elevated in animals and humans with cardiac dysfunction (16,17), and both chemokines and their receptors are up-regulated in the hearts of patients with congestive heart failure (CHF) (18). After myocardial infarction (MI), chemokines mediate the retention of stem cells (SC) that could potentially repair damaged myocardium (19), and re-establishing SDF-1α expression at a time remote from a MI may restore stem cell homing to the damaged cardiac tissue (20,21).
Although associated with cardiac dysfunction, the mechanisms by which chemokines and their receptors exert their effects are largely unknown. Since the role of chemokines in the cardiac response to inflammation may be quite distinct from that of cytokines (22), and the recent focus on using chemokines to modulate the SC milieu in the heart, there is an urgent need to quantify chemokine and chemokine receptor levels in vivo, non-invasively, and over time.
Our laboratory has previously developed a solid-phase pre-radiolabeling technique for rapid and efficient 99mTc labeling of small molecules and peptides under aprotic conditions (23). In the present study, we use this technique to prepare a biologically active SDF-1α radiotracer with high specific activity. We also demonstrate that CXCR4 levels can now be quantified in vivo, over time, and that CXCR4 is significantly over-expressed in myocardium subjected to infarction.
MATERIALS AND METHODS
Reagents
Recombinant murine stromal-cell derived factor-1α (SDF-1α) was purchased from Peprotech (Rocky Hill, NJ). The NHS ester of hydrazinonicotinamide (HYNIC-NHS) was the generous gift of J. L. Vanderheyden (Theseus Imaging). Ultra-dry dimethylsulfoxide (DMSO) was purchased from Acros Organics (Geel, Belgium). HPLC grade triethylammonium acetate, pH 7 (TEAA) was from Glen Research (Sterling, VA). HPLC grade water was from American Bioanalytic (Natick, MA). All other chemicals were purchased from Fisher Scientific (Hanover Park, IL) and were ACS or HPLC grade.
Synthesis and Purification of [99mTc-MAS3]-SDF-1α
The N-hydroxysuccinimide (NHS) ester of [99mTc-MAS3] was prepared to high purity (>99%) and high specific activity (average 1.1 × 108 MBq/mmol; 3 × 103 Ci/mmol) in DMSO using a 20 min protocol described in detail previously (23). For radiolabeling, SDF-1α dry powder was resuspended in DMSO at a concentration of 12.6 μM. 185 MBq (5 mCi; 0.45 nmol) of [99mTc-MAS3]-NHS (550 μL) was mixed with 50 μL (0.63 nmol) of SDF-1α followed by the addition of 2 μL (8 μmol) of 4 M triethylamine. Constant stirring at room temperature (RT) was maintained for 1 hr. Radiolabeled [99mTc-MAS3]-SDF-1α was purified by gel-filtration chromatography (GFC) using an 8 × 300 mm, 60 Å Diol (Catalog #DL06S053008WT; YMC, Kyoto, Japan) gel-filtration column and phosphate-buffered saline, pH 7.4 (PBS) as mobile phase. Details of the HPLC system and detectors have been described in detail previously (24). GFC-purified [99mTc-MAS3]-SDF-1α (specific activity 8.0 × 107 MBq/mmol; 2,166 Ci/mmol) was concentrated to ≈ 148 MBq/ml (4 mCi/ml) using a 5,000 MWCO Vivaspin (Catalog #VS0111, Sartorius Stedim Biotech, Aubagne, France) cartridge.
Synthesis of [99mTc-HYNIC]-Albumin
Covalent conjugation of the NHS ester of hydrazinonicotinamide (HYNIC-NHS) to albumin was performed by mixing 1 ml (72 nmol) of 5 mg/ml Cohn Fraction V bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) solution in PBS and 100 μL (320 nmol) of 3.2 mM HYNIC-NHS in DMSO. After incubation for 2 hr at RT, unreacted ligand was removed by the GFC on an ÄKTA prime pump and Superose-6 10/300 GL column (Amersham Biosciences, Piscataway, NJ) using 114 mM tricine, pH 6.6 as mobile phase, and a flow rate of 1 ml/min. Exchange labeling using tricine as weak ligand was performed by adding 1 mg of tricine powder to 1 ml of the derivatized albumin solution, followed by 25 μg of SnCl2.2H2O dissolved in 12.5 μL 0.01 N HCl and 100 μL (185–296 MBq; 5–8 mCi) of 99mTc-pertechnetate. After a 30 min incubation, radiolabeled albumin was purified and analyzed by GFC using an 8 × 300 mm, 200 Å Diol (Catalog #DL06S053008WT; YMC) column and PBS as mobile phase. GFC-purified [99mTc-HYNIC]-albumin (specific activity 1.8 × 107 MBq/mmol; 476 Ci/mmol) was concentrated to ≈ 148 MBq/ml (4 mCi/ml) using a 5,000 MWCO Vivaspin cartridge.
Quantification of Chemical Stability
[99mTc-MAS3]-SDF-1α was boiled for 5 min in PBS in the presence or absence of 1 mM dithiothreitol (DTT), and also incubated for 4 hr at 37°C in 100% calf serum. Stability and transmetallation were quantified using GFC and the 60 Å Diol column as described above. GFC molecular weight markers (Bio-Rad, Hercules, CA) were: M1 = thyroglobulin (670 kDa, rt = 4.4 min), M2 = γ-globulin (158 kDa, rt = 5.1 min), M3 = ovalbumin (44 kDa, rt = 6.9 min), M4 = myoglobin (17 kDa, rt = 8.0 min), and M5 = vitamin B12 (1.3 kDa, rt = 12.4 min).
Adenoviral Expression of Transgenes and Isolation of Neonatal Cardiomyocytes
Human prostate cancer cell line PC-3 was obtained from the ATCC (Manassas, VA). Adenoviruses co-expressing CXCR4 and green fluorescent protein (GFP), or human bone morphogenetic protein-2 (BMP-2) and GFP, were prepared as described previously (25). Cells were transduced at a multiplicity of infection of 500:1 and incubated for 48 hr at 37°C, under humidified 5% CO2, in RPMI 1640 medium (Mediatech Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Woodland, CA) and 5% penicillin/streptomycin (Cambrex Bioscience, Walkersville, MD).
Spontaneously beating cardiomyocytes were prepared from 1- to 2-day old Sprague-Dawley rat pups using the Neonatal Cardiomyocyte Isolation System (Worthington Biochemical, Lakewood, NJ). Cardiomyocytes were cultured in 96-well filter plates (model MSHAS4510, Millipore, Bedford, MA) at 20,000 cells/well in F-10 medium (Invitrogen, Carlsbad, CA) in the presence of 5% FCS and 10%) horse serum, 100 U/ml penicillin/streptomycin, and 2 mM L-glutamine over 48 hr.
High-Throughput Live Cell Binding Assay
To quantify absolute affinity and Bmax for the surface of living cells, a high-throughput homologous competition assay was employed as described in detail previously (23). Briefly, cells were split onto 96-well filter plates (model MSHAS4510, Millipore, Bedford, MA) and grown to 50% confluence (approximately 40,000 cells per well) over 48 hr. The assay was performed at 4°C to avoid internalization of the radioligand due to constitutive endocytosis. Cells were washed twice with ice-cold PBS or serum and incubated for 20 min at 4°C with 0.02 MBq (0.5 μCi) of radiotracer in the presence or absence of homologous cold compound. Cells were then washed 3 times with PBS or serum using a Millipore vacuum manifold (Catalog # MSVMHTS00) and the well contents transferred directly to 12 × 75 mm plastic tubes placed in gamma counter racks and counted on a model 1470 Wallac Wizard (Perkin Elmer, Wellesley, MA) ten-detector gamma counter. 96-well plate imaging was performed using an Isocam Technologies (Castana, IA) gamma camera equipped with a 1/2″ NaI crystal and a high-resolution (1 mm) low energy collimator.
Animal Model Systems
Animal studies were performed in accordance with approved Institutional Animal Care and Use Committee protocols. Male Sprague-Dawley inbred rats (200–250 gm; Charles River Labs, Wilmington, MA) were anesthetized with 50 mg/kg intraperitoneal (IP) pentobarbital, intubated, and mechanically ventilated with a rodent ventilator at 80 breaths per minute using room air. Following a left lateral thoracotomy, the left anterior descending (LAD) artery was ligated for 30 min with 6.0 silk suture (26). Myocardial infarction (MI) was confirmed by visualization of myocardial blanching and ballooning distal to the point of ligation. The incision was then closed by suturing all 3 layers (bone, muscle, and skin) and excess air was evacuated with a 22-gauge angiocath (BD, Franklin Lakes, NJ) to prevent pneumothorax. Rats were extubated when spontaneous breathing returned, and received 0.5 ml of 0.5% bupivicaine analgesia for the next 48 hr until used for radiotracer studies as described below. For the sham group, rats of comparable body weight and age underwent similar procedures with the exception of LAD ligation.
Blood Activity Curves, Biodistribution, and Clearance
37 MBq (1 mCi) of [99mTc-MAS3] or [99mTc-MAS3]-SDF-1α in 200 μL of saline was administered intravenously (IV) via the retro-orbital sinus. Blood was collected at 0, 1, 2, 5, 10, 15, 30, 60, 90, and 120 min post-injection from the tail vein using micro-capillary tubes, weighed, and counted as described above. Curve fitting was performed using Prism version 4.0a (GraphPad, San Diego, CA) software. For measurement of total body retention and clearance, at 2 hr post-injection, we ligated the ureters and urethra with silk sutures, removed the bladder en masse, and combined it with excreted urine and feces before measurement of radioactivity in a dose calibrator. The remaining carcass was also measured in a dose calibrator, then the heart, lungs, spleen, liver, kidneys, stomach, intestine, and brain were resected, washed twice in PBS, weighed, and counted as described above. In rats undergoing MI, the heart was separated into non-infarcted and infarcted tissue prior to quantitation. An additional cohort of rats was injected with 37 MBq (1 mCi) of 99mTc-labeled albumin in 200 μL of saline to measure perfusion. Statistical analysis was performed with an unpaired t-test using Prism.
Confocal Fluorescence Microscopy
In parallel with radiotracer experiments, an additional cohort of rats was sacrificed 24 hr after surgery by sodium pentobarbital overdose, and hearts were excised and frozen on dry ice. 16-μm cryo-sections were collected on Superfrost Plus slides, post-fixed with 2% paraformaldehyde/2% sucrose, and permeabilized with 0.3% Triton. Sections were blocked in 2% BSA in PBS (blocking solution). Mouse anti-SDF-1α monoclonal antibody (Catalog # MAB350, R&D Systems, Minneapolis, MN) or rabbit anti-rCXCR4 polyclonal antibody (Catalog # TP503, Torrey Pines Biolabs, Houston, TX) was diluted 1:80 or 1:100, respectively, in blocking solution, incubated overnight at 4°C with the specimen, and visualized using fluorophore-labeled goat anti-mouse IgG or goat anti-rabbit IgG secondary antibodies, respectively. F-actin was visualized with rhodamine phalloidin (Catalog # R415, Invitrogen). Negative controls were incubated with non-immune rabbit IgG (Catalog # AB-105-C, R&D Systems) and mouse IgG isotype control (Catalog # MAB002, R&D Systems). Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI). Photomicrographs were captured on a Leica TCS-SP (UV) confocal microscope at the Mount Sinai School of Medicine Microscopy Shared Research Facility.
RESULTS
Radiolabeling and Purification of [99mTc-MAS3]-SDF-1α
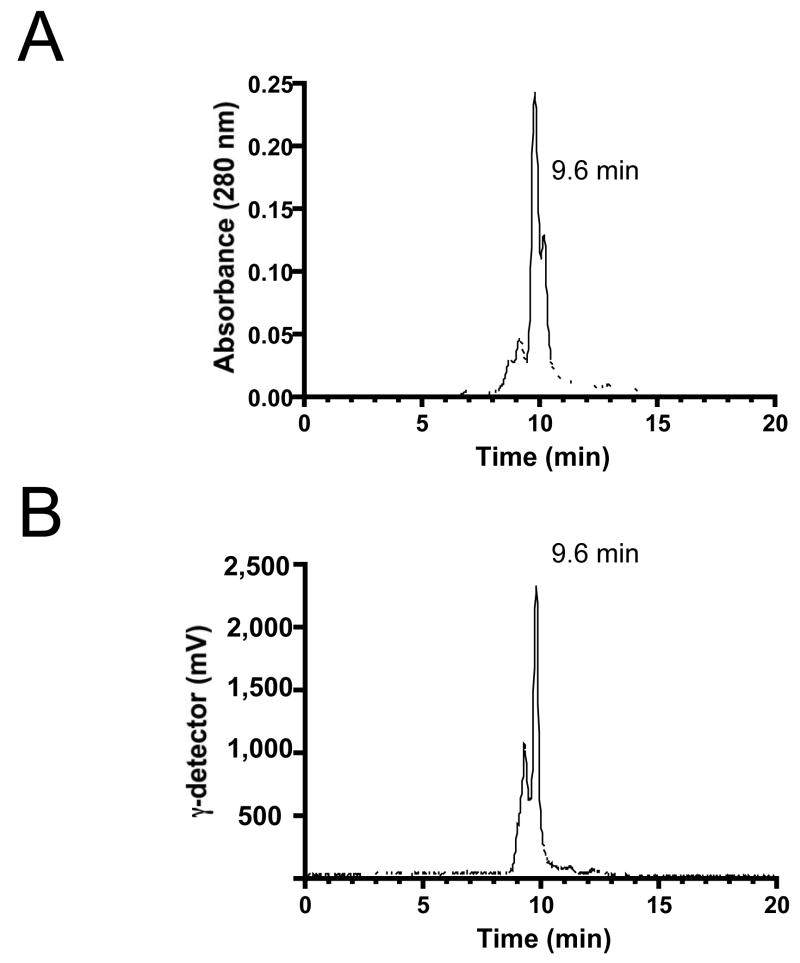
Using high specific-activity, solid-phase pre-loaded [99mTc-MAS3]-NHS, SDF-1α was labeled under non-aqueous conditions (Figure 1) and purified using GFC (Figure 2), resulting in a radiotracer with >98% radiopurity and a minimum specific activity of 8.0 × 107 MBq/mmol (2,166 Ci/mmol). With respect to the initial 99mTcO4−, the average radiolabeling yield was 70% [range 65–75%] for over 20 separate labeling procedures. With respect to [99mTc-MAS3]-NHS, radiolabeling yield was >98% for over 20 separate labeling procedures. Although the major peptide peak of recombinant SDF-1α has a retention time (rt) of 9.6 min and was labeled efficiently, other minor peaks were labeled with varying efficiency (Figure 2). Of note, the specific activity calculation is based on the entire mass of SDF-1α present in the labeling reaction, and is thus likely an underestimate of the true specific activity. No attempt was made to identify which of the 9 (1 alpha, 8 epsilon; Figure 1) possible primary amines were labeled, although the low molar ratio of [99mTc-MAS3]-NHS to SDF-1α (0.7:1) ensured that, on average, fewer than one radioatom per molecule was incorporated. Importantly, [99mTc-MAS3]-SDF-1α was readily separable from spontaneously hydrolyzed [99mTc-MAS3] at rt of 13.5 min (data not shown).
Figure 1. 99mTc Radiolabeling of SDF-1α.
Solid-phase pre-labeled [99mTc-MAS3]-NHS was conjugated covalently to recombinant SDF-1α in DMSO in the presence of base, releasing only NHS as the byproduct. Primary amines available for conjugation are indicated by * in the peptide sequence.
Figure 2. Purification and Stability of [99mTc-MAS3]-SDF-1α.
Purification of [99mTc-MAS3]-SDF-1α using gel-filtration chromatography and simultaneous monitoring of A) 280 nm absorbance and B) gamma-ray activity. Retention times of major peaks are also shown. Tracings are representative of N = 8 independent labeling reactions.
Stability of [99mTc-MAS3]-SDF-1α
Although one of the features of [99mTc-MAS3] complexes is high stability, and conjugation of the complex to SDF-1α was designed to be covalent, we tested the stability of [99mTc-MAS3]-SDF-1α under various conditions. There was no evidence of complex dissociation after boiling for 5 min in the presence or absence of 1 mM DTT, and no evidence of transmetallation when incubated with 100% serum for 4 hr at 37°C. Quantitation of the chromatographs (data not shown) suggested that >98% of [99mTc-MAS3]-SDF-1α remained intact under these conditions.
Affinity and Specificity of [99mTc-MAS3]-SDF-1α for CXCR4 on the Surface of Living Cells
Using a previously described live cell homologous competition assay (23), we were able to characterize the affinity and specificity of [99mTc-MAS3]-SDF-1α for CXCR4 on the surface of living cells. First, non-activated PC-3 human prostate cancer cells, which express low levels of CXCR4 mRNA (27) and no measurable protein, were transduced with an adenovirus co-expressing CXCR4 and GFP. GFP was used to confirm adenoviral transduction and to normalize levels of expression (data not shown). Negative controls included transduction with a similar adenovirus expressing BMP-2 and GFP, or untransduced cells. [99mTc-MAS3]-SDF-1α exhibited high specificity for CXCR4, an affinity of 1.0 ± 0.1 nM (mean ± S.D.), and a Bmax of 2.6 × 105 binding sites per cell. Indeed, cells could be visualized in situ on the filter plates by gamma-ray imaging (data not shown). Binding of [99mTc-MAS3]-SDF-1α to neonatal rat cardiomyocytes exhibited a slightly lower affinity (2.9 ± 0.5 nM) than adenovirus over-expressed CXCR4, but a relatively high Bmax of 4.8 × 104 binding sites per cell. This measured affinity is consistent with the previously published value of 8.3 ± 1.2 nM for SDF-1α binding to CXCR4 on human corneal fibroblasts (28). Results were nearly identical when the assay was performed in PBS (affinity of 2.9 ± 0.5 nM) or 100% serum (affinity of 2.7 ± 0.9 nM) with the same Bmax of 4.8 × 104 binding sites per cell, suggesting that serum does not contain natural inhibitors of the binding process.
Blood Half-Life, Biodistribution, and Clearance
After intravenous injection, [99mTc-MAS3] alone exhibits an extremely short blood half-life of 5.0 ± 1.0 min (Figure 3A) and rapid renal clearance (data not shown), consistent with previous reports (29). However, [99mTc-MAS3]-SDF-1α exhibits a prolonged blood half-life of 25.8 ± 4.6 min (Figure 3B). At 2 hr post-injection, 73.8 ± 6.1% injected dose (ID) was found in excrement, primarily urine, with 26.2 ± 6.1% ID remaining in the carcass (data not shown). Analysis of the major organs (Figure 3C) revealed relatively low uptake of less than 0.1% ID/g except for kidneys, the major site of excretion. No brain uptake was measured, suggesting that [99mTc-MAS3]-SDF-1α does not cross the blood brain barrier.
Figure 3. In Vivo Biodistribution and Clearance of [99mTc-MAS3]-SDF-1α.
A) Blood activity curves (mean ± S.D.) of [99mTc-MAS3] alone and B) [99mTc-MAS3]-SDF-1α after intravenous injection of 37 MBq (1 mCi) into sham-operated rats. Also shown are calculated half-lives (T1/2). Results are from N = 3 independent experiments. C) Total organ distribution (% ID/g; mean ± S.D.) in the rats from Figure 3A at 2 hr post injection.
Quantitation of Functional CXCR4 in Normal and Injured Myocardium
To quantify the expression of CXCR4 in the setting of MI, a rat model of ischemia-reperfusion was employed as described in Materials and Methods. Twenty-four hr after injury, and 2 hr after intravenous injection of [99mTc-MAS3]-SDF-1α, dramatic changes in CXCR4 expression were identified. As shown in Figure 4A, although normal myocardium from sham-operated rats, and non-infarcted myocardium from MI rats had consistently low uptake of [99mTc-MAS3]-SDF-1α, 0.11 ± 0.02% ID/g and 0.11 ± 0.1% ID/g, respectively, infarcted myocardium exhibited a 5-fold increase in CXCR4 expression (0.57 ± 0.04% ID/g), with p < 0.0001 for the comparison between infarcted and normal/non-infarcted tissue (Figure 4A). Since MI can cause changes in local blood perfusion, the experiment was repeated using 99mTc-labeled albumin. As shown in Figure 4B, there was no statistical difference in 99mTc-labeled albumin uptake ([99mTc-HYNIC]-albumin) in non-infarcted and infarcted myocardium from the same animal.
Figure 4. Quantitation of Functional CXCR4 Expression in Myocardial Infarction.
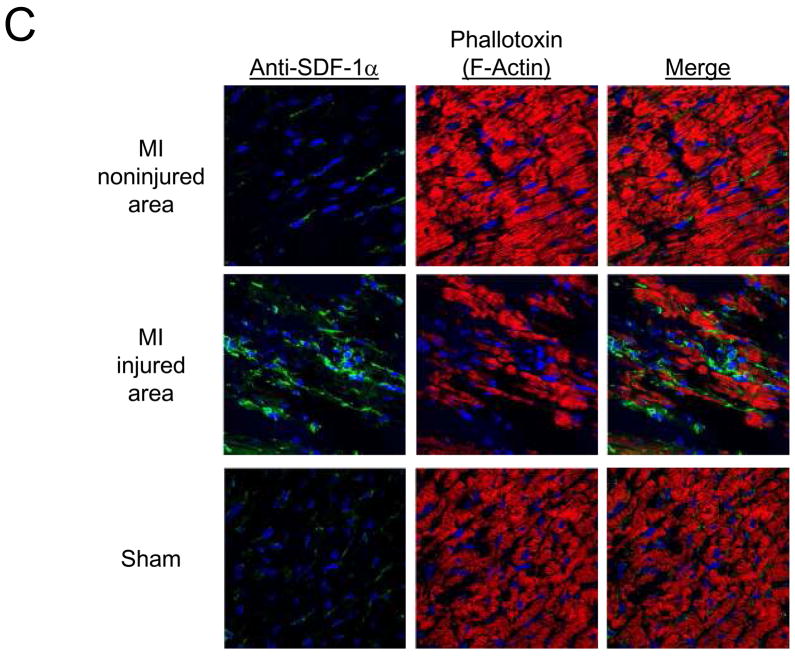
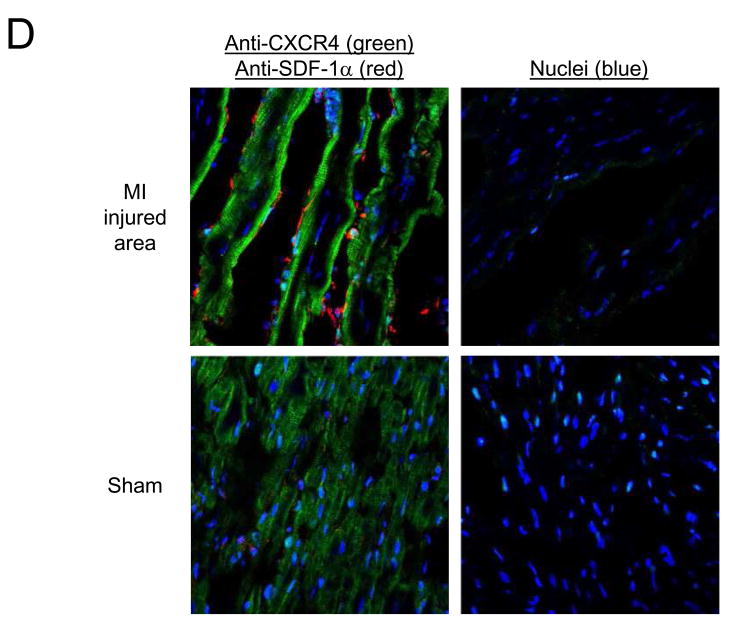
Uptake (% ID/g; mean ± S.E.M.) of A) [99mTc-MAS3]-SDF-1α and B) 99mTc-labeled albumin 24 hr after sham operation (Sham) or ischemia-reperfusion-induced myocardial infarction (MI) and 2 hr after intravenous injection of 37 MBq (1 mCi) of radiotracer. Normal myocardium and infarcted myocardium from MI hearts were quantitated separately. Results are from N = 4 rats per group. C) Confocal fluorescence microscopy of hearts from Figure 4A stained with mouse anti-SDF-1α antibody (green) and myocyte probe phalloidin (red). Nuclei are counterstained with DAPI (blue). D) Confocal fluorescence microscopy of hearts from Figure 4A stained with anti-CXCR4 antibody (green) and anti-SDF-1α antibody (red). Nuclei are counterstained with DAPI (blue).
Confocal fluorescence microscopy was then used to determine whether the quantitative changes seen using the [99mTc-MAS3]-SDF-1α radiotracer were consistent with endogenous expression levels (30). As shown in Figure 4C, endogenous SDF-1α (green) levels are increased in injured, but not non-injured or sham-operated myocardium, suggesting that SDF-1α is part of the natural response to MI. Most important, endogenous CXCR4 expression (green) increases significantly after MI (Figure 4D), along with SDF-1α (red), confirming that [99mTc-MAS3]-SDF-1α can be used to quantify CXCR4 levels in vivo.
DISCUSSION
Chemokines are a distinct class of inflammatory molecules that play a crucial role in a variety of developmental, physiologic and pathologic processes including heart disease (31). The ability to quantify CXCR4 levels, non-invasively and over time, enables the mechanisms underlying their effects to be more successfully explored. In this study, we have opted to label the endogenous ligand for CXCR4, SDF-1α, for use as a radiotracer. By utilizing a previously described solid-phase pre-labeling technique (23), we were able to achieve high specific activity, while retaining bioactivity. [99mTc-MAS3]-SDF-1α has a high affinity for CXCR4, and a high specificity on both over-expressing and endogenously expressing cells. Moreover, the radiotracer was remarkably stable under harsh conditions. In vivo, [99mTc-MAS3]-SDF-1α was cleared relatively quickly from the body via renal excretion, resulting in relatively low background tissue retention. Therefore, the in vivo quantification of CXCR4 levels using [99mTc-MAS3]-SDF-1α will likely significantly enhance studies to define the physiological and pathophysiological roles of SDF-1α and CXCR4 in critical disease states such as MI and cancer.
In this study, we focused on the role of CXCR4 in MI. Infarcted myocardium expressed 5-fold higher levels of CXCR4 when compared to non-infarcted areas at 24 hr post-injury. We confirmed, using identically labeled albumin that the increase in signal correlated specifically with CXCR4, and was not secondary to changes in myocardial perfusion. The reason CXCR4 (and SDF-1α) levels are markedly increased following ischemic injury is unknown, but is the focus of intense investigation (4,20). The development and experimental properties of [99mTc-MAS3]-SDF-1α described herein should assist future mechanistic studies by providing quantitation for the level and the kinetics of CXCR4 expression.
There are numerous potential clinical uses for [99mTc-MAS3]-SDF-1α as a single photon emission computed tomography (SPECT) radiotracer. First, it may be used to quantify the extent of injured myocardium in a patient following MI without the high liver uptake associated with many currently implemented radiodiagnostic agents (32–34). Second, it may be employed to titrate drugs specific for CXCR4 by non-invasively probing receptor occupancy in the target organ of interest. Third, given that CXCR4 levels have been demonstrated to be increased in patients with heart failure (22), this radiotracer has the potential to determine whether CXCR4 levels differ in patients with cardiac dysfunction, and thus stratify patients with respect to prognosis and treatment regimen. Finally, it could be used during stem cell therapy to ensure that the local environment for cellular retention is amenable to engraftment.
CONCLUSION
We describe the preparation of a high specific activity, 99mTc-labeled SDF-1α radiotracer with high radiochemical purity and stability. [99mTc-MAS3]-SDF-1α also possesses high affinity and specificity for its endogenous chemokine receptor, CXCR4, and can be used in vitro and in vivo as a non-invasive probe of CXCR4 expression levels. Using this radiotracer, we demonstrate that myocardial CXCR4 levels are increased 5-fold in myocardium subjected to ischemic injury compared to non-injured myocardium in the same heart. The potential clinical uses of the radiotracer in drug development and in patient management have been discussed.
Acknowledgments
Sources of Funding: This work was supported by NIH grants R01-CA-115296 (John V. Frangioni), R01-HL-073458 (Alison D. Schecter), and R01-HL-078691 (Roger J. Hajjar), and grants from the Lewis Family Fund (John V. Frangioni) and the Ellison Foundation (John V. Frangioni).
We thank Barbara L. Clough for editing and Eugenia Trabucchi and Alice Gugelmann for administrative assistance. This work was supported by NIH grants R01-CA-115296 (John V. Frangioni), R01-HL-073458 (Alison D. Schecter), and R01-HL-078691 (Roger J. Hajjar), and grants from the Lewis Family Fund (John V. Frangioni) and the Ellison Foundation (John V. Frangioni).
References
- 1.Chemokine/chemokine receptor nomenclature. Cytokine. 2003 Jan 7;21(1):48–49. doi: 10.1016/s1043-4666(02)00493-3. [DOI] [PubMed] [Google Scholar]
- 2.Doitsidou M, Reichman-Fried M, Stebler J, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002 Nov 27;111(5):647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 3.Hatse S, Princen K, Liekens S, Vermeire K, De Clercq E, Schols D. Fluorescent CXCL12AF647 as a novel probe for nonradioactive CXCL12/CXCR4 cellular interaction studies. Cytometry A. 2004 Oct;61(2):178–188. doi: 10.1002/cyto.a.20070. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003 Mar 11;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003 Sep 18;425(6955):307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 7.Berger EA. Introduction: HIV co-receptors solve old questions and raise many new ones. Semin Immunol. 1998 Jun;10(3):165–168. doi: 10.1006/smim.1998.0126. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraju K. Update on immunopathogenesis in inflammatory myopathies. Curr Opin Rheumatol. 2001 Nov;13(6):461–468. doi: 10.1097/00002281-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 9.MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999 Sep;19(5):266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- 10.Smith TK, Bader DM. Signals from both sides: Control of cardiac development by the endocardium and epicardium. Semin Cell Dev Biol. 2007 Feb;18(1):84–89. doi: 10.1016/j.semcdb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005 Apr 1;96(6):612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 12.Pyo RT, Sui J, Dhume A, et al. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006 Nov;41(5):834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007 May;97(5):730–737. [PubMed] [Google Scholar]
- 14.Aukrust P, Ueland T, Muller F, et al. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation. 1998 Mar 31;97(12):1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998 Jun;10(3):179–185. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 16.Behr TM, Wang X, Aiyar N, et al. Monocyte chemoattractant protein-1 is upregulated in rats with volume-overload congestive heart failure. Circulation. 2000 Sep 12;102(11):1315–1322. doi: 10.1161/01.cir.102.11.1315. [DOI] [PubMed] [Google Scholar]
- 17.Shioi T, Matsumori A, Kihara Y, et al. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997 Nov;81(5):664–671. doi: 10.1161/01.res.81.5.664. [DOI] [PubMed] [Google Scholar]
- 18.Aukrust P, Damas JK, Gullestad L, Froland SS. Chemokines in myocardial failure -- pathogenic importance and potential therapeutic targets. Clin Exp Immunol. 2001 Jun;124(3):343–345. doi: 10.1046/j.1365-2249.2001.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004 Nov 23;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 20.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003 Aug 30;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007 Oct;21(12):3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 22.Damas JK, Eiken HG, Oie E, et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000 Sep;47(4):778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 23.Misra P, Humblet V, Pannier N, Maison W, Frangioni JV. Production of multimeric prostate-specific membrane antigen small-molecule radiotracers using a solid-phase 99mTc preloading strategy. J Nucl Med. 2007 Aug;48(8):1379–1389. doi: 10.2967/jnumed.107.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humblet V, Misra P, Frangioni JV. An HPLC/mass spectrometry platform for the development of multimodality contrast agents and targeted therapeutics: prostate-specific membrane antigen small molecule derivatives. Contrast Media Mol Imaging. 2006 Sep;1(5):196–211. doi: 10.1002/cmmi.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Monte F, Lebeche D, Guerrero JL, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003 Jun 1;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 28.Bourcier T, Berbar T, Paquet S, et al. Characterization and functionality of CXCR4 chemokine receptor and SDF-1 in human corneal fibroblasts. Mol Vis. 2003 Apr 2;9:96–102. [PubMed] [Google Scholar]
- 29.Rusckowski M, Qu T, Gupta S, Ley A, Hnatowich DJ. A comparison in monkeys of (99m)Tc labeled to a peptide by 4 methods. J Nucl Med. 2001 Dec;42(12):1870–1877. [PubMed] [Google Scholar]
- 30.Czarnowska E, Gajerska-Dzieciatkowska M, Kusmierski K, et al. Expression of SDF-1 -CXCR4 axis and an anti-remodelling effectiveness of foetal-liver stem cell transplantation in the infarcted rat heart. J Physiol Pharmacol. 2007 Dec;58(4):729–744. [PubMed] [Google Scholar]
- 31.Viola A, Luster AD. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Annu Rev Pharmacol Toxicol. 2007 Sep 17; doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 32.Kailasnath P, Sinusas AJ. Comparison of T1-201 with Tc-99m-labeled myocardial perfusion agents: technical, physiologic, and clinical issues. J Nucl Cardiol. 2001 Jul–Aug;8(4):482–498. doi: 10.1067/mnc.2001.115078. [DOI] [PubMed] [Google Scholar]
- 33.Wackers FJ, Berman DS, Maddahi J, et al. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med. 1989 Mar;30(3):301–311. [PubMed] [Google Scholar]
- 34.Okada RD, Glover D, Gaffney T, Williams S. Myocardial kinetics of technetium-99m-hexakis-2-methoxy-2-methylpropyl-isonitrile. Circulation. 1988 Feb;77(2):491–498. doi: 10.1161/01.cir.77.2.491. [DOI] [PubMed] [Google Scholar]