Abstract
The American Joint Committee on Cancer (AJCC) staging of cutaneous melanoma is a continuously evolving system. The identification of increasingly more accurate prognostic factors has led to major changes in melanoma staging over the years, and the current system described in this review will likely be modified in the near future. Likewise, application of new imaging techniques has also changed the staging work-up of patients with cutaneous melanoma. Chest and abdominal computed tomography (CT) scanning is most commonly used for evaluation of potential metastatic sites in the lungs, lymph nodes and liver, and is indicated in patients with new symptoms, anaemia, elevated lactate dehydrogenase or a chest X-ray abnormality. CT scans should be restricted to patients with high-risk melanoma (stage IIC, IIIB, IIIC and stage IIIA with a macroscopic sentinel lymph node). Magnetic resonance imaging (MRI) of the brain is a mandatory test in patients with stage IV, optional in stage III and not used in patients with stage I and II disease. Positron emission tomography (PET)/CT is more accurate than CT or MRI alone in the diagnosis of metastases and should complement conventional CT/MRI imaging in the staging work-up of patients who have solitary or oligometastatic disease where surgical resection is most relevant.
Keywords: follow-up, imaging, melanoma, staging system, staging work-up
the current AJCC staging system
The AJCC staging system is based on evaluation of the primary tumour (T) and the presence or absence of regional lymphatic (N) and distant metastases (M). The system separates patients into four groups (Tables 1 and 2).
Table 1.
TNM classification of cutaneous melanoma (adapted from [1])
| Tumour (T) classification | |
| TX | Primary tumour cannot be assessed (e.g. shave biopsy, regressed primary) |
| Tis | Melanoma in situ |
| T1 | ≤1.00 mm |
| T1a | Without ulceration or level II/III |
| T1b | With ulceration or level IV or V |
| T2 | 1.01–2.00 mm |
| T2a | Without ulceration |
| T2b | With ulceration |
| T3 | 2.01–4.00 mm |
| T3a | Without ulceration |
| T3b | With ulceration |
| T4 | >4.00 mm |
| T4a | Without ulceration |
| T4b | With ulceration |
| Node (N) classification | |
| N1 | One lymph node |
| N1a | Micrometastasesa (clinically occult) |
| N1b | Macrometastasesb (clinically apparent) |
| N2 | Two to three lymph nodes |
| N2a | Micrometastasesa |
| N2b | Macrometastasesb |
| N2c | In-transit met(s)/satellite(s) without metastatic lymph nodes |
| N3 | Four or more lymph nodes, metastatic or matted, or in-transit met(s)/satellite(s) with metastatic lymph node(s) |
| Metastasis (M) classification | |
| M1a | Distant skin, subcutaneous or lymph node metastases, normal LDH |
| M1b | Lung metastases, normal LDH |
| M1c | All other visceral metastases, normal LDH; any distant metastases, elevated LDH |
Micrometastases are diagnosed after elective or sentinel lymphadenectomy.
Macrometastases are clinically detectable lymph node metastases confirmed by therapeutic lymphadenectomy, or lymph node metastases exhibiting gross extracapsular extension.
Table 2.
AJCC 2002 stage groupings for cutaneous melanoma (adapted from [1])
| Stage | Clinical stage grouping |
Pathologic stage grouping |
||||
| 0 | Tis | N0 | M0 | pTis | N0 | M0 |
| IA | T1a | N0 | M0 | pT1a | N0 | M0 |
| IB | T1b | N0 | M0 | pT1b | N0 | M0 |
| T2a | N0 | M0 | pT2a | N0 | M0 | |
| IIA | T2b | N0 | M0 | pT2b | N0 | M0 |
| T3a | N0 | M0 | pT3a | N0 | M0 | |
| IIB | T3b | N0 | M0 | pT3b | N0 | M0 |
| T4a | N0 | M0 | pT4a | N0 | M0 | |
| IIC | T4b | N0 | M0 | pT4b | N0 | M0 |
| III | Any T | N1–3 | M0 | |||
| IIIA | pT1–4a | N1a | M0 | |||
| pT1–4a | N2a | M0 | ||||
| IIIB | pT1–4b | N1a | M0 | |||
| pT1–4b | N2a | M0 | ||||
| pT1–4a | N1b | M0 | ||||
| pT1–4a | N2b | M0 | ||||
| pT1–4a/b | N2c | M0 | ||||
| pT1–4b | N1b | M0 | ||||
| IIIC | pT1–4b | N2b | M0 | |||
| Any T | N3 | M0 | ||||
| IV | Any T | Any N | M1 | Any T | Any N | M1 |
Stage I is limited to patients with low-risk primary melanomas without evidence of regional or distant metastases, and is divided into stages IA and IB. Stage IA includes primary lesions ≤1 mm thick, without ulceration of the overlying epithelium or invasion of the reticular dermis or subcutaneous fat (Clark level IV or V). Stage IB includes primary lesions ≤1 mm thick with epithelial ulceration or invasion into Clark level IV or V. It also includes primary lesions ≥1 mm and ≤2 mm thick without ulceration or invasion into Clark level IV or V.
Stage II includes high-risk primary tumours, without evidence of lymphatic disease or distant metastases, and is divided into three subcategories. Stage IIA includes lesions >1 mm and ≤2 mm thick with ulceration of the overlying epithelium and those >2 mm and ≤4 mm thick without epithelial ulceration. Stage IIB lesions are >2 mm and ≤4 mm thick with epithelial ulceration or >4 mm without ulceration. Stage IIC consists of primary lesions >4 mm with epithelial ulceration.
Stage III includes lesions with pathologically documented involvement of regional lymph nodes or the presence of in-transit or satellite metastases. Patients with one, two to three, or four or more affected lymph nodes are classified as having N1, N2 and N3 disease, respectively (Tables 1 and 2). Patients with in-transit or satellite metastases are classified as having N2 disease if lymph node involvement is not present, and as having N3 disease if lymph node involvement is present. In addition, microscopic versus macroscopic lymph node involvement is further subdivided as, for example, N1a versus N1b category, respectively. The presence of ulceration in the primary tumour remains an independent negative prognostic factor among patients with stage III disease, particularly those with microscopically involved nodes. Using these parameters, patients with stage III disease are divided into three subcategories: (a) stage IIIA includes patients with one to three microscopically involved lymph nodes (N1a or N2a) and with a non-ulcerated primary tumour; (b) stage IIIB includes patients with one to three microscopically involved lymph nodes (N1a or N2a) and with an ulcerated primary tumour, or patients with one to three macroscopically involved lymph nodes (N1b or N2b) and with a non-ulcerated primary tumour, or patients with in-transit and/or satellites without metastatic lymph nodes (N2c); (c) stage IIIC includes patients with four or more affected lymph nodes, matted lymph nodes or the presence of in-transit metastases or satellite lesions in conjunction with lymph node involvement (N3), or patients with one to three macroscopically involved lymph nodes (N2b) and with an ulcerated primary tumour. Patients with clinical evidence of regional lymph node metastasis without a full regional lymph node dissection are classified as clinical stage III, and no further staging is applied.
Stage IV is defined by the presence of distant metastases, and patients are divided into three subcategories based on the metastasis location: M1a is limited to distant skin, subcutaneous tissues or lymph nodes; M1b involves lung metastases; and M1c involves all other visceral sites. In addition, the presence of elevated serum lactate dehydrogenase (LDH) in conjunction with any distant metastasis classifies as M1c disease.
evolution of the staging system
The key new features of the current 2002 AJCC staging system when compared with the previous 1997 system include the following: (a) tumour thickness rather than level of invasion as a primary determinant for staging; (b) ulceration of the primary tumour as a highly significant and independent negative prognostic factor; (c) grouping together satellite and in-transit metastases as manifestations of lymphatic involvement rather than as an extension of the primary tumour; (d) number of lymph node metastases as a more reliable and reproducible predictor of prognosis than the size of the involved lymph nodes—the number of lymph node metastases is used to divide patients with stage III disease, and lymph node involvement is further subdivided into micro- or macrometastatic; (e) separation of lung metastases from other visceral sites of involvement, based on an observed longer survival; and (f) elevated serum LDH as a negative prognostic factor for patients with metastatic disease.
The AJCC Staging Task Force meets regularly to revise the staging system; the committee recently discussed the incorporation of mitotic rate, an important prognostic factor particularly in thin lesions, into the staging criteria. This parameter will likely be incorporated into the next staging system.
imaging techniques and laboratory investigations in staging work-up
Since the prognosis for patients with melanoma is determined by histology of the primary tumour and by the presence and extent of metastatic disease, imaging studies using radiographic and nuclear medicine techniques are an important component of the evaluation of patients with both localised and advanced melanoma (Table 3). However, the staging evaluations used at the time of initial diagnosis are often excessive. Few established guidelines for melanoma define the appropriate tests for the initial evaluation and subsequent follow-up.
Table 3.
Common practices for stage-specific and survival-related stage work-up
| Pathologic stage | ||||||||||
| 0 | I | II | III | IV | ||||||
| A | B | A | B | C | A | B | C | |||
| Ultrasound nodal basin | E | E | E | E | E | E | E | |||
| Chest X-ray | E | E | E | E | W | W | W | W | ||
| Ultrasound abdomen | E | E | E | E | E | E | E | E | ||
| LDH and S100B | E | E | E | E | E | E | E | W | ||
| CT chest, abdomen, pelvis | E | E | W | W | W | |||||
| Brain MRI | W | W | W | |||||||
| PET or PET/CT | E | E | R | |||||||
computed tomography scanning
Besides skin, subcutaneous tissue and lymph nodes, the lung is the most common site of melanoma metastases. Contrast-enhanced computed tomography (CT) scanning is the most reliable radiographic method for evaluating intrathoracic metastases [4–6]. CT also is superior to a chest X-ray in demonstrating the mediastinal and hilar adenopathy that often accompanies parenchymal lesions and/or the presence of lymphatic spread. CT is more specific than plain radiographs in the evaluation of lesions in the bone, and is particularly useful for the detection of purely lytic lesions not apparent by radionuclide bone scan [7]. CT has similar specificity to ultrasound for the detection of abdominal metastases, but its sensitivity is better (85% compared with 57%) [8].
magnetic resonance imaging
While CT and ultrasound are the preferential procedures for imaging the liver, magnetic resonance imaging (MRI) may be useful to differentiate between benign lesions such as haemangiomas and melanoma metastases [9–11]. MRI is also superior in delineation of vascular involvement and identification of additional hepatic lesions. It is the best modality for evaluating signal abnormalities in bone marrow and detecting accompanying features such as haemorrhage or soft-tissue masses but has relatively low specificity. MRI is significantly more sensitive than CT for the detection of metastatic disease to the brain [12], and also provides more detailed information about possible involvement of the spinal cord and leptomeninges.
positron emission tomography scan and PET/CT
The role of positron emission tomography (PET) and PET/CT in the management of patients with melanoma is rapidly evolving. PET/CT appears to be superior to stand-alone PET [13–16], and is rapidly replacing the use of PET alone for the detection of metastatic disease.
In patients with advanced melanoma, PET/CT is more sensitive than anatomic imaging modalities, such as CT or MRI, and at least equally specific [17–22]. Sensitivity is highest (≥90%) for metastases that are >1 cm in diameter, but tumour deposits as small as 0.6 cm can be reliably seen in areas of low background activity. In a retrospective study with 250 patients, PET/CT scans detected significantly more visceral and non-visceral metastases than either PET alone or CT alone (98.7%, 88.8% and 69.7%, respectively) [21]. In that series, PET/CT permitted more accurate staging of distant metastases than either PET or CT alone (98% compared with 93% and 84%, respectively); PET/CT was also more accurate than CT for staging regional lymph nodes (98% compared with 86%).
Fluorodeoxyglucose (FDG)-PET imaging alone has a lower sensitivity than PET/CT (43% compared with 97%) and is less sensitive for the detection of melanoma metastases in the liver and brain compared with that in other sites [17, 22, 23].
New data indicate that PET/CT may be the most accurate test for the diagnosis of bone metastases [7, 24, 25]. Overall, PET/CT is more accurate than CT or MRI alone in the diagnosis of metastasis, but there is no convincing evidence that the improved accuracy of PET/CT over CT scanning alone improves patient outcome [26]. Unfortunately, PET and PET/CT are not sufficiently available or reimbursed in many countries.
lymph node ultrasound
Nodal metastases are frequently the initial manifestation of metastatic spread [27]. Complete and accurate determination of nodal status is important not only for prognosis but also for decision-making regarding sentinel lymph node biopsy (SNB) and adjuvant therapy.
Physical examination is an inaccurate predictor of nodal metastases: 20% of clinically node-negative patients have metastatic deposits, whereas 20% of those who are clinically node-positive have pathologically negative nodes. Furthermore, CT scanning is insensitive to the presence of small nodal metastases and also is complicated by false-positive results [28].
Several studies show the benefit of nodal ultrasound as compared with palpation and in combination with fine needle aspiration cytology (FNAC) [29, 30]. Ultrasound is routinely used, especially in some European countries, in the staging work-up before SNB and during follow-up. Sensitivity for the detection of a positive sentinel node by ultrasound before a surgical procedure ranges between 39% and 79%, and specificity is ∼100%. In patients with a positive finding by cytology, SNB can be avoided by directly performing therapeutic lymph node dissection.
lymphoscintigraphy and SNB
The pattern of tumour cell spread to sentinel lymph nodes (SLNs) can be most accurately demonstrated by a combination of lymphoscintigraphy and SNB. When performed correctly, the SNB can provide important information that directly influences patient management and prognosis [31–36]. Besides histopathology of the primary melanoma, SNB is the most important staging tool. In experienced hands, the false-negative rate for the SLN procedures is typically ≤5% [37, 38]. Problems are often encountered with head and neck lesions where the detection rate is lower.
bone scintigraphy
Bony metastatic disease may appear on a plain radiograph [39]. Although most metastases are lytic, the activity of osteoblasts at the site of a bony metastasis can often be detected by bone scintigraphy months before changes are seen on plain radiographs [40].
Despite its sensitivity, bone scanning is not routinely ordered in patients with primary melanoma unless symptoms indicating bone metastases are present. The low specificity of this technique, combined with the low incidence of metastases to the bone early in the disease, results in an unacceptable number of false-positive findings.
If bone pain develops in a patient with melanoma and plain radiographs are negative, the bone scan offers a sensitive test to help rule out metastatic disease.
laboratory investigations
LDH and S100B have been found more valuable than any other blood serum markers. LDH has been widely accepted as a prognostic marker in stage IV and was reported to be suitable to detect liver metastases, although its increased concentration is not very specific for that kind of metastasis. Several authors found increased serum LDH in most patients with progressive distant metastases. An increased concentration of LDH was found to be a first sign of metastases in 12% of patients [41]. However, an increase in LDH can be caused by many other factors, and, in general, determination of LDH has low sensitivity and specificity for the detection of distant melanoma metastases.
S100B is found in primary melanoma and is positively correlated with the invasiveness of the tumour. It has been shown that S100B levels correlate with clinical staging, the extent of metastatic spread and disease progression [42, 43]. The value of measuring serum S100B for staging work-up and follow-up is controversial, especially in the early stages of melanoma, as its sensitivity ranges between 30% and 60% and depends on the stage of the disease [44]. The analysis of both serum parameters is fairly inexpensive when compared with most imaging techniques.
staging work-up in different clinical stages
stage I and IIA/B
Melanoma has the potential to metastasise to any organ: common sites of dissemination include the skin, subcutaneous tissues, lymph nodes, liver, bone, lung, brain and visceral organs. Because of this metastatic potential, patients with localised (stage I and II) primary melanoma commonly undergo unnecessary, extensive radiological evaluations searching for distant metastases.
A routine physical examination, including a full skin assessment and palpation of regional lymph nodes, and taking the patient's history is the basis of every staging work-up in cutaneous melanoma. Palpation and patient self-examination remain the most important tools in the detection of locoregional metastases. Up to 70% of first recurrences of primary melanomas are locoregional [45], and most imaging techniques do not evaluate the regional area very well.
Asymptomatic patients with clinical stage I or II lesions should be differentiated based on their risk of relapse. In patients with a lesion <1.0 mm thick, an imaging work-up is not recommended, since the cure rate is >90% and the likelihood of detecting asymptomatic metastases at the time of primary diagnosis is virtually zero. Most imaging studies are not indicated even in patients with primary melanoma thicker than 1.0 mm, since the detection of distant metastases is also rare. In these patients, SNB is the most important staging tool and should be performed routinely [1].
Although a chest X-ray is routinely ordered in patients with stage I and II melanoma, it serves primarily as baseline information for future comparison as the detection yield of metastatic disease is negligible. For instance, in a series of 876 asymptomatic patients in stage I/II, only one patient (0.1%) had a true-positive chest film demonstrating pulmonary metastases; in contrast, false-positive tests occurred in ∼15% of cases [46].
Despite the aggressive nature of melanoma and the potential for early disease dissemination, extensive imaging (e.g. CT of the chest, abdomen, pelvis and brain) of asymptomatic patients with stage I or II melanoma is usually not indicated, since the detection rate is low and the frequency of false-positive findings is unacceptably high (10–20%) [25, 28, 47].
Recent studies using ultrasound of the regional lymph node basin showed convincingly that this technique is an inexpensive tool for detection of early lymph node metastasis [48]. We recommend the inclusion of lymph node ultrasound in staging investigations, which offers the opportunity to perform ultrasound-guided FNAC for cytological evaluation. Since physical examination is an inaccurate predictor of nodal metastases and 20% of clinically node-negative patients have metastatic deposits, SNB can be avoided in patients with suspicious nodal lesions confirmed by FNAC [29, 30]. These patients should directly undergo complete lymph node dissection.
PET has not been useful in the staging work-up of patients with stage I and II disease due to its low sensitivity for regional lymphatic disease and occult metastases [49–51]. In addition, there is a relatively high rate of false-positive examinations. In ∼1% of patients, PET may detect an incidental second primary tumour [52].
Serum S100B and LDH have limited sensitivity and specificity, especially in the early stages of melanoma. These blood tests may provide baseline information for patients with primary melanoma >1.0 mm Breslow depth.
Patients with a melanoma thicker than 4.0 mm and with an ulcerated primary tumour (stage IIC) have an unfavourable outcome with a median 5-year survival of ∼40%, which is lower than that of patients with stage IIIA disease. We recommend a similar staging work-up for these patients to that for those with stage IIIB melanoma.
stage IIC and III
A similarly poor clinical outcome is observed for stages IIC and III. Patients with positive SLNs often undergo comprehensive imaging evaluation before completing lymphadenectomy, or following surgery when considered for an adjuvant therapy or a trial. However, despite the frequent use of such studies, routine staging with CT, MRI or PET does not appear to be useful in this setting, as the techniques usually do not show early metastatic disease. The low frequency of detectable metastases found with extensive imaging is illustrated by three large, single-institution series; in these trials, the detection rate of occult metastases with chest CT, abdomen CT, or brain CT or MRI was between 0.5% and 3.7%. All metastasis-positive patients had macrometastatic disease in the sentinel node and thick, or thick and ulcerated primary lesions. If CT imaging had been restricted to those patients, the true-positive rate would have been up to 13%. The false-positive rate was up to 12% [47, 53, 54]. This indicates that, for the purpose of a staging work-up, patients with positive SLNs should be further differentiated into a group with macroscopic nodal disease and a group with only microscopic disease that should not undergo an extensive imaging work-up.
Patients with stage IIC and III melanoma, including those with local, satellite, in-transit or macroscopic nodal disease, have a >50% risk of systemic recurrence [55]. For these patients, a complete blood analysis, serum LDH and chest X-ray for future reference should be obtained. Although advanced imaging studies (CT, MRI, PET) have a relatively low yield for detecting distant metastases in asymptomatic stage IIC and III patients, metastases are detected more frequently than in those with stage I and II disease, indicating that additional imaging studies are warranted. Patients who develop a second locoregional recurrence represent a higher risk subset and should undergo staging evaluations as if they had distant metastases.
CT imaging of the chest and abdomen is commonly performed in patients with locoregional disease. Although the yield of these tests (particularly abdominal CT) in detecting distant metastases in asymptomatic patients is low, they often identify false-positive abnormalities and thereby function as an important baseline for future studies in this high-risk population. CT scanning of the pelvis is generally performed for patients with a locoregional recurrence below the waist [56, 57], and the neck is generally imaged for a recurrence in the head and neck region. Nodal ultrasound is an alternative to CT scanning of the head and neck region.
The necessity of performing routine brain imaging in asymptomatic patients with advanced locoregional disease is controversial. Some clinicians perform the procedure only in symptomatic patients to rule out central nervous system (CNS) involvement, while others recommend brain imaging before definitive local therapy in accordance with a report showing a rate of asymptomatic CNS metastases as high as 6% [58].
PET/CT is widely used at many centres in the USA, and the role of PET/CT in clinical stage III melanoma is rapidly evolving [59]. However, unresolved issues remain regarding the optimal technique (use of p.o. or i.v. contrast, respiratory gating). Like CT or PET alone, the interpretation of PET/CT is complicated by false-negatives, false-positives and the identification of second primaries. No studies assess the value of integrated PET/CT imaging in patients with the disease limited to a positive sentinel node. PET/CT may be particularly valuable for patients with regionally advanced disease, where identification of additional regional disease would alter planned surgical strategy. In addition, PET/CT may be used for evaluation of suspicious or equivocal findings identified by conventional imaging.
The sensitivity of serum S100B in patients with stage III and IV melanoma ranges between 30% and 60%, while the specificity is ∼90%. Therefore, baseline examination of LDH and S100B may be valuable in that group.
clinical stage IV melanoma
Detection of early stage IV metastasis plays a role in palliative care but has not been associated with a better treatment outcome. Patients with known systemic metastases (stage IV) should be evaluated more comprehensively because the likelihood of detecting additional asymptomatic lesions is high. These patients should be staged with MRI of the brain and CT of the chest and abdomen. CT of the pelvis is indicated for patients with a history of primary tumours below the waist or with symptoms indicating metastatic involvement. Other imaging studies should be ordered based on symptoms (e.g. bone scan for patients with bone pain, small-bowel follow-through for patients with anaemia indicating iron-deficiency). Serum LDH should be determined in all patients as it carries prognostic significance (Table 1).
PET scans often show a greater sensitivity for the detection of metastases when compared with conventional radiographic studies [17, 21, 60]. In a series of 100 patients with stage IV disease, 415 metastatic lesions were evaluated with PET and routine CT scans [60]. The PET scan detected 93% of lesions and, in 20 patients, it detected 24 metastases up to 6 months earlier than conventional imaging or physical examination [60]. However, PET without concurrent CT may only complement routine imaging studies rather than replace them. Therefore, we generally recommend complementing conventional CT/MRI imaging with integrated PET/CT in the staging work-up of patients who have solitary or oligometastatic disease where the issue of surgical resection is most relevant.
conclusion
Melanoma can metastasise to a number of sites throughout the body, and the route and pattern of metastatic disease is unpredictable. The AJCC staging system provides the basis for identification of the risk of relapse and a guide to patient management. The AJCC Staging Task Force meets regularly to revise the staging system and to make it more accurate; mitotic rate will likely be incorporated into the staging system in the near future.
Since up to 70% of the first recurrence is found in the locoregional area, routine physical examination, including full skin assessment and palpation of regional lymph nodes, remains the most important tool to detect locoregional metastases. In several countries in Europe, ultrasound of the nodal basin and abdominal ultrasound are part of the routine staging work-up. However, more data are needed to prove the value of these studies.
Lymphoscintigraphy and SNB are integral components of primary management and staging for patients with cutaneous melanoma >1.0 mm thick who are clinically node negative. Ultrasound of the nodal basin in combination with fine needle aspiration cytology before SNB can avoid SNB by sending the patient directly to radical lymph node dissection in the case of a positive cytological finding.
Investigations that include cost–efficacy analyses need to be carried out to formally prove the benefit of modern imaging approaches. Chest and abdominal CT scanning is most commonly used for evaluation of potential metastatic sites in the lungs, lymph nodes and liver, and is indicated in any patient with new symptoms, physical findings, anaemia, elevated LDH or a chest X-ray abnormality. CT scans should be restricted to patients with high-risk melanoma (stage IIC, IIIB, IIIC and stage IIIA with a macroscopic SLN).
MRI of the brain is the procedure of choice for symptoms related to the CNS. It is a mandatory test in patients with stage IV, optional in patients with stage III and not recommended in patients with stage I and II disease. Although early detection of brain metastases may identify a limited number of patients who are eligible for more aggressive local therapies, no available data demonstrate that screening for brain metastases results in a survival benefit for patients.
We recommend that bone scintigraphy should not be part of a routine stage work-up unless there is clinical suspicion of bone metastases.
The role of PET/CT in the evaluation and management of patients with advanced locoregional or metastatic disease is evolving rapidly. Overall, PET/CT is more accurate than CT or MRI alone in the diagnosis of metastases. PET/CT is most useful for identifying all metastatic sites of disease before embarking on a metastasectomy of an apparently isolated lesion, or for clarifying the nature of a suspicious lesion identified by the CT scan.
conflict of interest disclosures
The authors declare no conflict of interest.
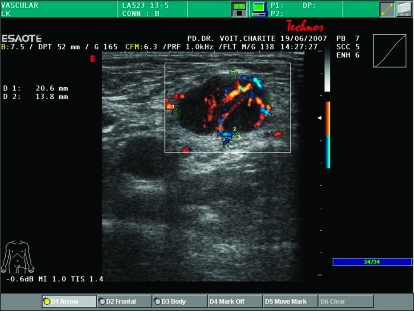
Figure 1.
Ultrasound image of a metastatic lymph node. The lesion reveals the typical appearance of a lymph node metastasis during follow-up showing a balloon shape, and irregular and mostly peripherally located hyper-perfusion corresponding to neo-angiogenesis. The suspicious lymph node can easily be evaluated with FNAC.
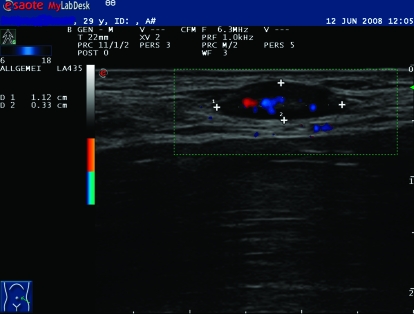
Figure 2.
Ultrasound of a benign lymph node. Both oval-shaped lesions show an enlarged but normal reactive lymph node with central echoes in B-mode and peripheral lack of echoes according to the parenchyma and centrally located perfusion. These lesions can safely be validated by ultrasound alone.
Acknowledgments
We thank Christiane Voit, Department of Dermatology at Charité Berlin, for ultrasound images of the nodal basin, and Pavel Kramata, ScienceFirst, LLC, Cedar Knolls, NJ 07927, USA, for writing support and coordination during preparation of the manuscript.
References
- 1.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Natl Compr Canc Netw. 2006;4:666–684. [Google Scholar]
- 2.Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393–399. doi: 10.1097/CMR.0b013e3282f05039. [DOI] [PubMed] [Google Scholar]
- 3.Dummer R, Panizzon R, Bloch PH, Burg G. Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Dermatology. 2005;210:39–44. doi: 10.1159/000081482. [DOI] [PubMed] [Google Scholar]
- 4.Davis SD. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology. 1991;180:1–12. doi: 10.1148/radiology.180.1.2052672. [DOI] [PubMed] [Google Scholar]
- 5.Heaston DK, Putman CE, Rodan BA, et al. Solitary pulmonary metastases in high-risk melanoma patients: a prospective comparison of conventional and computed tomography. AJR Am J Roentgenol. 1983;141:169–174. doi: 10.2214/ajr.141.1.169. [DOI] [PubMed] [Google Scholar]
- 6.Kostrubiak I, Whitley NO, Aisner J, et al. The use of computed body tomography in malignant melanoma. JAMA. 1988;259:2896–2897. [PubMed] [Google Scholar]
- 7.Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:229–236. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann PM, Crone-Munzebrock W. Tumor follow-up using sonography and computed tomography in the abdominal region of patients with malignant melanoma. Aktuelle Radiol. 1992;2:81–85. [PubMed] [Google Scholar]
- 9.Ferrucci JT. Liver tumor imaging: current concepts. AJR Am J Roentgenol. 1990;155:473–484. doi: 10.2214/ajr.155.3.2117343. [DOI] [PubMed] [Google Scholar]
- 10.Paley MR, Ros PR. Hepatic metastases. Radiol Clin North Am. 1998;36:349–363. doi: 10.1016/s0033-8389(05)70027-0. [DOI] [PubMed] [Google Scholar]
- 11.Ghanem N, Altehoefer C, Hogerle S, et al. Detectability of liver metastases in malignant melanoma: prospective comparison of magnetic resonance imaging and positron emission tomography. Eur J Radiol. 2005;54:264–270. doi: 10.1016/j.ejrad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Yock DH. Magnetic Resonance Imaging of CNS. Mosby Yearbook. 1995 [Google Scholar]
- 13.Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–1023. doi: 10.1016/j.athoracsur.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 14.Halpern BS, Schiepers C, Weber WA, et al. Presurgical staging of non-small cell lung cancer: positron emission tomography, integrated positron emission tomography/CT, and software image fusion. Chest. 2005;128:2289–2297. doi: 10.1378/chest.128.4.2289. [DOI] [PubMed] [Google Scholar]
- 15.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 16.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 17.Holder WD, Jr, White RL, Jr, Zuger JH, et al. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–769. doi: 10.1097/00000658-199805000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gritters LS, Francis IR, Zasadny KR, Wahl RL. Initial assessment of positron emission tomography using 2-fluorine-18-fluoro-2-deoxy-D-glucose in the imaging of malignant melanoma. J Nucl Med. 1993;34:1420–1427. [PubMed] [Google Scholar]
- 19.Paquet P, Hustinx R, Rigo P, Pierard GE. Malignant melanoma staging using whole-body positron emission tomography. Melanoma Res. 1998;8:59–62. doi: 10.1097/00008390-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Rinne D, Baum RP, Hor G, Kaufmann R. Primary staging and follow-up of high risk melanoma patients with whole-body 18F-fluorodeoxyglucose positron emission tomography: results of a prospective study of 100 patients. Cancer. 1998;82:1664–1671. doi: 10.1002/(sici)1097-0142(19980501)82:9<1664::aid-cncr11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Steinert HC, Huch Boni RA, Buck A, et al. Malignant melanoma: staging with whole-body positron emission tomography and 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;195:705–709. doi: 10.1148/radiology.195.3.7753998. [DOI] [PubMed] [Google Scholar]
- 22.Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–653. doi: 10.1007/BF02574480. [DOI] [PubMed] [Google Scholar]
- 23.Tyler DS, Onaitis M, Kherani A, et al. Positron emission tomography scanning in malignant melanoma. Cancer. 2000;89:1019–1025. [PubMed] [Google Scholar]
- 24.Aydin A, Yu JQ, Zhuang H, Alavi A. Detection of bone marrow metastases by FDG-PET and missed by bone scintigraphy in widespread melanoma. Clin Nucl Med. 2005;30:606–607. doi: 10.1097/01.rlu.0000174200.67064.28. [DOI] [PubMed] [Google Scholar]
- 25.Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4:252–258. doi: 10.1007/BF02306618. [DOI] [PubMed] [Google Scholar]
- 26.Iagaru A, Quon A, Johnson D, et al. 2-Deoxy-2-[F-18]fluoro-D-glucose positron emission tomography/computed tomography in the management of melanoma. Mol Imaging Biol. 2007;9:50–57. doi: 10.1007/s11307-006-0065-0. [DOI] [PubMed] [Google Scholar]
- 27.Balch CM, Soong SJ, Shaw H, et al. An analysis of prognostic factors in 4000 patients with cutaneous melanoma. In: Balch C, Milton G, editors. Cutaneous Melanoma. Philadelphia: JB Lippincott; 1985. p. 321. [Google Scholar]
- 28.Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11:638–643. doi: 10.1200/JCO.1993.11.4.638. [DOI] [PubMed] [Google Scholar]
- 29.Rossi CR, Mocellin S, Scagnet B, et al. The role of preoperative ultrasound scan in detecting lymph node metastasis before sentinel node biopsy in melanoma patients. J Surg Oncol. 2003;83:80–84. doi: 10.1002/jso.10248. [DOI] [PubMed] [Google Scholar]
- 30.Voit CA, van Akkooi AC, Schäfer-Hesterberg G, et al. Role of ultrasound (US) and US-guided fine needle aspiration cytology (US-FNAC) prior to sentinel lymph node biopsy (SLNB) in 500 melanoma patients: reduction of need for SNLB by high US-FNAC SN positive identification rate. 2007 ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007:25. Abstr 8512. [Google Scholar]
- 31.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–466. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Dufresne EN, Kaplan WD, Zimmerman RE, Rose CM. The application of internal mammary lymphoscintigraphy to planning of radiation therapy. J Nucl Med. 1980;21:697–699. [PubMed] [Google Scholar]
- 33.Jones D, Hanelin L, Christopherson D, et al. Radiotherapy treatment planning using lymphoscintigraphy. Int J Radiat Oncol Biol Phys. 1986;12:1707–1710. doi: 10.1016/0360-3016(86)90300-7. [DOI] [PubMed] [Google Scholar]
- 34.Siddon RL, Chin LM, Zimmerman RE, et al. Utilization of parasternal lymphoscintigraphy in radiation therapy of breast carcinoma. Int J Radiat Oncol Biol Phys. 1982;8:1059–1063. doi: 10.1016/0360-3016(82)90178-x. [DOI] [PubMed] [Google Scholar]
- 35.Campisi C, Boccardo F. Role of microsurgery in the management of lymphoedema. Int Angiol. 1999;18:47–51. [PubMed] [Google Scholar]
- 36.Gannon CJ, Rousseau DL, Jr, Ross MI, et al. Accuracy of lymphatic mapping and sentinel lymph node biopsy after previous wide local excision in patients with primary melanoma. Cancer. 2006;107:2647–2652. doi: 10.1002/cncr.22320. [DOI] [PubMed] [Google Scholar]
- 37.Uren RF, Howman-Giles RB, Shaw HM, et al. Lymphoscintigraphy in high-risk melanoma of the trunk: predicting draining node groups, defining lymphatic channels and locating the sentinel node. J Nucl Med. 1993;34:1435–1440. [PubMed] [Google Scholar]
- 38.Patten RM, Shuman WP, Teefey S. Subcutaneous metastases from malignant melanoma: prevalence and findings on CT. AJR Am J Roentgenol. 1989;152:1009–1012. doi: 10.2214/ajr.152.5.1009. [DOI] [PubMed] [Google Scholar]
- 39.Patten RM, Shuman WP, Teefey S. Metastases from malignant melanoma to the axial skeleton: a CT study of frequency and appearance. AJR Am J Roentgenol. 1990;155:109–112. doi: 10.2214/ajr.155.1.2112830. [DOI] [PubMed] [Google Scholar]
- 40.Fon GT, Wong WS, Gold RH, Kaiser LR. Skeletal metastases of melanoma: radiographic, scintigraphic, and clinical review. AJR Am J Roentgenol. 1981;137:103–108. doi: 10.2214/ajr.137.1.103. [DOI] [PubMed] [Google Scholar]
- 41.Finck SJ, Giuliano AE, Morton DL. LDH and melanoma. Cancer. 1983;51:840–843. doi: 10.1002/1097-0142(19830301)51:5<840::aid-cncr2820510516>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Buer J, Probst M, Franzke A, et al. Elevated serum levels of S100 and survival in metastatic malignant melanoma. Br J Cancer. 1997;75:1373–1376. doi: 10.1038/bjc.1997.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauschild A, Michaelsen J, Brenner W, et al. Prognostic significance of serum S100B detection compared with routine blood parameters in advanced metastatic melanoma patients. Melanoma Res. 1999;9:155–161. doi: 10.1097/00008390-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Banfalvi T, Boldizsar M, Gergye M, et al. Comparison of prognostic significance of serum 5-S-cysteinyldopa, LDH and S-100B protein in stage III-IV malignant melanoma. Pathol Oncol Res. 2002;8:183–187. doi: 10.1007/BF03032392. [DOI] [PubMed] [Google Scholar]
- 45.Haffner AC, Garbe C, Burg G, et al. The prognosis of primary and metastasising melanoma. An evaluation of the TNM classification in 2,495 patients. Br J Cancer. 1992;66:856–861. doi: 10.1038/bjc.1992.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 47.Aloia TA, Gershenwald JE, Andtbacka RH, et al. Utility of computed tomography and magnetic resonance imaging staging before completion lymphadenectomy in patients with sentinel lymph node-positive melanoma. J Clin Oncol. 2006;24:2858–2865. doi: 10.1200/JCO.2006.05.6176. [DOI] [PubMed] [Google Scholar]
- 48.Blum A, Schlagenhauff B, Stroebel W, et al. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma: results of a prospective study of 1288 patients. Cancer. 2000;88:2534–2539. doi: 10.1002/1097-0142(20000601)88:11<2534::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Bastiaannet E, Oyen WJ, Meijer S, et al. Impact of [18F]fluorodeoxyglucose positron emission tomography on surgical management of melanoma patients. Br J Surg. 2006;93:243–249. doi: 10.1002/bjs.5174. [DOI] [PubMed] [Google Scholar]
- 50.Wagner JD, Schauwecker D, Davidson D, et al. Inefficacy of F-18 fluorodeoxy-D-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer. 2005;104:570–579. doi: 10.1002/cncr.21189. [DOI] [PubMed] [Google Scholar]
- 51.Fink AM, Holle-Robatsch S, Herzog N, et al. Positron emission tomography is not useful in detecting metastasis in the sentinel lymph node in patients with primary malignant melanoma stage I and II. Melanoma Res. 2004;14:141–145. doi: 10.1097/00008390-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752–757. [PubMed] [Google Scholar]
- 53.Miranda EP, Gertner M, Wall J, et al. Routine imaging of asymptomatic melanoma patients with metastasis to sentinel lymph nodes rarely identifies systemic disease. Arch Surg. 2004;139:831–837. doi: 10.1001/archsurg.139.8.831. [DOI] [PubMed] [Google Scholar]
- 54.Gold JS, Jaques DP, Busam KJ, et al. Yield and predictors of radiologic studies for identifying distant metastases in melanoma patients with a positive sentinel lymph node biopsy. Ann Surg Oncol. 2007;14:2133–2140. doi: 10.1245/s10434-007-9399-3. [DOI] [PubMed] [Google Scholar]
- 55.Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15:1039–1051. doi: 10.1200/JCO.1997.15.3.1039. [DOI] [PubMed] [Google Scholar]
- 56.Shirkhoda A, Albin J. Malignant melanoma: correlating abdominal and pelvic CT with clinical staging. Radiology. 1987;165:75–78. doi: 10.1148/radiology.165.1.3628793. [DOI] [PubMed] [Google Scholar]
- 57.Buzaid AC, Tinoco L, Ross MI, et al. Role of computed tomography in the staging of patients with local-regional metastases of melanoma. J Clin Oncol. 1995;13:2104–2108. doi: 10.1200/JCO.1995.13.8.2104. [DOI] [PubMed] [Google Scholar]
- 58.Johnson TM, Fader DJ, Chang AE, et al. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol. 1997;4:396–402. doi: 10.1007/BF02305552. [DOI] [PubMed] [Google Scholar]
- 59.Choi EA, Gershenwald JE. Imaging studies in patients with melanoma. Surg Oncol Clin N Am. 2007;16:403–430. doi: 10.1016/j.soc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Damian DL, Fulham MJ, Thompson E, Thompson JF. Positron emission tomography in the detection and management of metastatic melanoma. Melanoma Res. 1996;6:325–329. doi: 10.1097/00008390-199608000-00008. [DOI] [PubMed] [Google Scholar]