Abstract
Melanoma can be considered an emerging chronic disease that may considerably affect patients’ lives. The authors systematically reviewed the available literature on health-related quality of life (HRQOL) and melanoma. Of reviews and the selected studies, reference lists were hand-searched. The quality of the eligible studies was appraised based on 14 previously published criteria. Of the 158 abstracts, 44 articles were appraised, resulting in 13 selected studies written in English (published between 2001 and 2008). Most studies assessed patients from specialised centres with varying, but relatively advanced, disease stages. The most commonly used instruments were the SF-36 and EORTC QLQ-C30. Recently, a melanoma-specific HRQOL questionnaire [FACT-Melanoma (FACT-M)] was introduced for clinical trial purposes. It showed that approximately one-third of melanoma patients experienced considerable levels of distress, mostly at the time of diagnosis and following treatment. Systemic therapies affected HRQOL negatively in the short term, but to a lesser extent in the long term. Health status and patients’ psychological characteristics are associated with higher levels of HRQOL impairment. The authors found that the impact of melanoma on patients’ HRQOL is comparable to that of other cancers. Accurately assessing HRQOL impairment in melanoma patients is pivotal, as it may affect disease management, including therapy and additional counselling, future preventive behaviour and perhaps even prognosis.
Keywords: cutaneous, health status, health status indicators, melanoma, quality of life (QOL), skin, survival analysis
introduction
The incidence of cutaneous melanoma has increased dramatically in the western world in the last few decades (4% for women and 5% for men, annually) [1]. Despite this rise in incidence, the mortality rate seems to be levelling off due to earlier diagnosis [2, 3]. Approximately 70% of melanoma patients have a Breslow thickness of <1.5 mm, with a 5-year survival rate of 95%. When lymph nodes are involved, the survival rate drops to 20–40% [1]. Melanoma affects relatively young and middle-aged people. Patients with melanoma are more at risk of developing a new primary melanoma (1.2–8.2%), and their first-degree relatives have a twofold higher risk of developing melanoma [4]. Despite tremendous efforts, melanoma treatment has not changed substantially; in most cases it comprises surgery with or without (sentinel) lymph node resection. A small group of patients with metastasised disease receive chemotherapy and/or interferon therapy (often in clinical trial settings).
About 80% of patients will survive melanoma [1, 3] but remain at risk for disease progression for many years, for which there is no successful therapy. Therefore, melanoma can be considered a chronic, life-threatening disease. Additionally, patients are aware that UV exposure is an important risk factor for the development of melanoma, which may affect their lifestyles as well as their social and professional activities [5].
Altogether, these observations indicate that melanoma may have a considerable impact on patients’ lives, including their health-related quality of life (HRQOL), which the World Health Organization (WHO) defines as ‘an individual's perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards and concerns. This broad ranging concept is in a complex way affected by the person's physical health, psychological state, level of independence, social relationships, personal beliefs and their relationship to salient features of their environment. Because in ∼75% of patients local surgical excision is an adequate therapy, HRQOL impairment is predominantly determined by psychological aspects and, to a lesser extent, by (long-term) therapy-induced events, as is often observed in other cancers. The psychosocial issues facing patients with an early stage, highly curable disease such as melanoma differ from other types of cancer with profound loco-regional effects or distant metastases. Although >85% of melanoma patients rated follow-up surveillance to be worthwhile, they also indicated that little attention had been paid to their well-being during surveillance [6].
The objective of this review is to evaluate, summarise and discuss the available literature concerning HRQOL in patients with cutaneous melanoma.
methods
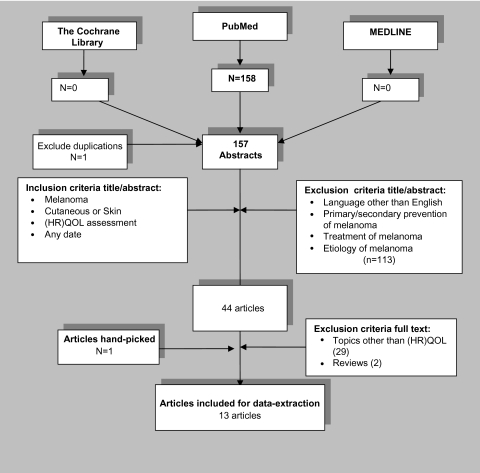
Systematic searches were conducted in PubMed, MEDLINE and the Cochrane Library with the assistance of a medical librarian. The search algorithm of MeSH-used terms was the following: (cutaneous OR skin) AND melanoma AND (quality of life OR life) AND (health status OR health status indicators OR survival analysis OR health-related OR health) NOT ‘non-melanoma’. Restrictions were not placed on publication dates or language. Several inclusion and exclusion criteria were used (Figure 1). The quality of the eligible studies was assessed using criteria for assessing the methodological quality of studies in HRQOL published previously (Table 2) [7]. In brief, these criteria focus on the methodological aspects of HRQOL studies, e.g. whether inclusion and exclusion criteria are well formulated, whether responders and non-responders are reported and whether the socio-demographics of the responders are known and the score range is between 0 and 14. The criteria are quite strict; if a certain criterion is not explicitly mentioned, the default is 0.
Figure 1.
Flow-chart of the systematic search.
Table 2.
HRQOL instruments used in eligible studies
| Instrument | Type | Goals | Domains/subscales |
| Brief Symptom Inventory (BSI) | Generic | Measure of emotional distress | Nine clinical scales and three summary scales. Global severity index (GSI): sensitive measure of overall distress. |
| Chronic Strains Survey (CSS) | Generic | Measure of persistent stressful conditions | Items concerning the existence and perceived burden of economical and social difficulties, strains in work life, alcohol or drug abuse, other chronic diseases, etc. |
| EuroQol Group (EQ-5D) | Generic | A five-dimensional health state classification | Five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. |
| Health and Activities Limitation Index (HALex) | Generic | Provides a HRQOL utility score | A single numerical value for the health state based on patients’ perceived health status in conjunction with any activity limitation they might experience. 0 = near death state to 1 = perfect health with no limitations. Previously validated against a large population of subjects. |
| Short Form-36 (SF-36) | Generic | Assesses health functioning; often used as a general measure of HRQOL | Eight subscales: physical functioning, vitality, social functioning, general health, bodily pain, physical role, emotional role and mental health. |
| Two component summary scales: physical and mental component summary scales (PCS and MCS). | |||
| European Organization for Research and Treatment of Cancer (EORTC QLQ-C30) | Cancer specific | Assesses the HRQOL of cancer patients participating in international clinical trials | Five functional scales: physical (PF), role (RF), cognitive (CF), emotional (EF) and social (SF). |
| Three symptom scales: fatigue (FA), pain (PA), nausea and vomiting (NV). | |||
| A global health status / QOL scale (QL). | |||
| Six single items assessing additional symptoms commonly reported by cancer patients: dyspnoea (DY), loss of appetite (AP), insomnia (SL), constipation (CO), diarrhoea (DI). | |||
| A single item on the perceived financial impact of the disease (FI). | |||
| Functional Assessment of Cancer Therapy—Melanoma (FACT-M) | Melanoma specific | Assesses the HRQOL of melanoma patients participating in clinical trials | Fact-G items (general) questions. |
| Melanoma-specific sub-scale: 24 items encompassing three HRQOL domains: physical, emotional and social well-being. | |||
| Cassileth Scar Questionnaire | Therapy specific | Investigates patients’ opinion on the size and cosmetic implications of their excisions | 12 items, each scored from 1 to 4 (minimum to maximum negative cosmetic impact). The questionnaire also contains outline drawings of the human body on which patients are asked to indicate the size, shape and location of their scar [16]. |
results
included studies
Of the 158 abstracts extracted from PubMed, 114 were excluded primarily because they did not focus on HRQOL; 44 full articles were reviewed, but only 13 were eligible for this review (Figure 1). The articles were all in English and published between 2001 and 2008.
quality of included studies
The included studies varied in quality according to the quality criteria used (Table 1) [7]. The scores of the eligible studies ranged between 8 and 12 out of a maximum of 14 (67% and 86%, respectively) (Table 3).
Table 1.
List of 14 criteria assessing the methodological quality of HRQOL studies of melanoma patients
| A | Socio-demographic and medical data are described (e.g. age, race, employment status, educational status, tumour stage at diagnosis, etc.) |
| B | Inclusion and/or exclusion criteria are formulated |
| C | The process of data collection is described (e.g. interview or self-report, etc.) |
| D | The type of cancer is described. |
| E | The results are compared between two groups or more (e.g. health population, groups with different cancer treatment or age, comparison with time at diagnosis, etc.). |
| F | Mean or median and range or standard deviation of time since diagnosis or treatment is given. |
| G | Participation and response rates for patient groups have to be described and have to be >75%. |
| H | Information is presented about patient/disease characteristics of respondents and non-respondents or if there is no selective response. |
| I | A standardised or valid QOL questionnaire is used. |
| J. | Results are described not only for QOL but also for the physical, psychological and social domains. |
| K. | Mean, median, standard deviations or percentages are reported for the most important outcome measures. |
| L. | An attempt is made to find a set of determinants with the highest prognostic value. |
| M. | Patient signed an informed consent form before study participation. |
| N. | The degree of selection of the patient sample is described. |
Table 3.
Summary of eligible and reviewed studies on melanoma and HRQOL impairment
| Country, year [Ref.] | Research type | Study population | No. patients | HRQOL instrument | Conclusions | Quality score (range 0–14) |
| USA, 2008 [8] | Cohort prospective 12 months | MD Anderson Cancer Center: patients recruited with new melanoma or within the first 3 years of follow-up. | 225 | FACT, EORTC QLQ, POMS, MCSDS | Reliable QOL questionnaire for patients with melanoma in clinical trials. | 9 |
| aEurope, 2004 [9] | Randomised prospective 2 months | Centres throughout Europe: patients with non-ocular melanoma, with or without brain metastases; phase III study comparing fotemustine (F) and dacarbazine (D) (one in each of the patient arms); metastasised disease. | 229 (112F, 117D) | EORTC QLQ-C30 | ‘No significant difference in QOL between two groups. The general tendency in the selected QOL dimensions was degradation over time in both arms.’ | 10 |
| Finland, 2007 [10] | Cohort prospective 3–4 months after diagnosis | Oncology Clinic of Tampere University Hospital, Finland: patients with cutaneous melanoma with localised disease. Included patients with Clarke II, II or IV. Breslow 0.20–7.00 mm. Excluded patients with in situ melanoma, Clark V and orbital melanoma. | 59 | WOC, SSF, AX, LES, CSS, RSCL, DEPS | ‘Women tend to have slightly more psychological symptoms (P = 0.085). Patients reported self-perceived QOL as quite good to good.’ | 8 |
| UK, 2006 [11] | Randomised prospective 60 months | Weston Park Teaching Hospital, Sheffield: melanoma patients, all stages. | 674 | EORTC QLQ-C30, EQ5D | ‘Patients in observational group had significantly higher mean QOL than interferon patients.’ | 8 |
| Poland, 2005 [12] | Controlled trial, prospective minimum of 56 days after surgery | Department of Soft Tissue and Bone Cancer, Institute of Oncology, Warsaw: two equal groups of 110 patients after radical surgery for melanoma. One group received supplementary IFN-α2b therapy. Stage unclearly mentioned. | 220 | EORTC QLQ-C30 | ‘The IFN-α2b significantly affected the emotional, social and physical health of the patients. In spite of adverse effects of treatment, patients scored their QOL as good.’ | 10 |
| Finland, 2005 [13] | Cohort prospective 3 months after diagnosis | Oncology Clinic of Tampere University Hospital: patients with melanoma and breast cancer patients. Localised disease, newly diagnosed. | 175 (melanoma 72) | WOC, SFSS, AX, LES, RSCL, DEPS, EORTC-QLQ (breast cancer module) | ‘QOL of newly diagnosed cancer patients is highly associated with psychosocial factors. Non-cancer life stresses seem to be very important in the QOL in newly diagnosed cancer patients. Adjuvant treatment may compromise supportive psychosocial factors that enhance QOL in cancer.’ | 9 |
| UK, 2006 [14] | Cohort prospective 6 months | Pigmented Lesion Clinic: malignant and non-malignant skin lesions, all stages. | 195 (melanoma 10) | EORTC QLQ-C30, HAD, STAI-SSF | ‘QOL pre-diagnosis was excellent. Emotional functioning, insomnia and global health status deteriorated throughout diagnostic process for patients with malignant melanoma.’ | 12 |
| USA 2004 [15] | Cohort prospective 9 months | Multidisciplinary Melanoma Clinic: melanoma patients with stages I–III. | 351 | MOC, BSI, SF-36, WOC, STAI | The healthy cluster reported a significantly higher HRQOL than the unhealthy clusters when confronted with melanoma. | 11 |
| USA 2003 [16] | Cohort prospective 3 months | Anderson Cancer Centre, Houston: population with metastatic renal cell carcinoma or metastatic melanoma. Phase I/b trial for trial vaccination. | 53 (melanoma 24) | ISEL, IES, BSI, SF 36 | ‘The results suggest that social support buffers the negative association between intrusive thoughts/avoidance and psychological adjustment. Overall the results are consistent with a social-cognitive processing model of post-trauma reactions among cancer patients.’ | 10 |
| USA, 2003 [17] | Randomised prospective 6 months | Melanoma Clinic: melanoma patients with stages I–III | 48 | BSI SF-36 STAI | ‘Distress significantly reduced after 4 CBI sessions, with an increase in HRQOL in patients with medium-high distress.’ | 10 |
| USA, 2003 [18] | Cross-sectional retrospective | Population-based study; patients with melanoma, breast, colon or lung cancer <1 year, 1–5 years and >5 years, stage unknown. | 692 (melanoma = 92) | HALex | ‘Health utility score lowest directly after treatment and improve over time. Long term (>5 years) survivors have the highest score.’ | 9 |
| USA, 2001 [19] | Cohort | Multidisciplinary Melanoma Clinic: melanoma patients with stages I–III. | 287 | BSI, SF-36, WOC, STAI | ‘A significant minority of patients are distressed and rely heavily on non-beneficial coping strategies.’ | 10 |
France, Germany, Norway, Hungary, Spain, Slovakia, Austria.
Abbreviations: AX, Anger Expression Scale; BSI, Brief Symptom Inventory; CSS, Chronic Strains Survey; DEPS, Depression Anxiety Scale; EQ-5D, EuroQol group; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer; FACT-M, Functional Assessment of Cancer Therapy—Melanoma; HAD, Hospital Anxiety and Depression; HALex, Health and Activities Limitation Index; IES, Impact of Event Scale; ISEL, Interpersonal Support Evaluation List; LES, Life Experience Survey; MCSDS, Marlowe–Crowne Social Desirability Scale, POMS, Profile of Mood States; PAIS-SR, Psychosocial Adjustment of Illness Scale—Self Report; RSCL, Rotterdam Symptom Checklist; SF-36, Short Form-36; SFSS, Structural–Functional Social Support Scale; SSF, Social Support Survey; STAI, State Trait Anxiety Inventory; WOC, Ways of Coping.
study design and population
Of the 13 studies that assessed HRQOL in melanoma patients, seven were cohort studies, five were clinical trials and one was a cross-sectional population-based study. Three studies investigated the effects of therapy such as surgery, interferon or vaccination on patients’ HRQOL. Of the 10 prospective studies, eight had a study duration of ≤4 months. Only two studies followed patients for >2 years after melanoma diagnosis. Six studies were conducted in the USA, of which three included patients from specialised melanoma clinics. The remaining European studies most often included patients from tertiary oncology departments. One randomised clinical trial from the UK and one cross-sectional study from the USA were both population based. The study size varied between 10 and 674 patients. Five of 13 studies included <100 patients (of which two had <50 patients), and four studies had >250 patients. The tumour stages varied, but most studies focused on localised disease, and only a few focused on metastasised melanoma (Table 3).
HRQOL measures
A total of 20 different instruments were used in the 13 studies; seven measured HRQOL, of which five were generic instruments, one was cancer specific and one was melanoma specific (Tables 2 and 3). The most frequently used generic and cancer-specific HRQOL tools were the SF-36 (5/13) and EORTC QLQ-C30 (6/13), respectively. In three studies, a combination of generic and cancer-specific tools was used. In two studies, health utilities (HALex and EQ-5D) were used as a measure of HRQOL, and in one study a melanoma-specific (FACT-M) tool was used. Recently, the FACT-M questionnaire was proposed as ‘a reliable and valid instrument for patients with melanoma that can be used for the assessment of QOL in clinical trials’ [8, 20].
In addition to HRQOL, all included studies reported on other psychological and social constructs, such as social support and personality structure, but these instruments were beyond the scope of this review.
HRQOL impairment in melanoma
The results of the different studies indicated that there are three distinct periods of HRQOL impact during the melanoma experience: diagnosis, treatment and follow-up [19, 21]. The immediate period following diagnosis (i.e. acute survival phase) was often associated with high levels of HRQOL impairment [14, 18]. Patients reported more pain, less energy and more interference of stressors (physical and emotional) on social activities. Importantly, patients also gave worse evaluations of overall personal health. Acute survival is followed by extended survival, which is dominated more by fears of recurrence and less by the physical limitations the cancer or its associated therapies create [21]. In the follow-up phase, psychological distress can interfere with screening recommendations and preventive behaviours [19].
A recent study showed that before the definite diagnosis of melanoma, patients reported an excellent HRQOL. During the diagnostic process, however, insomnia increased, while emotional functioning and global health status deteriorated, as measured by standard anxiety scales (HADS, STAI-SFF) and the EORTC QLQ-C30 for patients ultimately diagnosed with melanoma [14].
In a surgical randomised clinical trial (RCT) of high-risk melanoma patients, patients with a 3 cm excision margin had significantly poorer mental and physical functioning (SF-36) compared with those with a 1 cm excision margin [22]. After 3–6 months, the difference in HRQOL impact between these two patient groups was no longer significant, except for a persisting poor scar perception in the 3 cm excision group. Predictors of poor scar perception were younger age, female gender, a 3 cm excision margin and poor physical and mental health post-surgery. Additionally, patients with a 3 cm margin had more complications, a longer hospital stay, more skin grafts and greater physical impairment.
Three clinical trials assessed the effect of chemotherapeutics and/or interferon therapy on patients’ HRQOL [9, 11, 12]. Patients who received interferon therapy scored significantly lower on their HRQOL in terms of functioning and symptoms compared with the (placebo) control group. The EORTC QLQ-C30 instrument showed that patients had significantly worse mean scores in role functioning (RF), emotional functioning (EM), cognitive functioning (CF), social functioning (SF) and global health status compared with patients in a placebo group [11]. Although systemic drugs (e.g. interferon) decreased patients’ HRQOL during treatment, the overall gain in HRQOL was favourable in some patients, especially those with a poor prognosis [12].
In one study, cognitive behavioural intervention significantly decreased distress (i.e. BSI and STAI scores) in patients with medium to high baseline levels of distress [17].
predictors of HRQOL impairment
Several studies have demonstrated that overall health is the most important predictor of melanoma's impact on HRQOL [8, 13, 15]. Patients with poor health are significantly more likely to report higher levels of HRQOL impairment or lower levels of health utility than healthier patients. Patients who report poor physical health have a comparable impact to those with poor psychological health, and those who experienced both reported the lowest HRQOL scores [8, 13]. Not only is global health a predictor of HRQOL, it also deteriorates after the diagnosis of melanoma due to an increase in insomnia and pain [8, 14, 15].
In addition to health status, several psychological factors such as non-cancer life stresses, wishful thinking and maladaptive coping styles were important determinants of melanoma's level of impact on HRQOL [19]. In patients with metastasised melanoma, low levels of social support were significantly associated with greater psychological distress and poorer mental HRQOL. Additionally, low social support decreased the likelihood of HRQOL adjustment 1 month after treatment [16].
No studies have investigated the HRQOL across gender, age and all tumour stages, except in a surgical RCT in which older individuals were significantly more likely to report lower levels of physical functioning, as is expected [22]. Women reported significantly higher levels of anxiety than men surrounding the diagnosis [14].
discussion
About one-third of patients with melanoma have reported clinically significant levels of distress, which is in accordance with findings in other cancers. Distress was highest around the time of diagnosis and immediately post-treatment, and decreased over time [14, 18, 22]. Although increased levels of HRQOL impairment are associated with poor recovery and an increase in morbidity and maybe even disease progression [6, 8, 10, 13, 14, 17–21], relatively few studies have investigated HRQOL in melanoma patients. The reviewed studies focused mainly on populations from specialised (melanoma or cancer) centres that received additional therapy during short periods of time. The impact of melanoma on individuals from the general population who have survived this skin cancer for many years is not well documented.
Although the SF-36 is the most widely accepted generic HRQOL tool, its psychometric properties have not been tested in melanoma patients. A generic instrument allows for comparability between melanoma patients, other diseases and cancers, and the general population, as normative data exist for many countries. As generic instruments may not be very sensitive in detecting effects that are associated with specific diseases (e.g. sun avoidance behaviour in melanoma patients affects social functioning), the combination of a generic and specific HRQOL instrument is most informative [23].
FACT-M, which is a melanoma-specific HRQOL instrument that focuses on physical domains and was designed for clinical trial settings, has recently been validated in 273 melanoma patients and shown to be reliable, responsive and have a good construction and convergent validity [8, 20]. Because the majority of patients diagnosed with melanoma will most likely experience few limitations in their physical functioning and be much more affected mentally, the content of the FACT-M seems especially appropriate for patients with advanced melanomas and less for those from the general population. Fear of recurrence and changed lifestyles, due to UV avoidance, are likely to be important HRQOL domains that are not fully assessed in any of the available HRQOL instruments, which indicates a lack of content validity. Altogether, the selection of an appropriate HRQOL instrument remains a trade-off between the psychometric properties of the instrument, the study population and the research objectives of the study [23].
The reported effects of systemic treatment (e.g. interferon) on HRQOL may be influenced by the treated patient population. Patients who agree to participate in a clinical trial and/or receive therapy may be more motivated and optimistic, and thus be more likely to report benefit and endure more drug-induced toxicity compared with those not receiving systemic treatment. The latter issue is confirmed by the fact that some patients on interferon therapy, which is associated with severe flu-like symptoms, scored their HRQOL during treatment as good [12, 24].
In cancers that are treated with relatively non-aggressive therapies, have relatively good survival rates and have a high number of years lived with disability, patients’ perspectives are very important. In addition to clinical therapy, psychological surveillance after diagnosis and therapy may be needed, especially among patients with poor global health status, those with certain personality structures (e.g. maladaptive coping styles), and those without a social network [16, 19]. Although a pilot study indicated that a psycho-educational intervention might improve survival in melanoma patients, a large Danish RCT demonstrated no increased survival or recurrence-free interval among those who were offered six weekly 2-hour sessions of psycho-education compared with the control group [25, 26]. In contrast, a few studies have indicated that HRQOL impairment, psychological factors and personality structure may affect the survival rate of melanoma patients [16, 21]. Patients with a ‘type C’ personality (i.e. tending to be cooperative, unassertive, and repress negative emotions through anger-avoidance) may also be less likely to survive melanoma [16, 21].
conclusion
The proportion of patients with melanoma who report high levels of HRQOL impairment is comparable to that observed in other cancers. Optimal measurement of HRQOL in patients with melanoma is challenging because of the fairly unique characteristics of the tumour and its often straightforward treatment. More specific HRQOL measures could assist physicians in identifying patients who are in need of psychological counselling, which may affect treatment, follow-up and patients’ well-being.
conflict of interest disclosures
The authors declare no conflict of interest.
References
- 1.de Vries E, Houterman S, Janssen-Heijnen ML, et al. Up-to-date survival estimates and historical trends of cutaneous malignant melanoma in the south-east of The Netherlands. Ann Oncol. 2007;18:1110–1116. doi: 10.1093/annonc/mdm087. [DOI] [PubMed] [Google Scholar]
- 2.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107:119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 3.Karim-Kos HE, de Vries E, Soerjomataram I, et al. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Stam-Posthuma JJ, van Duinen C, Scheffer E, et al. Multiple primary melanomas. J Am Acad Dermatol. 2001;44:22–27. doi: 10.1067/mjd.2001.110878. [DOI] [PubMed] [Google Scholar]
- 5.De Vries E, Dore JF, Autier P, et al. Patients’ perception of the cause of their melanoma differs from that of epidemiologists. Br J Dermatol. 2002;147:388–389. doi: 10.1046/j.1365-2133.2002.48893.x. [DOI] [PubMed] [Google Scholar]
- 6.Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol. 2005;6:608–621. doi: 10.1016/S1470-2045(05)70283-7. [DOI] [PubMed] [Google Scholar]
- 7.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41:2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Cormier JN, Ross MI, Gershenwald JE, et al. Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer. 2008;112:2249–2257. doi: 10.1002/cncr.23424. [DOI] [PubMed] [Google Scholar]
- 9.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 10.Lehto US, Ojanen M, Dyba T, et al. Baseline psychosocial predictors of survival in localized melanoma. J Psychosom Res. 2007;63:9–15. doi: 10.1016/j.jpsychores.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer. 2006;94:492–498. doi: 10.1038/sj.bjc.6602973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rataj D, Jankowiak B, Krajewska-Kulak E, et al. Quality-of-life evaluation in an interferon therapy after radical surgery in cutaneous melanoma patients. Cancer Nurs. 2005;28:172–178. doi: 10.1097/00002820-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lehto US, Ojanen M, Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16:805–816. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 14.Al-Shakhli H, Harcourt D, Kenealy J. Psychological distress surrounding diagnosis of malignant and nonmalignant skin lesions at a pigmented lesion clinic. J Plast Reconstr Aesthet Surg. 2006;59:479–486. doi: 10.1016/j.bjps.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Trask PC, Griffith KA. The identification of empirically derived cancer patient subgroups using psychosocial variables. J Psychosom Res. 2004;57:287–295. doi: 10.1016/j.jpsychores.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Devine D, Parker PA, Fouladi RT, Cohen L. The association between social support, intrusive thoughts, avoidance, and adjustment following an experimental cancer treatment. Psychooncology. 2003;12:453–462. doi: 10.1002/pon.656. [DOI] [PubMed] [Google Scholar]
- 17.Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854–864. doi: 10.1002/cncr.11579. [DOI] [PubMed] [Google Scholar]
- 18.Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res. 2003;114:1–5. doi: 10.1016/s0022-4804(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 19.Trask PC, Paterson AG, Hayasaka S, et al. Psychosocial characteristics of individuals with non-stage IV melanoma. J Clin Oncol. 2001;19:2844–2850. doi: 10.1200/JCO.2001.19.11.2844. [DOI] [PubMed] [Google Scholar]
- 20.Cormier JN, Davidson L, Xing Y, et al. Measuring quality of life in patients with melanoma: development of the FACT-melanoma subscale. J Support Oncol. 2005;3:139–145. [PubMed] [Google Scholar]
- 21.Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;19:70–77. doi: 10.1053/sonu.2003.50006. [DOI] [PubMed] [Google Scholar]
- 22.Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152–159. doi: 10.1046/j.1087-0024.2003.09118.x. [DOI] [PubMed] [Google Scholar]
- 23.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- 24.Crott R. Cost effectiveness and cost utility of adjuvant interferon alpha in cutaneous melanoma: a review. Pharmacoeconomics. 2004;22:569–580. doi: 10.2165/00019053-200422090-00002. [DOI] [PubMed] [Google Scholar]
- 25.Boesen EH, Boesen SH, Frederiksen K, et al. Survival after a psychoeducational intervention for patients with cutaneous malignant melanoma: a replication study. J Clin Oncol. 2007;25:5698–5703. doi: 10.1200/JCO.2007.10.8894. [DOI] [PubMed] [Google Scholar]
- 26.Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]