Abstract
Handwriting impairments in Parkinson’s disease (PD) have been associated with micrographia, i.e. diminished letter size. However, dyscoordination of the wrist and fingers may also contribute to handwriting deterioration in PD. To investigate this hypothesis, right-handed PD patients and controls were tested in performance of three types of cyclic wrist and finger movements: drawing of two lines and a circle. The line drawing was performed with either simultaneous flexion and extension of the wrist and fingers (equivalent pattern resulting in a right-tilted line) or with wrist flexion/extension accompanied with finger extension/flexion (nonequivalent pattern resulting in a left-tilted line). Circle drawing required a specific phase difference between wrist and finger motions. Movements were performed with an inkless pen on a digitizer-tablet at two frequency levels. Consistent deformations of the circle into right-tilted ovals and lower variability in equivalent compared with nonequivalent lines revealed preference to produce right-tilted shapes. This preference became more apparent with increased movement speed and it was amplified in PD patients. Analysis revealed that the circle deformation emerged mainly due to reduction in relative phase, while wrist and finger amplitudes remained unchanged. The results suggest that PD causes deficit characterized by strong tendency to produce certain coordination patterns between wrist and finger motions. This deficit may significantly contribute to handwriting impairments in PD by reducing the dexterity in the production of the variety of shapes of the cursive letters. Furthermore, the deficiency revealed in wrist and finger coordination may represent a more general deficit affecting control of various multi-joint movements in PD.
Introduction
Parkinson’s disease (PD) is characterized by specific features of limb movements such as shortened movement amplitude, delays in movement initiation, slowness during motion, high variability, and tremor (Brown & Marsden, 1991; Flash, Inzelberg, Schechtman, & Korczyn, 1992; Sheridan, Flowers, & Hurrell, 1987). While these and other features of Parkinsonian movements have been recognized and extensively studied, deficits in movement control that result in these features remain largely unknown. In this study, the effect of PD on movement control is examined in relation to wrist and finger movements like those involved in handwriting.
Handwriting deterioration is typical of PD (Jankovic, 2008) and often is the first manifestation of this disease (Mclennan, Tyler, Schwab, & Nakano, 1972). The debilitating effect of PD on handwriting is usually described as micrographia, i.e. diminished letter size (Lewitt, 1983). Micrographia has become widely recognized as a dysfunction caused by PD, probably, because it is easily observable and is consistent with hypometria, a well-known tendency in PD to shorten movement amplitude (Contreraz-Vidal, Teulings, & Stelmach, 1995; Desmurget, Grafton, Vindras, Grea, & Turner, 2003; Flash et al., 1992; Margolin & Wing, 1983; Van Gemmert, Teulings, Contreras-Vidal, & Stelmach, 1999). Nevertheless, another deficit, namely, dyscoordination of wrist and finger motions, may also contribute to impaired handwriting in PD (Teulings, ContrerasVidal, Stelmach, & Adler, 1997; Van Gemmert, Adler, & Stelmach, 2003). Although this deficit has obtained less attention than micrographia, it may be as important. The contribution of this factor to impaired production of graphical output in PD is the focus of the present study.
Without considering proximal arm joints, the hand-finger biomechanical system has at least ten degrees of freedom. Nevertheless, limitations imposed by the need for the fingers to hold the pen and for the entire hand to move on the horizontal surface reduce the actual number of degrees of freedom during handwriting to two (Teulings, 1996). One degree of freedom arises from simultaneous flexion/extension of all finger joints and results in the movement of the pen tip to and from the hand palm. The other degree of freedom is rotation of the entire hand about the wrist as a combination of palmar flexion/extension and radial abduction/dorsal flexion and ulnar abduction, depending on the level of supination/pronation of the forearm (Teulings, Thomassen, & Maarse, 1988). Thus, although multiple joints are involved in handwriting, the variety of shapes in cursive letters is produced with only two degrees of freedom associated with the wrist and finger motions. These two degrees of freedom have been named as main (principal, natural) axes (Dooijes, 1983; Maarse, Schmaker, & Thomassen, 1986; Meulenbroek, Thomassen, van Lieshout, & Swinnen, 1998; Plamondon & Lamarche, 1986; Schomaker & Plamondon, 1990; Teulings et al., 1988). The present study examines the influence of PD on coordination between these two degrees of freedom. Revealing difficulties that PD patients have in coordination of the wrist and finger motions during production of graphical output may shed light on basic dysfunctions associated with movement control in PD.
Evidence that the coordination of wrist and finger motions is impaired in PD has been provided by (Teulings et al., 1997) who found that strokes produced with motion of both wrist and fingers are more variable as compared with strokes produced predominantly by motion of a single degree of freedom, that is, either the fingers holding the pen or the wrist. It was hypothesized in that study that PD patients have difficulty in simultaneous control of more than one degree of freedom. According to this hypothesis, the number of degrees of freedom involved in motion is the factor that determines the level of difficulty associated with movement performance (Seidler, Alberts, & Stelmach, 2001). This hypothesis predicts that all types of handwriting movements produced with simultaneous motion of the wrist and fingers would cause similar difficulties in PD patients.
Here we promote an alternative hypothesis that PD differentially affects handwriting movements, depending on the coordination pattern (i.e., relative phase) of wrist and finger motions. This hypothesis follows from our previous finding that quality of performance of graphical movements in young adults depends on coordination pattern of wrist and finger motions (Dounskaia, Van Gemmert, & Stelmach, 2000). Several combinations of wrist and finger motions resulting in cyclic drawing of a circle and lines of different orientations were examined in that study. Increases in cycling frequency caused consistent deformation of the circle into a right-tilted oval in right-handed subjects. Analysis of wrist and finger motions revealed that circle drawing required a specific value of relative phase. The circle deformation observed with the cycling frequency increases was accompanied with decreases in relative phase, while wrist and finger amplitudes remained constant. This result pointed to a bias towards coordination of wrist and finger motions with low relative phase as a reason for the circle deformation at high cycling frequencies.
Results of line drawing supported this conclusion. Variability of line drawing was lower when the wrist and fingers simultaneously flexed and extended (the “equivalent” pattern performed with 0° relative phase) compared with a movement during which wrist flexion was accompanied by finger extension and wrist extension by finger flexion (the “nonequivalent” pattern performed with 180° relative phase). Since the equivalent pattern was performed with 0° relative phase, and its graphical outcome was a right-tilted line, the results for line and circle drawing together pointed to a preference in young adults to draw right-tilted shapes that required low relative phase between the wrist and finger motions. The revealed biases demonstrated that complexity of performance varies across wrist and finger coordination patterns. Biomechanical properties of the hand holding a pen that facilitate production of the right-tilted graphical shapes and cause difficulties during production of other shapes were discussed as a possible reason for the obtained results. This hypothesis was supported by mirror-symmetric biases (for the left tilt) found in left-hand subjects.
Taking into account deficient wrist and finger movements in PD ((H. L. Teulings et al., 1997)), it can be predicted that complexity associated with performance of certain wrist and finger coordination patterns and corresponding coordination biases would be amplified in movements of PD patients, which may significantly contribute to the impairments observed in patient’s handwriting. This hypothesis was tested in the present study by examining performance of the three coordination patterns of wrist and finger motions, equivalent, nonequivalent, and resulting in circle drawing, by PD patients and age-matched controls. Based on the results for young adults (Dounskaia et al., 2000), it was hypothesized in the present study that the bias to deform the circle into the tilted oval would be amplified by PD. For the line drawing, it was hypothesized that increases in movement variability characteristic of PD would be more pronounced during the nonequivalent than equivalent pattern. Movements were tested at two cyclic frequency levels, 1 and 3 Hz, representing slow and fast movement speeds. It was expected that since increases in movement speed exacerbate the coordination biases (Dounskaia et al., 2000), they will emphasize the differential influence of PD on the performance of the three coordination patterns.
Methods
Participants
Nine healthy control subjects (5 males, 4 females, mean age 71.3 years, SD = 5.8 years) and nine subjects with idiopathic Parkinson’s disease (6 males, 3 females, mean age 69.6 years, SD = 4.9 years) participated in the experiment. All subjects were right-handed. After an explanation of the experiment, subjects signed informed consent approved by the Human Subjects Institutional Review Board of Arizona State University. Patients and controls met study criteria as follows: normal or corrected vision, and the presence of full range of motion in the finger and wrist joints. In addition, patients and controls met a cutoff score of 25 on the Mini-Mental State Exam (Folstein, Folstein, & Mchugh, 1975). Elderly controls did not have a history of any central nervous system (CNS) disease.
Patients diagnosed with idiopathic PD as designated by a history of levodopa responsiveness and the presence of two of three cardinal symptoms of PD (tremor, bradykinesia, rigidity) were eligible to participate (Calne, Snow, & Lee, 1992). In addition, eligible patients could not have a history of any other CNS disorder. All patients were tested in the OFF condition, i.e. at least 12 hours following their last intake of PD medication. Patients were Hoehn and Yahr stages 2 or 3 (Hoehn & Yahr, 1967) and averaged 27.7 (SD=6.6) points on Subscale III (motor exam) of the Unified Parkinson’s Disease Rating Scale (UPDRS, (Fahn & Elton, 1987). These tests were performed prior to the initiation of the experiment. Patients who had any noticeable action tremor and/or severe resting tremor (quantified by a score of 3 or greater on the UPDRS-III) were excluded from the analysis. A summary of patient characteristics is presented in Table 1.
Table 1.
PD patient characteristics.
| Patient No. | Age (yrs) | Gender | Disease Duration (yrs) | Hoehn&Yahr Stage | UPDRS Motor Exam |
|---|---|---|---|---|---|
| 1 | 74 | F | 7 | 3 | 31.5 |
| 2 | 77 | M | 6 | 2.5 | 21.0 |
| 3 | 67 | M | 10 | 2 | 21.5 |
| 4 | 65 | M | 11 | 2.5 | 28.5 |
| 5 | 66 | M | 12 | 2 | 18.0 |
| 6 | 69 | M | 3 | 2.5 | 37.5 |
| 7 | 70 | M | 3 | 2.5 | 27.5 |
| 8 | 75 | F | 14 | 3 | 35.5 |
| 9 | 63 | F | 11 | 2.5 | 28.0 |
M - Male, F - Female
Procedure
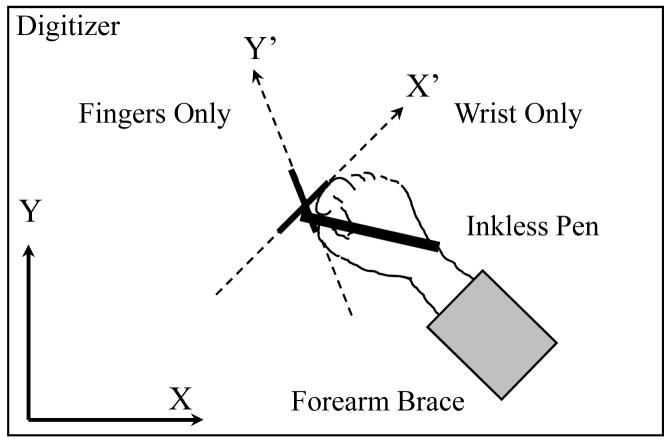
A computer controlled digitizer-tablet (WACOM Intuos 12×18) and an inkless pen (WACOM Intuos GP-110) were used to record hand movements. The digitizer was positioned on a table in front of the subject. Pen displacements along the X- and Y-axis were acquired at a sampling frequency of 200 Hz. The X-axis was oriented in the medio-lateral direction. The Y-axis was oriented in the anterio-posterior direction, perpendicular to the X-axis. The spatial accuracy of registration was 0.02 mm. Subjects were comfortably seated with both forearms positioned on the table. They were asked to adopt the most comfortable writing posture. The right forearm was immobilized at the angle of 130° relative to the X-axis by an arm rest attached to the table. Thus, only the two degrees of freedom of the hand along the main axes (Teulings, 1996) remained available for motion, flexion/extension at the wrist and flexion/extension of the fingers holding the pen (Fig. 1). Subjects performed repetitive drawing of three shapes, a circle and two lines, with no visible trace left by the inkless pen. Initially, the circle drawing was performed. Subjects were presented a sample circle of 2.5 cm diameter out of the field of drawing. They were instructed to repetitively draw circles of approximately the same size in the counterclockwise direction one on top of each other. After this task was completed, the sample circle was located at the place on the digitizer where the subject previously drew the circle. Subjects were instructed to draw the lines back and forth within the sample circle. Although the size of the drawn lines and the circle was defined by the diameter of the sample circle, the accuracy of size production was deemphasized, and the subject’s attention was focused on joint coordination and temporal characteristics of movements, as explained next. The trial duration was 15 sec.
Figure 1.
Schematic presentation of the experimental set-up and the coordinate systems used for movement analysis. Subjects were holding a pen, moving it on a digitizer. The forearm was immobilized with a brace. Pen motion was recorded in the orthogonal coordinate system with the X-axis oriented in the medio-lateral direction. The nonorthogonal X′Y′ system represented the “main” axes, i.e. the directions in which motion was produced during finger-only and wrist-only movements.
The line drawing tasks were formulated in terms of coordination of the wrist and finger movements. One pattern consisted of simultaneous flexion and simultaneous extension of the wrist and fingers (the equivalent coordination pattern). The other pattern included the combination of wrist flexion with finger extension and wrist extension with finger flexion (the nonequivalent coordination pattern). Thus, subjects focused on the production of the required combinations of wrist and finger movements, and not on spatial orientation of the emergent lines. All types of movements were performed continuously in a cyclic manner. Each cycle included drawing a circle or a line back and forth per auditory signal.
In addition, drawing of trajectories emerging from cyclical movements of the wrist only and fingers only were recorded. The fingers-only movements produced straight-line trajectories. The wrist-only movements were rotational, and the emergent pen trajectories had an arc-shape. However, the arcs had low curvature and were close to straight lines. For this reason, the trajectories of wrist-only movements were approximated with straight lines connecting the extreme points of the arcs. The lines emerging from the wrist-only and fingers-only movements were used to establish the “main” axes and to determine wrist and finger amplitude and relative phase during circle drawing, as described in the Data Analysis section. To help subjects to perform the movement of the wrist only, a light thin bar was attached along the thumb. During the movement of the fingers only, the light thin bar was attached to the wrist along the forearm axis. This bar did not physically restrain movement of the wrist or fingers but it moved when there was motion in the joints that were required to be stationary. This visual cue helped subjects to accurately perform the wrist-only and finger-only movements. Prior to recording movements, practice trials were performed until subjects could easily produce the required wrist and finger movement patterns at the comfortable pace. Usually, one or two practice trials were sufficient for a subject to acquire each of the required coordination patterns. The method of defining movement directions through a coordination pattern of joint motions used here for line drawing has been extensively employed in studies of multi-joint arm movements (Dounskaia, Wisleder, & Johnson, 2005; Gribble & Ostry, 1999; Sainburg, Ghilardi, Poizner, & Ghez, 1995; Seidler et al., 2001).
Movements were performed at two levels of cycling frequency, 1 Hz (slow speed) and 3 Hz (fast speed), paced with computer-generated auditory beats. The frequency manipulation was included in the experiment because Dounskaia et al. (2000) demonstrated that increases in cyclic frequency make preferences for specific wrist and finger coordination patterns (those involved in drawing of the equivalent line and right-tilted oval) more pronounced. Thus, it was expected that higher difficulties experienced by PD patients in production of the nonequivalent lines compared with the equivalent lines would be more explicit during fast than slow movements. Results presented further demonstrate that PD patients were capable to produce the required movements at the 3 Hz frequency level. Two trials were performed in each condition, i.e. for each combination of movement type and speed level. Each characteristic computed for data analysis was averaged between the two trials to result in a single value for each subject and each condition.
Data Analysis
The X- and Y-digitizer data were smoothed with a Butterworth low-pass filter with a 15 Hz frequency cut-off. Pen displacement was divided into separate lines within each line-drawing trial and into circles within each circle-drawing trial. The lines were distinguished by determining the coordinates of the extreme (reversal) points. Local maxima and minima of the Y-coordinate were used to detect the extreme points during the equivalent pattern because the orientation of the equivalent lines was close to the Y-axis. In contrast, the orientation of the nonequivalent lines was close to the X-axis. Local extrema of the X-coordinate were therefore used to distinguish these lines. Maximal values of the Y-coordinate were used to divide circle-drawing trials into separate circles.
The data obtained for the four lines were used to calculate cycle duration, line length, and line orientation with respect to the X-axis. Additionally, stability of wrist and finger coordination was compared between the equivalent and nonequivalent lines by calculating two characteristics, line variability and standard deviation (SD) of line orientation. The line variability was computed within each trial as variability of each line end averaged between the two ends. The variability of each line end was computed as mean distance of the extreme points of the lines from the average extreme point at this end, i.e. as (1/n)Σ√[(xi−xm)2+(yi−ym)2], where i is the number of the line within the trial, xi, yi are coordinates of the line end, xm is mean of xi, ym is mean of yi, n is the total number of lines within the trial, and Σ signifies summation across the line number i=1, …, n.
To evaluate the quality of circle drawing, the maximal diameter of the path contour within each cycle was determined by finding within each cycle the two points at maximal distance from each other. A tendency to deform the circular shape into an oval was analyzed with the use of the ratio between the maximal diameter and the perpendicular diameter. This ratio is equal to 1.0 for a perfect circle and it is higher than 1.0 for oval shapes. The orientation of the maximal diameter was also computed to examine whether the emergent oval shapes had consistent orientation.
Deformations of the circle into ovals could occur due to changes either in wrist and finger amplitudes, or in relative phase between these motions, or both. To investigate these changes, a nonorthogonal coordinate system X′Y′ of the main axes (Teulings et al., 1988) associated with the wrist and finger movements was determined for each subject (Fig. 1). The X′, Y′ axes were determined as average orientations of the lines produced at the slow speed (the easy speed condition) with wrist-only and fingers-only movements, respectively. Since X′, Y′ axes represented the two degrees of freedom of hand motion, each graphical shape was represented by amplitudes of the displacements along X′ and Y′ and relative phase between these displacements. To determine the displacements along X′ and Y′, angles θw, θf were calculated, where θw was the angle between the X-axis and the average line produced during the slow movements of the wrist only (representing the X′-axis), and θf was the angle between the Y-axis and the average line produced by each subject during the slow movements of the fingers only (representing the Y′-axis). With the use of these angles, pen motion was represented in the main, nonorthogonal coordinates x′ and y′ calculated as x′= xCosθw + ySinθf, y′= xCosθf + ySinθw, where x, y are coordinates of the orthogonal XY coordination system. The x′ and y′ coordinates were used to calculate amplitude of wrist and finger motions and relative phase between them during the circle-drawing task.
Relative phase between wrist and finger movements was computed by subtracting the phase of the time series of y′ (finger) displacements from the phases of the time series of the x′ (wrist) displacements. Each time series of displacement was a periodic, sinusoidal-like signal. The phase of each signal was computed using the formula φi = arctan(vi/Δi) proposed by Schmidt, Treffner, Shaw, & Turvey (1992). Here vi is the derivative of the signal at sample i divided by the cyclic frequency during the trial, and Δi is the value of the signal at sample i minus the average value of the signal within the trial. Relative phase was normalized for each subject relative to the angle between the X′ and Y′ axes (the wrist-fingers angle). The following considerations demonstrate the necessity for this normalization. Ideal circle drawing represented in the orthogonal XY coordinates requires 90° relative phase between the X- and Y-displacements. However, when nonorthogonal coordinates X′, Y′ are used, circle drawing requires relative phase equal to the angle between the nonorthogonal axes. In the case of drawing an ideal circle with the wrist and fingers, the required relative phase is equal to the angle between X′ and Y′, i.e. to the wrist-fingers angle. However, this angle varies among individuals. The normalization of relative phase was therefore performed for each subject to take into account individual differences in the anatomical structure of the hand and to allow averaging of relative phase across subjects in each group. The normalized relative phase was calculated by dividing relative phase by the wrist-fingers angle. In this normalization, relative phase equal to 1.0 corresponds to drawing a perfect circle. Deviations of the circular shape may emerge either because of deviations of the normalized relative phase from 1.0, or because of changes in wrist and finger amplitudes, or both. Thus, the computation of the wrist and finger amplitudes and normalized relative phase allowed us to examine changes in wrist and finger control underlying circle deformations in each group.
Statistical analysis addressed the effect of Parkinson’s disease and movement speed on the drawing movements. It included a 2 × 2 (group × speed) ANOVA with repeated measures on the second variable that was applied to cycle duration, the diameter ratio, wrist and finger amplitude, and relative phase obtained from circle drawing. The same type of analysis was applied to the line orientation data separately for each line due to the distinct orientations of the four lines. Line length and cycle duration during line drawing were analyzed with a 2 × 2 × 4 (group × speed × line) ANOVA with repeated measures on the second and third variable. In these analyses, group was related to PD patients and controls, speed was related to the two levels of cyclic frequency, and line was related to lines produced with the equivalent, nonequivalent, wrist-only, and fingers-only coordination patterns. The analyses of line variability and line orientation SD were applied only to the lines produced with the two-degree-of-freedom (equivalent and non-equivalent) patterns to establish whether variability differed between the movement patterns including the same number of degrees of freedom. These analyses were performed with a 2 × 2 × 2 (group × speed × line) ANOVA with repeated measures on the second and third variable.
Results
Table 2 presents mean and SD of cycle duration at each speed level and in each group during line and circle drawing. Cycle duration was close to that determined by the metronome (333 and 1000 ms for the fast and slow movements, respectively). ANOVA applied to the cycle duration separately during drawing the circle and the four lines at each frequency level revealed that speed was the only factor in both analyses that caused significant effects [F(1,16) = 452.3, p<0.001 and F(1,16) = 663.7, p<0.001 for circle and line drawing, respectively]. Other main effects and interactions were not significant (p>0.1). Thus, PD patients were capable to produce both required levels of movement speed. The further analysis focused on quality of drawing of the required shapes in the two groups.
Table 2.
Mean (and SD) of cycle duration (ms) during circle and line drawing
| Controls | Patients | |||
|---|---|---|---|---|
| Line | Circle | Line | Circle | |
| Slow | 869 (78) | 915 (60) | 886 (128) | 918 (133) |
| Fast | 324 (19) | 321 (20) | 335 (68) | 345 (49) |
Circle Drawing
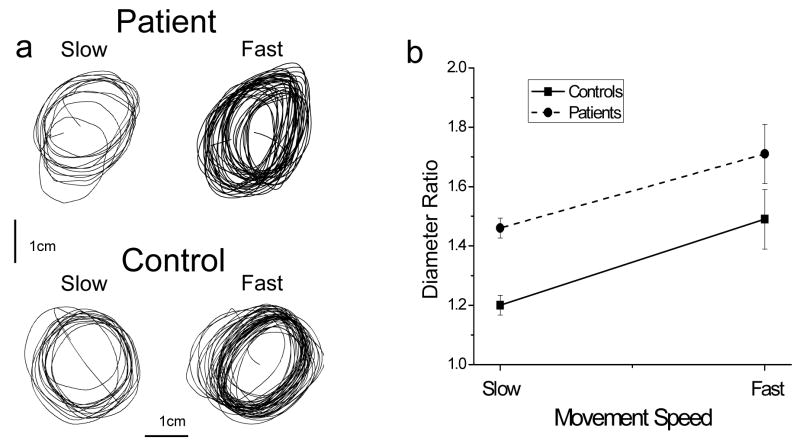
A representative example of individual circle drawing is shown in Fig. 2a. As demonstrated in this example and confirmed by visual analysis of other circle-drawing trajectories, cycle-to-cycle variability of circle drawing was higher in PD patients than in controls, which is consistent with well-recognized variability of Parkinsonian movements. Further, this variability was predominantly represented by drift of the produced contours in the plane of drawing, whereas the shape of the contours was relatively stable across the cycles. An observation that is most important for the goals of the present study is that there was a tendency to transform the circle into a right-tilted oval. Furthermore, this tendency became more pronounced with increases in movement speed and it was amplified by PD. Indeed, during the low movement speed, the circle deformation is apparent in Fig. 2a in the movement of PD patient but not of the control. Although both subjects deformed the circle at the high speed level, these changes were stronger in the patient than in the control. These observations were quantified by computing the ratio of the maximal and perpendicular diameter calculated for each cycle of circle drawing and averaged across cycles of each trial. This ratio is equal to 1.0 for a perfect circle and its values are higher for ovals. Fig. 2b shows that this characteristic increased with increases in movement speed in both groups [F(1,16) = 19.4, p<0.001]. However, the circle deformation was consistently higher in PD patients than in controls [F(1,16) = 7.3, p<0.05].
Figure 2.
Circle drawing. (a) Representative examples of circle drawing in a patient and control during two speed levels. The thin bars indicate the scale of actual drawings. The control subject was able to draw the circle accurately at the lower speed level and demonstrated a tendency to deform the circle into a right-tilted oval at the higher speed level. This tendency was observed in the patient already at the lower speed level and it became stronger with the increase in movement speed. (b) Diameter ratio obtained during circle drawing. The ratio is equal to 1.0 during drawing a perfect circle. The increases in the ratio demonstrate that both subject groups deformed the circle into an oval shape, and this tendency was more apparent with increases in movement speed. The circle deformation was significantly greater in PD patients than in controls.
It is also observed in Fig. 2a that the produced oval was tilted to the right. This tilt was apparent in both groups, specifically at the higher movement speed. Indeed, mean and SD of the orientation of the maximal diameter was 65.0° (36.5°) and 74.0° (48.89°) at the lower speed and 63.9° (13.8°) and 76.4° (14.8°) at the higher speed in patients and controls, respectively. To investigate the changes in the wrist and finger coordination pattern accompanying the deformation of the circle into the right-tilted oval, the wrist and finger amplitude and relative phase were examined in the two groups at the two speed levels.
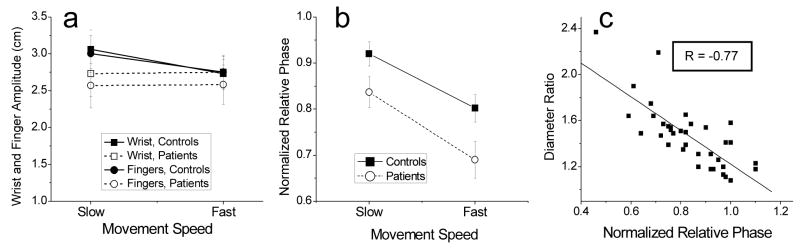
To analyze the characteristics of wrist and finger motions, the pen-tip path represented by the orthogonal X- and Y- components was decomposed into wrist and finger displacements, as described in the Data Analysis section. The time series of the wrist and finger displacements were then used to compute wrist and finger amplitude and normalized relative phase mean values of which are shown in Fig. 3a, b. Both the wrist and finger amplitude slightly decreased with decreases in movement speed in control subjects (Fig. 3a). It is unlikely that these decreases accounted for the bias towards the right-tilted oval because they were small and because they were equal in both degrees of freedom. Furthermore, no decreases in amplitude were observed in PD patients, who had a stronger bias towards the tilted oval. The results of the statistical analyses were consistent with these observations. No significant differences between the two groups were found in the amplitude data. The main effect of speed was significant only for the finger amplitude [F(1,16) = 5.0, p<0.05] and not for the wrist amplitude (p>0.1). The group by speed interaction was also significant for the finger amplitude because it decreased with increases in speed only in controls and not in patients. The interaction was not significant for the wrist amplitude (p>0.05). Thus, no decreases in either the wrist or finger amplitude were found that could account for the transformation of the circular shape into the tilted oval, specifically in movements of PD patients.
Figure 3.
Movement characteristics during circle drawing. (a) Wrist and finger movement amplitude. Amplitude at both joints remained largely the same in the two groups during the two speed conditions. Although the decreases in finger amplitude observed in movements of controls with increases in speed were found significant, the absolute changes were small. (b) Normalized relative phase between wrist and finger motions. Relative phase decreased with increases in movement speed and it was consistently lower in patients than in controls. The changes in relative phase match the changes in the circle diameter ratio shown in Fig. 2b. This is confirmed in panel (c) that shows results of correlation analysis between relative phase and the diameter ratio.
Contrary to the wrist and finger amplitude, relative phase (Fig. 3b) markedly decreased in both groups with increases in movement speed [F(1,16) = 49.5, p<0.001]. This result shows that both groups had a tendency to decrease the phase lag of finger movements with respect to shoulder movements required for circle drawing. In addition, group effect was significant [F(1,16) = 4.5, p<0.05]. Relative phase was consistently lower in PD patients than in controls. The interaction was not significant (p>0.1). The dependence of relative phase on movement speed and group match the changes found in the diameter ratio, suggesting that the deformation of the circular shape emerged due to a bias towards the coordination of wrist and finger motions with a certain value of relative phase that was lower than that required for circle drawing. This conclusion was supported by significant correlation (R = −0.77, p<0.01) between relative phase and the diameter ratio (Fig. 3c).
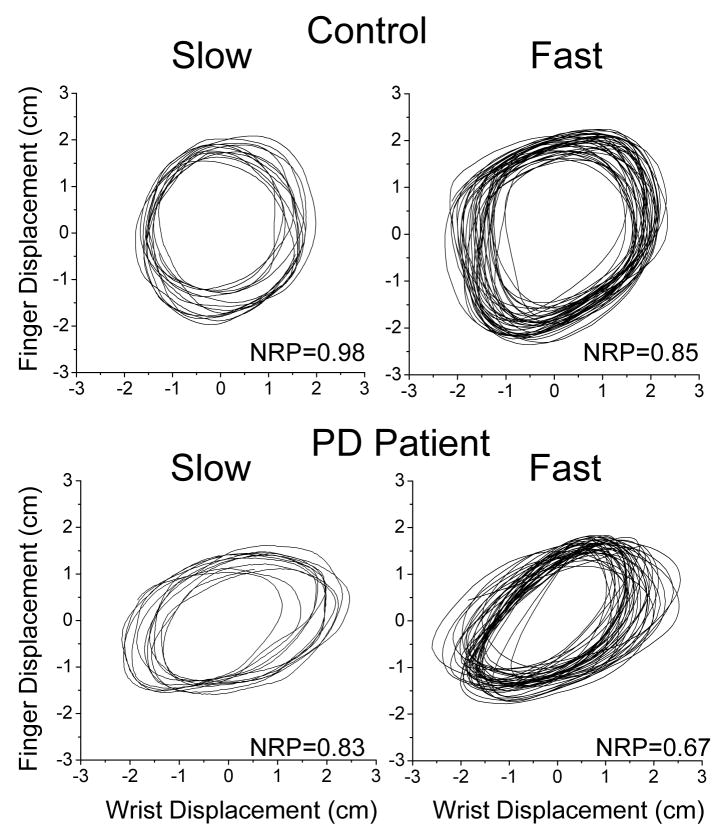
Biased coordination of wrist and finger motions caused by the increases in movement speed and by PD was further investigated by examining plots of finger displacements against wrist displacements for each subject in each condition. Since wrist and finger amplitudes were similar, graphs in these plots showed a circular shape when normalized relative phase was close to 1.0 (as required for circle drawing). Changes in coordination represented by decreases in relative phase resulted in ovals approaching the right-diagonal line. These coordination changes were observed with increases in movement speed and to a larger degree in PD patients than in controls. Fig. 4 shows examples of such plots for a representative control and a PD patient. In both subjects, increases in cycling frequency were accompanied with changes in the coordination of wrist and finger corresponding to the decreases in relative phase. However, the deviation of wrist and finger coordination from the required circular pattern was more pronounced in the PD patient.
Figure 4.
Individual examples of plots of finger displacements versus wrist displacements that demonstrate biases in wrist and finger coordination during circle drawing. The presented data were obtained from a representative control and PD patient in the slow and fast condition. The transformation of the circular shape of the graph into right-diagonal ovals demonstrates changes in coordination between wrist and finger motions driven by the increases in movement speed and by PD.
Line Drawing
Mean and SD of the orientation and length of the lines produced with the four coordination patterns of wrist and finger movements in each group and averaged across the two speed levels are provided in Table 3. Orientation and length of the four lines were similar to those reported by Dounskaia et al. (2000) for young adults. Each of the four coordination patterns resulted in a distinct line orientation, demonstrating that the equivalent and nonequivalent lines were distinct from the lines produced with a single degree of freedom (the wrist of fingers only), and thus involved coordinated motion of the wrist and fingers. Neither group nor movement speed significantly affected orientation of each line and line length. Line length was also statistically non-distinguishable across the four lines.
Table 3.
Mean (and SD) of line orientation and length
| Controls | Patients | |||
|---|---|---|---|---|
| Orientation (deg) | Length (cm) | Orientation (deg) | Length (cm) | |
| Equivalent | 62 (8) | 2.6 (0.2) | 59 (9) | 2.7 (1.2) |
| Nonequivalent | 158 (12) | 2.6 (0.4) | 131 (55) | 2.4 (1.2) |
| Wrist Only | 32 (8) | 2.5 (0.2) | 29 (10) | 2.7 (1.3) |
| Fingers Only | 109 (11) | 2.6 (0.3) | 107 (11) | 2.3 (0.5) |
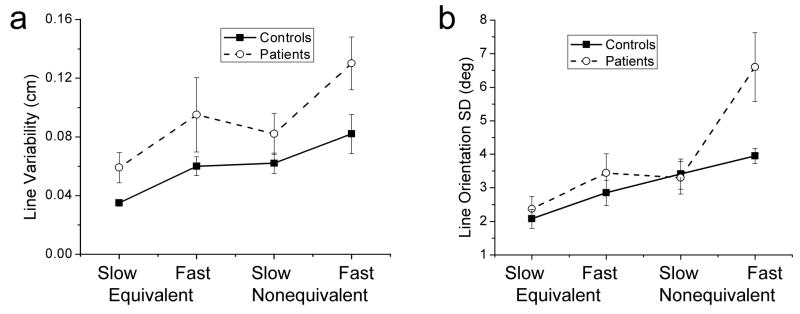
To examine whether the quality of wrist and finger coordination depends on coordination pattern, variability of line drawing was compared between the equivalent and nonequivalent lines (i.e. the lines involving motion of both the wrist and fingers). An example of drawing of these two lines at the fast speed by representative subjects is shown in Fig. 5. The quality of line drawing was different for different groups and conditions. It was assessed with the use of two characteristics, general line variability represented by “cloudiness” of the extreme points and variability of the line slope represented by line slope SD. The dependence of each factor on group, line, and movement speed was analyzed. The results obtained for line variability are shown in Fig. 6a. All three main effects were significant. Variability was higher in PD patients than in controls [F(1,16) = 6.3, p<0.05], for the nonequivalent compared to equivalent lines [F(1,16) = 9.0, p<0.01], and during the fast speed in contrast to the slow speed [F(1,16) = 11.4, p<0.005]. The interactions were not significant (p>0.1).
Figure 5.
Examples of drawings of the equivalent and nonequivalent lines in patients and controls during fast movements. The thin bars indicate the scale of actual drawings. Note the significant deterioration of the nonequivalent line drawing in the PD patient.
Figure 6.
Characteristics of line drawing variability. (a) Line variability represented by the variability of the extreme points of the lines. Line variability was significantly higher in PD patients than in controls, for the nonequivalent than equivalent line, and during fast than slow speed. (b) Line orientation SD that characterizes the ability of subjects to maintain steady orientation of the lines. This characteristic revealed a marked deterioration of performance in PD patients during fast drawing of the nonequivalent line but not the equivalent line. The vertical bars in this and the following figures represent standard error (SE).
In addition to the consistently higher performance variability in patients compared with controls, the line orientation SD demonstrated that the debilitating effect of PD on line drawing was not the same across conditions, being more pronounced during production of the nonequivalent line at the fast speed than during the other conditions. This result is apparent from Fig. 6b. Accordingly, statistical analysis did not show a significant effect of group (p>0.1). However, the speed by group and the three factor interactions were significant [F(1,16) = 6.6, p<0.05 and F(1,16) = 4.7, p<0.05, respectively]. Also, the main effects of line and speed were significant. Line orientation SD was higher for the nonequivalent than equivalent line [F(1,16) = 18.2, p<0.001] and during fast compared with slow speed [F(1,16) = 23.0, p<0.001]. To summarize, the results for line drawing suggest that performance variability was generally higher in patients than in controls. In addition, difficulties experienced by patients were not equal across conditions, being specifically challenging during drawing of the nonequivalent line, as revealed by the breakdown in the variability of the slope of this line at the high frequency level.
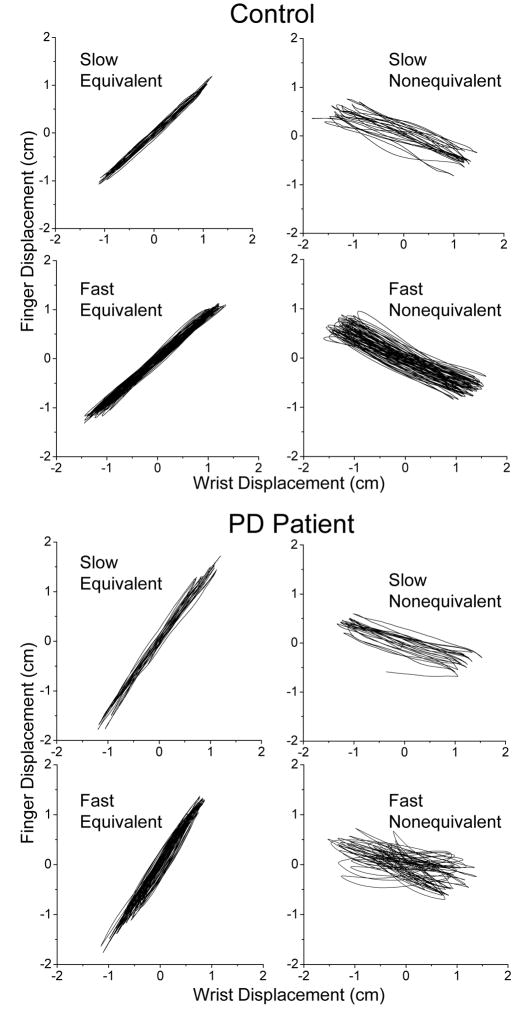
The fact that the difficulties in drawing the nonequivalent line amplified by movement speed and by PD were associated with the required pattern of wrist and finger coordination was verified by examining plots of finger displacements against wrist displacements. Fig. 7 shows examples of these plots obtained for a representative control subject and a PD patient. These examples demonstrate consistently lower quality of wrist and finger coordination for the nonequivalent than equivalent pattern and during fast than slow movements. The debilitating influence of the nonequivalent pattern and fast movement speed is specifically apparent in the PD patient. In this case, dyscoordination was represented by marked irregularity of the slope of the graph lines. Also, the patient had difficulty to synchronize reversals at the wrist and fingers, as revealed by slightly rounded graph lines at the extreme points.
Figure 7.
Individual examples of plots of finger displacements versus wrist displacements that demonstrate wrist and finger dyscoordination during line drawing. The shown data were obtained from a representative control and PD patient during slow and fast drawing of the equivalent and nonequivalent line. Wrist and finger dyscoordination was especially apparent in the PD patient during fast drawing of the nonequivalent line, as suggested by high variability of the graph lines and of the slope of these lines, as well as by the lack of coordinated reversals of the wrist and finger motions.
The dyscoordination of movements of PD patients during the fast speed was quantitatively assessed by computing the slope SD of the lines representing wrist and finger coordination. This characteristic was computed in the same way as line orientation SD. Results of statistical analysis obtained for the slope SD were similar to those reported above for line orientation SD. Namely, group effect was not significant (p>0.1) but the speed by group and the three factor interactions were significant [F(1,16) = 5.7, p<0.05 and F(1,16) = 4.6, p<0.05, respectively]. The line and speed main effects were significant. The slope SD was higher during production of the nonequivalent compared with equivalent line [F(1,16) = 20.1, p<0.001] and during fast compared with slow speed [F(1,16) = 25.1, p<0.001].
The increased difficulties associated with drawing of the nonequivalent as compared with equivalent line observed in both groups and specifically in PD patients was consistent with the results obtained for circle drawing. The tendency during circle drawing was to deform the circular shape into the right-tilted oval, i.e. the oval orientation of which was close to the orientation of the equivalent line (which was about 60°, see Table 3). The similarity in the orientation between the emergent oval and the line of low variability suggests that there was a common bias towards a specific combination of wrist and finger movements that resulted in the right-tilted traces of the pen, and PD amplified this bias.
Discussion
We examined the influence of PD on performance of cyclic handwriting-like movements involving three types of coordination of wrist and finger motions, a pattern with relative phase corresponding to circle drawing and the equivalent and nonequivalent patterns resulting in drawing of lines of distinct orientations. The circle drawing task revealed a tendency in both groups to draw a right-tilted oval instead of the circle. However, this tendency was magnified in PD patients (Fig. 2). Indeed, the circle deformation was apparent in patients but not in controls during slow movements. Although both groups deformed the circle during fast movements, the deformation was more pronounced in patients than in controls.
To establish changes in control underlying the observed circle deformation, motions at the wrist and fingers, the two degrees of freedom involved in production of handwriting-like movements, were examined. The oval shape could emerge due to significant decreases in amplitude of one degree of freedom relative to the other or due to changes in relative phase. The analysis demonstrated that there were no decreases in amplitude of either the wrist or fingers that could account for the circle deformation in either of the two groups (Fig. 3a). Although there was a significant effect of speed on the finger amplitude in control subjects, the absolute changes were small. Also, no significant decreases in the wrist or finger amplitude were found in PD patients who provided substantially greater deformation of the circle than controls.
In contrast, pronounced decreases in relative phase with increases in movement speed were observed in both groups (Fig. 3b). In addition, relative phase was significantly lower in PD patients than in controls. The correspondence between the changes in relative phase and in the circle diameter ratio suggests that the circle deformation emerged due to a bias to produce a specific coordination pattern between the wrist and fingers, and that PD patients were more susceptible to this bias than controls. This conclusion was confirmed by significant correlation between relative phase and the diameter ratio (Fig. 3c). It was further supported by examining plots of finger displacement against wrist displacement obtained for each subject. The changes in wrist and finger coordination corresponding to decreases in relative phase were represented by changes in the shape of the graphs in the plots in response to the increases in movement speed and the PD effect (Fig. 4).
The PD-related deficit in wrist and finger coordination was further supported with the results for line drawing. The line variability data revealed that in both groups, the nonequivalent line was more variable than the equivalent line. In addition, line variability was higher in movements of patients than controls (Fig. 6a), which is consistent with recognized variability of movements in PD (Flowers, 1976; Sheridan et al., 1987). In addition to this well-known effect of PD, another characteristic of line drawing variability, the orientation SD, revealed that increases in movement speed escalated the deterioration of performance in PD, specifically for the nonequivalent line (Fig. 6b). The increased variability during the nonequivalent compared with equivalent coordination pattern points to the wrist and finger coordination as a factor influencing line drawing in both groups but more so in PD patients. Dyscoordination of wrist and finger motions accompanying increases in line variability was confirmed by the visual analysis of the plots of finger displacement versus wrist displacement (Fig. 7). Together, the results of line drawing suggest that although PD causes elevated difficulties in the production of both coordination patterns, these difficulties were markedly higher during the nonequivalent pattern compared with the equivalent pattern.
Similarity of the wrist and finger coordination patterns during the most preferred movement types, the equivalent line and the oval tilted right, clarify the observed coordination biases. The equivalent line was drawn with simultaneous flexion and extension of the wrist and fingers. The oval drawing differed from this pattern in that it included relatively small phase difference between the wrist and finger movements. Due to the similarity, the graphical output in both patterns resulted in a right tilt. Thus, our data show that PD causes increased biases towards the production of right-tilted cursive shapes. This finding reveals a new deficit in handwriting-like movements caused by PD. It suggests that impairments caused by PD vary across wrist and finger coordination patterns, being pronounced for some patterns and minor for other patterns.
This conclusion differs from the previously formulated hypothesis that impaired coordination of wrist and finger movements in PD is associated with the need to simultaneously control motion of more than a single degree of freedom (Teulings et al., 1997; Van Gemmert et al., 2003). The number of degrees of freedom as the major source of dyscoordination in PD has also been proposed in the relation to other multi-degree-of-freedom movements (Alberts, Tresilian, & Stelmach, 1998; Seidler et al., 2001). This hypothesis is consistent with impaired coordination in PD reported with respect to various motor actions (Gross et al., 2008; Hocherman, Alexandrovsky, Badarny, & Honigman, 1998; Muratori, McIsaac, Gordon, & Santello, 2008; Plotnik, Giladi, & Hausdorff, 2007; Poizner et al., 2000). However, studies of bimanual coordination also suggest that the debilitating effect of PD is not equal across coordination patterns but is escalated in patterns that require overcoming certain constraints, such as the natural tendency for homologous muscle activation (Byblow, Summers, Lewis, & Thomas, 2002; Johnson et al., 1998; Ponsen et al., 2006). This finding is consistent with the differential impairments of wrist and finger coordination patterns revealed here.
The deficit related to the amplified biases in the production of graphical shapes may significantly contribute to the handwriting impairments in PD. In addition to hampering the production of the variety of shapes included in cursive letters, the biases may indirectly contribute to micrographia. In this study, we did not observe any trend in PD patients to reduce the size of the drawn shapes associated with micrographia. This is not surprising, since micrographia is observed in only 10%–15% of patients (Mclennan et al., 1972), and our patient selection criteria did not include this deficit. In addition, the sample circle shown to subjects during the drawing tasks may have served as a visual cue that aided patients in maintaining the required size of the shapes (Oliveira, Gurd, Nixon, Marshall, & Passingham, 1997). Although no tendency to decrease movement amplitude was observed in this study, it can be speculated that the deficient ability to produce various wrist and finger coordination patterns may evoke micrographia as a compensatory strategy that reduces the size of “difficult” strokes. Other handwriting impairments in PD, such as delays in initiation and slowness of stroke production, may also be a consequence of the difficulties associated with drawing of specific shapes because careful planning and slow performance of joint motions may partially compensate for these difficulties.
A possible reason for the observed biases is the biomechanical properties of the hand holding a pen (Meulenbroek & Thomassen, 1991; Van Sommers, 1984). Driven by this hypothesis, Dounskaia et al. (2000) revealed biases in wrist and finger movements of young adults similar to those (although less pronounced) as observed in the present study. They argued that in right-handed subjects, drawing right-tilted lines and ovals may be more compliant with biomechanical interactions between wrist and finger motions than drawing other shapes. The minimal effort required for overcoming these interactions makes right-tilted shapes the most preferred graphical output. This hypothesis was supported by demonstration of mirror-symmetric biases in left-handed subjects.
The role of biomechanical factors in organization of multi-joint movements has been extensively studied for arm movements. The two most influential biomechanical properties during arm movements are passive “interaction” torques arising at the joints because of mechanical interactions between adjacent segments of the arm (Hollerbach & Flash, 1982) and resistance to arm’s inertia (Hogan, 1985). Each of these factors varies across different patterns of shoulder and elbow motions, causing variations in control complexity (Gribble & Ostry, 1999). The variations in control complexity result in biases towards performance of joint coordination patterns that require minimal active resistance to interaction torque (Dounskaia, Ketcham, & Stelmach, 2002; Dounskaia, Swinnen, Walter, Spaepen, & Verschueren, 1998; Goble, Zhang, Shimansky, Sharma, & Dounskaia, 2007). They also result in higher speed and lower variability during movements characterized by low inertial resistance compared with movements during which inertial resistance is high (Gordon, Ghilardi, Cooper, & Ghez, 1994). These studies show that difficulties associated with regulation of biomechanical factors during multi-joint arm movements vary across joint coordination patterns. It has also been shown that the difficulties associated with regulation of biomechanical factors are increased in PD patients (Dounskaia, Ketcham, Leis, & Stelmach, 2005; Fradet, Lee, Stelmach, & Dounskaia, published on-line).
Similar to the arm joints, motions of the wrist and fingers holding a pen also influence each other. However, biomechanical factors influencing wrist and finger movements may be more diverse than those influencing movements of the shoulder-elbow linkage. In addition to interaction torques and hand inertia, elastic forces caused by physical limits of joint displacements can be influential in the wrist and fingers. Also, activity of a number of finger muscles provides synergistic effect on wrist motion. The anatomical properties of the hand make it difficult to directly evaluate the effect of biomechanical factors on production of handwriting movements through analysis of movement dynamics. Nevertheless, the biomechanical effects predict differential complexity of various wrist and finger movements and difficulties in the production of some but not other coordination patterns like those observed in the present study.
The influence of biomechanical factors on joint control also predicts that PD boosts the tendency to comply with biomechanical effects because regulation of these effects may require generation of high muscular effort, specifically during fast movements (Dounskaia et al., 1998). Evidence that force generated for movement execution as well as underlying levels of muscle activity are reduced in PD has been reported (Hallett & Khoshbin, 1980; Marsden, 1982; Pfann, Buchman, Comella, & Corcos, 2001). The deficit in force generation may result in the tendency in PD patients to acquire “easy” coordination patterns during which muscular effort for overcoming the biomechanical effects is minimized. This interpretation of the enhanced biases in PD observed in the present study is consistent with “rate” models of basal ganglia (e.g., the parallel pathways hypothesis and the selectivity hypothesis) that suggest that underproduction of dopamine causes decreased rate of basal ganglia discharge to the frontal cortex, thus reducing movement activation (Albin, Young, & Penney, 1989; Delong, 1990; DeLong & Wichmann, 2007; Mink, 1996).
In addition to differential muscular effort required for the production of various wrist and finger coordination patterns, the biases may also emerge due to differential demands for precision of control across the coordination patterns. For instance, accuracy of muscle force magnitude and timing required to regulate biomechanical effects may vary across coordination patterns. A deficit in fine regulation of force has been recognized for PD patients (Stelmach, Teasdale, Phillips, & Worringham, 1989; Van Gemmert et al., 1999; Wing, 1988). Thus, the inability to accurately regulate muscle force may be another reason for differential difficulties experienced by PD patients in the production of various wrist and finger coordination patterns. This inability is consistent with recent models that have increased the understanding that altered patterns of basal ganglia discharge may play a more important role than discharge rate (see for recent reviews (DeLong & Wichmann, 2007; Gale, Amirnovin, Williains, Flaherty, & Eskandar, 2008; Utter & Basso, 2008).
Specifically, it has been recognized that abnormal oscillation, increased synchronization of neurons, and other changes in the patterns of neuronal activity within the basal ganglia contribute to Parkinsonian movement features. According to these models, dopamine depletion results in the lack of finely tuned basal ganglia activity required for selection and execution of motor commands. The “noisy” basal ganglia output may prevent cortical motor areas from producing timely and accurate descending signals to the muscles necessary for regulation of biomechanical effects during multi-joint movements (Bevan, Magill, Terman, Bolam, & Wilson, 2002; Gatev, Darbin, & Wichmann, 2006; Mink, 2003; Van Gemmert & Stelmach, 2002; Van Gemmert, Teulings, & Stelmach, 1998). With respect to the wrist and finger movements, this deficit may cause PD patients to use “simple” coordination patterns that comply with the factors causing biases, and therefore, do not require high accuracy of muscle force for regulation of these factors.
Other deficiencies in motor control typical of PD, not related to regulation of biomechanical factors, may have also contributed to the differences observed here between the two groups. For instance, the ability to maintain required movement timing may be decreased in PD (Freeman, Cody, & Schady, 1993), although auditory cues like that used in the present study are known to improve this ability (Georgiou et al., 1993; Kritikos et al., 1995). The formulation of the tasks as specific wrist and finger coordination patterns may have increased cognitive demands for movement production, contributing to the deterioration of performance in PD patients. Nevertheless, all these PD-related factors would provide equal effect on all movement types (such as general increases in movement variability) and would not be able to account for the enhanced biases in wrist and finger coordination revealed in PD patients. Thus, biomechanical properties of the multi-joint structure of the hand are the most plausible causes of the biased wrist and finger coordination in PD.
The stronger biases in PD patients than in controls revealed here for wrist and finger movements may represent a deficit common for various multi-joint movements in PD. This would be the case, for example, if the biases are caused by biomechanical interactions between adjacent limb segments that are present during all types of multi-joint movements. The deficit in PD patients associated with augmented biases in multi-joint movements has not been recognized. Nevertheless, it may significantly contribute to the Parkinsonian movement features. Indeed, the need to overcome the biases would likely cause delays in movement initiation, movement slowness, rigidity, and other well-documented features of movements in PD such as increased variability, shortened movement amplitude, reliance on vision, and submovements (Brown & Marsden, 1991; Cooke, Brown, & Brooks, 1978; Flash et al., 1992; Rand, Stelmach, & Bloedel, 2000; Stelmach, Worringham, & Strand, 1986). Thus, investigation of the biased coordination of multi-joint movements in PD and factors causing these biases may significantly contribute to understanding of the motor disorder caused by PD.
In conclusion, this study reveals strong biases in wrist and finger coordination during handwriting-like movements performed by PD patients. A possible reason for these biases is that PD patients may be unable to properly regulate influence of biomechanical factors on wrist and finger motion. Although precise reasons for the amplified difficulty of specific coordination patterns still need to be determined, the biased coordination of wrist and finger motions revealed in this study may significantly contribute to impaired handwriting in PD. Moreover, the effect of amplified coordination biases may not be limited to deteriorations in handwriting but may contribute to PD-related impairments in other multi-joint movements. Our results open up possibilities for clinical applications, suggesting that training programs that focus on “difficult” coordination patterns of multi-joint movements have perspectives for alleviating the debilitating influence of PD on performance of everyday motor tasks.
Acknowledgments
The study was supported by National Institutes of Health [grant number NS43502] awarded to George Stelmach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts JL, Tresilian JR, Stelmach GE. The co-ordination and phasing of a bilateral prehension task. The influence of Parkinson’s disease. Brain. 1998;121(Pt 4):725–742. doi: 10.1093/brain/121.4.725. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The Functional-Anatomy of Basal Ganglia Disorders. Trends in Neurosciences. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in Neurosciences. 2002;25(10):525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Dual Task-Performance and Processing Resources in Normal Subjects and Patients with Parkinsons-Disease. Brain. 1991;114:215–231. [PubMed] [Google Scholar]
- Byblow WD, Summers JJ, Lewis GN, Thomas J. Bimanual coordination in Parkinson’s disease: deficits in movement frequency, amplitude, and pattern switching. Mov Disord. 2002;17(1):20–29. doi: 10.1002/mds.1281. [DOI] [PubMed] [Google Scholar]
- Calne D, Snow B, Lee C. Criteria for diagnosing Parkinson’s disease. Annals of Neurology. 1992;32(125–127) doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Contreraz-Vidal JL, Teulings HL, Stelmach GE. Micrographia in Parkinson’s disease. Neuroreport. 1995;6:2089–2092. doi: 10.1097/00001756-199510010-00032. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown JD, Brooks VB. Increased Dependence on Visual Information for Movement Control in Patients with Parkinsons-Disease. Canadian Journal of Neurological Sciences. 1978;5(4):413–415. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- Delong MR. Primate Models of Movement-Disorders of Basal Ganglia Origin. Trends in Neurosciences. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Experimental Brain Research. 2003;153(2):197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- Dooijes EH. Analysis of Handwriting Movements. Acta Psychologica. 1983;54(1–3):99–114. [Google Scholar]
- Dounskaia N, Ketcham CJ, Leis BC, Stelmach GE. Disruptions in joint control during drawing arm movements in Parkinson’s disease. Experimental Brain Research. 2005;164(3):311–322. doi: 10.1007/s00221-005-2251-8. [DOI] [PubMed] [Google Scholar]
- Dounskaia N, Van Gemmert AWA, Stelmach GE. Interjoint coordination during handwriting-like movements. Experimental Brain Research. 2000;135(1):127–140. doi: 10.1007/s002210000495. [DOI] [PubMed] [Google Scholar]
- Dounskaia N, Wisleder D, Johnson T. Influence of biomechanical factors on substructure of pointing movements. Experimental Brain Research. 2005;164(4):505–516. doi: 10.1007/s00221-005-2271-4. [DOI] [PubMed] [Google Scholar]
- Dounskaia NV, Ketcham C, Stelmach GE. Influence of biomechanical constraints on horizontal arm movements. Motor Control. 2002;6(4):366–387. doi: 10.1123/mcj.6.4.366. [DOI] [PubMed] [Google Scholar]
- Dounskaia NV, Swinnen SP, Walter CB, Spaepen AJ, Verschueren SMP. Hierarchial control of different elbow-wrist coordination patterns. Experimental Brain Research. 1998;121(3):239–254. doi: 10.1007/s002210050457. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. United Parkinson’s disease rating scale. In: Fahn S, Marsden CD, editors. Recent developments in Parkinson’s disease. Vol. 2. New Jersey: MacMillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Flash T, Inzelberg R, Schechtman E, Korczyn AD. Kinematic Analysis of Upper Limb Trajectories in Parkinsons-Disease. Experimental Neurology. 1992;118(2):215–226. doi: 10.1016/0014-4886(92)90038-r. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Visual Closed-Loop and Open-Loop Characteristics of Voluntary Movement in Patients with Parkinsonism and Intention Tremor. Brain. 1976;99(Jun):269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fradet L, Lee GS, Stelmach GE, Dounskaia N. Parkinson’s disease disrupts joint control during line-drawing and point-to-point arm movements . Experimental Brain Research (Published on-line) [Google Scholar]
- Fradet L, Lee GS, Stelmach GE, Dounskaia N. Parkinson’s disease disrupts joint control during line-drawing and point-to-point arm movements (submitted) [Google Scholar]
- Freeman JS, Cody FW, Schady W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:1078–1084. doi: 10.1136/jnnp.56.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JT, Amirnovin R, Williains ZM, Flaherty AW, Eskandar EN. From symphony to cacophony: Pathophysiology of the human basal ganglia in Parkinson disease. Neuroscience and Biobehavioral Reviews. 2008;32(3):378–387. doi: 10.1016/j.neubiorev.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Movement Disorders. 2006;21(10):1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of inertial cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- Goble JA, Zhang Y, Shimansky Y, Sharma S, Dounskaia NV. Directional biases reveal utilization of arm’s biomechanical properties for optimization of motor behavior. Journal of Neurophysiology. 2007;98(3):1240–1252. doi: 10.1152/jn.00582.2007. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of Planar Reaching Movements.2. Systematic Extent Errors Resulting from Inertial Anisotropy. Experimental Brain Research. 1994;99(1):112–130. doi: 10.1007/BF00241416. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Ostry DJ. Compensation for interaction torques during single- and multijoint limb movement. Journal of Neurophysiology. 1999;82(5):2310–2326. doi: 10.1152/jn.1999.82.5.2310. [DOI] [PubMed] [Google Scholar]
- Gross RD, Atwood CW, Jr, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia. 2008;23(2):136–145. doi: 10.1007/s00455-007-9113-4. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A Physiological Mechanism of Bradykinesia. Brain. 1980;103(Jun):301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hocherman S, Alexandrovsky L, Badarny S, Honigman S. L-DOPA improves visuo-motor coordination in stable Parkinson’s disease patients. Parkinsonism Relat Disord. 1998;4(3):129–136. doi: 10.1016/s1353-8020(98)00034-0. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism - Onset Progression and Mortality. Neurology. 1967;17(5):427. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hogan N. The Mechanics of Multi-Joint Posture and Movement Control. Biological Cybernetics. 1985;52(5):315–331. doi: 10.1007/BF00355754. [DOI] [PubMed] [Google Scholar]
- Hollerbach JM, Flash T. Dynamic Interactions between Limb Segments during Planar Arm Movement. Biological Cybernetics. 1982;44(1):67–77. doi: 10.1007/BF00353957. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of Neurology Neurosurgery and Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Cunnington R, Bradshaw JL, Phillips JG, Iansek R, Rogers MA. Bimanual co-ordination in Parkinson’s disease. Brain. 1998;121(Pt 4):743–753. doi: 10.1093/brain/121.4.743. [DOI] [PubMed] [Google Scholar]
- Kritikos A, Leahy C, Bradshaw JL, Iansek R, Phillips JG, Bradshaw JA. Contingent and Non-contingent Auditory Cueing in Prkinson’s Diseaase. Neuropsychologia. 1995;33:1193–1204. doi: 10.1016/0028-3932(95)00036-3. [DOI] [PubMed] [Google Scholar]
- Lewitt PA. Micrographia as a Focal Sign of Neurological Disease. Journal of Neurology Neurosurgery and Psychiatry. 1983;46(12):1152–1153. doi: 10.1136/jnnp.46.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse FJ, Schmaker LRB, Thomassen AJWM. The influence of changes in the effector coordinate system on handwriting movements. In: Kao HSR, Van Galen GP, Hoosain R, editors. Graphonomics: Contemporary Research in handwriting. Amsterdam: Elsevier Science Publishers B. V; 1986. pp. 31–46. [Google Scholar]
- Margolin D, Wing AM. Agraphia and micrographia: Clinical manifestations of motor programming and performance disorders. Acta Psychologica. 1983;54:263–283. doi: 10.1016/0001-6918(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The Mysterious Motor Function of the Basal Ganglia - the Robert Wartenberg Lecture. Neurology. 1982;32(5):514–539. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- Mclennan JE, Tyler HR, Schwab RS, Nakano K. Micrographia in Parkinsons Disease. Journal of the Neurological Sciences. 1972;15(2):141. doi: 10.1016/0022-510x(72)90002-0. [DOI] [PubMed] [Google Scholar]
- Meulenbroek RGJ, Thomassen AJWM. Stroke-Direction Preferences in Drawing and Handwriting. Human Movement Science. 1991;10(2–3):247–270. [Google Scholar]
- Meulenbroek RGJ, Thomassen AJWM, van Lieshout PHHM, Swinnen SP. The stability of pen-joint and interjoint coordination in loop writing. Acta Psychologica. 1998;100(1–2):55–70. doi: 10.1016/s0001-6918(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements - Impaired inhibition of competing motor patterns. Archives of Neurology. 2003;60(10):1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Muratori LM, McIsaac TL, Gordon AM, Santello M. Impaired anticipatory control of force sharing patterns during whole-hand grasping in Parkinson’s disease. Exp Brain Res. 2008;185(1):41–52. doi: 10.1007/s00221-007-1129-3. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Micrographia in Parkinson’s disease: the effect of providing external cues. Journal of Neurology Neurosurgery and Psychiatry. 1997;63(4):429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Movement Disorders. 2001;16(6):1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- Plamondon R, Lamarche F. Modelization of handwriting: A system approach. In: Kao HSR, Van Galen GP, Hoosain R, editors. Graphonomics: Contemporary Research in Handwriting. Amsterdam: Elsevier Science Publishers B.V; 1986. pp. 169–183. [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res. 2007;181(4):561–570. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- Poizner H, Feldman AG, Levin MF, Berkinblit MB, Hening WA, Patel A, et al. The timing of arm-trunk coordination is deficient and vision-dependent in Parkinson’s patients during reaching movements. Exp Brain Res. 2000;133(3):279–292. doi: 10.1007/s002210000379. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Daffertshofer A, van den Heuvel E, Wolters E, Beek PJ, Berendse HW. Bimanual coordination dysfunction in early, untreated Parkinson’s disease. Parkinsonism Relat Disord. 2006;12(4):246–252. doi: 10.1016/j.parkreldis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rand MK, Stelmach GE, Bloedel JR. Movement accuracy constraints in Parkinson’s disease patients. Neuropsychologia. 2000;38(2):203–212. doi: 10.1016/s0028-3932(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995;73(2):820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RC, Treffner PJ, Shaw BK, Turvey MT. Dynamic Aspects of Learning an Interlimb Rhythmic Movement Pattern. Journal of Motor Behavior. 1992;24(1):67–83. doi: 10.1080/00222895.1992.9941602. [DOI] [PubMed] [Google Scholar]
- Schomaker LRB, Plamondon R. The Relation between Pen Force and Pen-Point Kinematics in Handwriting. Biological Cybernetics. 1990;63(4):277–289. [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Multijoint movement control in Parkinson’s disease. Experimental Brain Research. 2001;140(3):335–344. doi: 10.1007/s002210100829. [DOI] [PubMed] [Google Scholar]
- Sheridan MR, Flowers KA, Hurrell J. Programming and Execution of Movement in Parkinsons-Disease. Brain. 1987;110:1247–1271. doi: 10.1093/brain/110.5.1247. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Teasdale N, Phillips J, Worringham CJ. Force Production Characteristics in Parkinsons-Disease. Experimental Brain Research. 1989;76(1):165–172. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ, Strand EA. Movement Preparation in Parkinsons-Disease - the Use of Advance Information. Brain. 1986;109:1179–1194. doi: 10.1093/brain/109.6.1179. [DOI] [PubMed] [Google Scholar]
- Teulings HL. Handwriting movement control. In: Keele SW, Heuer H, editors. Handbook of perception and action, vol 2 Motor Skills. London: Academic Press; 1996. pp. 561–613. [Google Scholar]
- Teulings HL, ContrerasVidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Experimental Neurology. 1997;146(1):159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Thomassen AJWM, Maarse FJ. A description of handwriting in terms of main axes. In: Plamondon R, Suen CY, Simner M, editors. Computer and Human Recognition of Handwriting. Singapore: World Scientific Publishing Co; 1988. pp. 69–82. [Google Scholar]
- Utter AA, Basso MA. The basal ganglia: An overview of circuits and function. Neuroscience and Biobehavioral Reviews. 2008;32(3):333–342. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AWA, Adler CH, Stelmach GE. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(11):1502–1508. doi: 10.1136/jnnp.74.11.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gemmert AWA, Stelmach GE. Increased motor processing demands reduce stroke size in parkinsonian handwriting. Journal of Sport & Exercise Psychology. 2002;24:16–16. [Google Scholar]
- Van Gemmert AWA, Teulings HL, Contreras-Vidal JL, Stelmach GE. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia. 1999;37(6):685–694. doi: 10.1016/s0028-3932(98)00122-5. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AWA, Teulings HL, Stelmach GE. The influence of mental and motor load on handwriting movements in Parkinsonian patients. Acta Psychologica. 1998;100(1–2):161–175. doi: 10.1016/s0001-6918(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Van Sommers P. Descriptive and experimental studies of graphic production processes. Cambridge: University Press; 1984. [Google Scholar]
- Wing AM. A Comparison of the Rate of Pinch Grip Force Increases and Decreases in Parkinsonian Bradykinesia. Neuropsychologia. 1988;26(3):479–482. doi: 10.1016/0028-3932(88)90100-5. [DOI] [PubMed] [Google Scholar]