Abstract
Asymmetric stem cell division is a mechanism widely employed by the cell to maintain tissue homeostasis, resulting in the production of one stem cell and one differentiating cell. However, asymmetric cell division is not limited to stem cells and is widely observed even in unicellular organisms as well as in cells that make up highly complex tissues. In asymmetric cell division, cells must organize their intracellular components along the axis of asymmetry (sometimes in the context of extracellular architecture). Recent studies have described cell asymmetry in many cell types and in many cases such asymmetry involves the centrosome (or spindle pole body in yeast) as the center of cytoskeleton organization. In this review, I summarize recent discoveries in cellular polarity that lead to an asymmetric outcome, with a focus on centrosome function.
Key words: stem cell, asymmetric division, niche, centrosome, spindle orientation
Introduction
The centrosome is the major microtubule-organizing center (MTOC) within a eukaryotic cell and plays fundamental roles in organizing the cytoskeletal network and the mitotic spindle, and in coordinating the cell cycle.1–4 Furthermore, problems with the centrosome have been implicated in cancer, as many cancer cells show an increase in centrosome number, although it remains debatable if a numerical increase in centrosomes in cancer cells is a result or a cause of tumorigenesis.
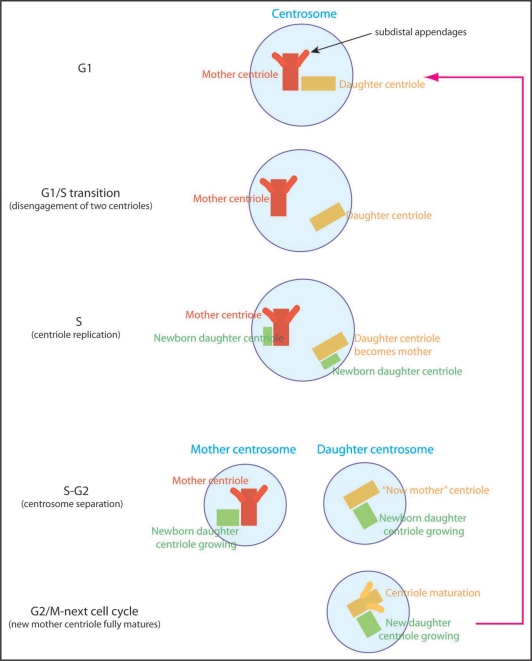
The centrosome is composed of a pair of centrioles and the surrounding pericentriolar material (PCM). Since centrioles require more than one cell cycle to mature, they have “age difference,” such that individual centrioles have different ages based on how many cell cycles they have endured (Fig. 1). Similarly, centrosomes can be distinguished based on the age of their centrioles. Mother centrosomes contain one centriole that is more than one cell cycle old, and another that was assembled during the current cell cycle. Daughter centrosomes contain one centriole that was assembled in the previous cell cycle and one that was assembled in the current cell cycle. Functionally, older, more mature centrioles develop a structure called the subdistal appendages, which is a major microtubule-anchoring site within the centrosome.5–7 Therefore, mother centrosomes typically have a greater capacity for microtubule anchoring.
Figure 1.
Centriole and centrosome duplication cycle. In G1 phase of the cell cycle, cells have a single centrosome containing one mother centriole and one daughter centriole. The mother centriole (red) can be distinguished from the daughter (yellow) because of its “subdistal appendages”. At G1/S transition, two centrioles are separated from each other (disengagement) and start nucleating daughter centrioles (green). Now the daughter centriole becomes the mother for the first time in her life, but it has not yet mature enough to have the subdistal appendages. Growth of new centrioles (green) lasts S and G2 phase, and at some point two pairs of centrioles separate from each other, becoming two centrosomes. Here, the centrosome containing the older mother centriole (red) is the mother centrosome and the other containing the younger mother centriole (yellow) is the daughter centrosome. Then, toward the end of the cell cycle (or even sometimes in the next round of cell cycle), the younger mother centriole (yellow) becomes fully matured by developing the subdistal appendages.
Recent progress in centrosome biology has revealed striking functional asymmetry between mother and daughter centrosomes, implying that centrosome asymmetry goes beyond differences in microtubule anchoring activity. Here, I first review the asymmetry between mother and daughter centrosomes at the cellular level, then describe such asymmetry in developmental contexts. Finally, I discuss examples of asymmetry observed during cell division, which is potentially governed by the centrosome.
Centrosome Asymmetry
The centrosome is a subcellular organelle that has its own duplication cycle that is synchronized with the DNA replication cycle: it duplicates at the G1/S transition, being regulated by the same set of Cdk-cyclins as DNA replication.1 Due to this duplication cycle and the fact that centrosome maturation takes more than one cell cycle, two centrosomes (the mother and the daughter) are not the same. In general, the mother centrosome is more able to anchor microtubules than the daughter centrosome, due to at least in part to the subdistal appendages. Recent discoveries in cell biology have suggested several cellular asymmetries, establishment of which involves centrosome asymmetry.
Cell biology of centrosome asymmetry.
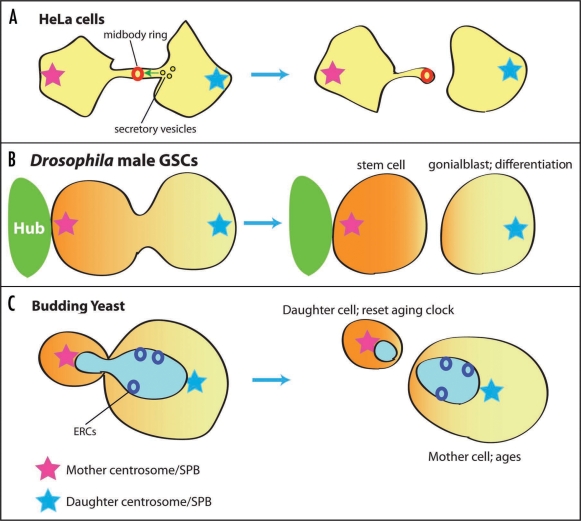
Mother and daughter centrosomes/centrioles show different characteristics during the cell cycle and cell division. For instance, mother and daughter centrioles have different motility within the cell, where in G1 cells, the mother centriole is typically less motile than the daughter, presumably because of microtubules that the mother anchors.8 Piel et al. further demonstrated that mother centrioles move very close to the midbody, right before the abscission.9 Abscission, or the final stage of cytokinesis, occurs only from one side, where secretory vesicles carrying membrane/protein components required for abscission are carried only from the daughter centrosome-containing cell, leaving a midbody ring in the cell with the mother centrosome (Fig. 2A). As a result of such asymmetric inheritance of the midbody ring, some HeLa cells contain multiple remnant rings.10 Recently, Pohl et al. demonstrated that these ring remnants are typically digested by autophagy in normal cells, such that normal cells do not have multiple rings.11 As such, possession of multiple ring remnants might be a feature of abnormal (ex. cancerous) cells. Elaborate mechanisms to remove midbody rings in normal cells suggest its importance in the maintenance of cellular homeostasis. It is also possible that the midbody ring is associated with unwanted cellular material, such as cell cycle regulators that drive cells into undesired cell cycle arrest or proliferation, or cell fate determinants (see below for details).
Figure 2.
Centrosome segregation during asymmetric cell division. (A) Abscission of HeLa cell cytokinesis occurs asymmetrically from the side that contains the daughter centrosome, leaving a midbody ring in the cell with the mother centrosome. (B) Drosophila male germ line stem cells (GSCs) divide asymmetrically under the influence of signaling from the hub cells. The mother centrosome is always inherited by the stem cell. (C) Budding yeast segregate the mother SPB to the bud. Extrachromosmal ribosomal DNA circles (ERCs) are segregated specifically to the nucleus of the mother cell, resetting the aging clock of the daughter (bud) cell.
Developmental biology of centrosome asymmetry.
Recently, the asymmetric behavior of mother and daughter centrosomes was described in the context of developmental biology.12,13 We have demonstrated that, in Drosophila male germ line stem cells (GSCs) always divide asymmetrically, where the mother centrosome is always inherited by the stem cell, and the daughter centrosome by the differentiating cell (Fig. 2B).14 During interphase of GSCs, the mother centrosome stays close to the apical side of the stem cell, where GSCs attach to hub cells, which are the major component of the stem cell niche. The daughter centrosome migrates to the opposite side of the GSCs. Such stereotypical behavior of the mother and daughter centrosomes prepares spindle orientation, which is perpendicular toward the hub. Since hub cells secrete a key signaling ligand (called Upd) that activates the JAK-STAT pathway within the GSC and specifies stem cell identity, the perpendicularly oriented mitotic spindle places one daughter cell inside and the other outside the range of the Upd secretion. In this manner, the outcome of GSC division becomes asymmetric.15 At the EM level, the mother centrosome always firmly associates with astral microtubules, which connect the centrosome to the hub-GSC interface, while the daughter is associated with only a few astral microtubules.14 A lack of substantial anchoring of the daughter centrosome by microtubules may explain why it is motile. Details of the daughter centrosome motility remain to be identified, but it is possible that an actin-based motor, such as myosin, is responsible for this movement as shown in cultured cell.16 It also remains to be determined if the mother or daughter centrosome is associated with any factors such as fate determinants. Indeed, during early embryogenesis of the mollusk, fate-determining mRNA is associated with only one centrosome during division.17 In Drosophila larval neuroblasts, one centrosome is associated with a robust array of microtubules, while the other remains dormant until immediately prior to mitosis by being stripped of any detectable PCM.18,19 Drosophila neuroblasts divide asymmetrically by segregating cortically-associated fate determinants,20 and it would be interesting to examine if the segregation/polarization of such fate determinants is somehow correlated with the differences between mother and daughter centrosomes.
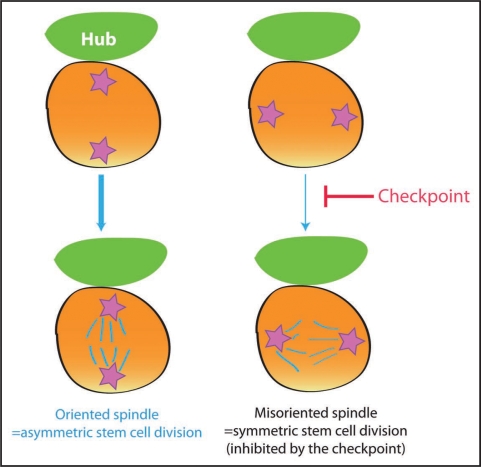
Cells may have additional layers of regulation that ensure asymmetric cell division: not only is spindle precisely oriented/aligned to ensure the asymmetric stem cell divisions, such orientation/alignment might be carefully monitored to back up the accuracy of the process. Recently, we proposed that Drosophila male GSCs might have the checkpoint that monitors the correct positioning of the centrosomes (Fig. 3); GSCs that do not have correct centrosome positioning (i.e., neither of the two centrosomes are juxtaposed to the hub) do not commit to mitosis.21 Live observation revealed that GSCs with misoriented centrosomes stay in G2 for a prolonged time period, and as soon as the centrosomes orientation is corrected, they proceed to mitosis. Interestingly, the frequency of such “misoriented” (and thus delayed in cell cycle) GSC populations increases with age, explaining at least in part why spermatogenesis declines with age.21 The accumulation of misoriented GSCs with age is not due to general disintegration of GSCs due to organismal aging, since misoriented GSCs are still capable of cell division, generating differentiating progeny. Furthermore, we have shown that misoriented GSCs originate (at least in part) from dedifferentiation of partially differentiated spermatogonia.21 We speculate that dedifferentiated GSCs either cannot re-establish the anchoring of the centrosome to the hub-GSC junction or take long time to do so. As long as dedifferentiated GSCs are misoriented (i.e., forever or a very long time), these cells activate the orientation checkpoint, thus being delayed/arrested in the cell cycle, producing less progeny. However, it should be noted that dedifferentiation still helps maintaining the number of GSCs with age, thus producing more sperms, the end product of GSCs, even though dedifferentiated GSCs are not as capable in producing differentiating cells as native GSCs. We speculate that, if this kind of checkpoint exists in mammalian stem cells, it could be a link between the tumor suppressor mechanism and tissue aging; while rigid control on asymmetric stem cell division may help preventing tumorigenesis due to overproliferation of stem cells (via symmetric stem cell divisions), it eventually leads to tissue aging by delaying stem cell division. The molecular identity of the centrosome orientation checkpoint remains to be determined. However, studies in the budding yeast may provide a direction (see below).
Figure 3.
Model of the centrosome orientation checkpoint in the Drosophila male GSCs. GSCs with misoriented centrosomes do not commit to mitosis due to the checkpoint.
What can we learn from the unicellular organism, budding yeast?
Although unicellular, budding yeast divide asymmetrically in many ways; for example, the mother cell generates the smaller bud cells (size asymmetry), only the mother cell will switch the mating type after the division (fate asymmetry). Since the budding yeast determines the division axis by forming a bud before nuclear division, spindle orientation/position must be tightly coordinated with the preformed division axis for a successful division. To ensure the coordination between cell polarity and the cell division axis, budding yeast have the spindle position checkpoint (SPOC)22 (Fig. 2C). The SPOC inhibits the mitotic exit network (MEN) until one spindle pole body (SPB, yeast equivalent of centrosome) enters the bud. Typically, this bud-bound SPB is the mother SPB and the daughter SPB stays in the mother cell23 (Fig. 2C). Many components of the SPOC and MEN specifically localize to the bud-bound (i.e., mother) SPB, thereby controlling and coordinating the cell cycle progression.24–28
Another asymmetry in budding yeast is asymmetric segregation of extra-chromosomal ribosomal DNA circles (ERCs) to the mother cell. The ERCs are derived from ribosomal DNA and accumulate each cell cycle. Since the accumulation of ERCs leads to the aging/senescence, asymmetric segregation of ERCs into the mother cell resets the age of the daughter (bud cell) at the expense of mother's aging29 (Fig. 2C). It was recently demonstrated that septin-dependent, lateral diffusion barriers form at the nuclear envelope, and ERCs are confined only in the mother cell nucleus.30 It would be interesting to examine if there is any correlation between mother and daughter SPB segregation pattern and the ERC inheritance pattern, for example after the randomization of the mother/daughter SPB due to temporal depolymerization of microtubles.23
More Cellular Asymmetries: What is the Role of the Centrosome?
Inspired by the fact that centrosomes participate in asymmetry defined by cell biological architecture or developmental biological fates, here I would like to discuss a few examples of other cellular asymmetry. Although there is no evidence thus far that these asymmetries are related to centrosomal asymmetry, it is tempting to speculate that centrosomes and possibly their age difference are involved.
The aggresome.
The aggresome is an inclusion body that confines highly denatured protein aggregates and associates with the centrosome.31,32 Aggresomes are asymmetrically inherited by only one cell upon division, both in culture cells and in Drosophila neuroblasts.33 How mother and daughter centrosomes are utilized in this type of asymmetric division is not known, but tight association of aggresomes with one centrosome suggests that the centrosome is somehow involved in aggresome inheritance.34 If such an association is combined with stereotypical positioning of the mother versus the daughter centrosome in the context of tissue architecture or development, it is likely that the inheritance of aggresomes is asymmetric such that only one cell inherits denatured proteins in an effort to protect the other cell.
Prominin (CD133).
In mouse neuroblasts, or neuronal stem cells, prominin-1 (CD133) displays very intriguing inheritance and localization patterns. When neuroblasts are dividing in a symmetrical manner during early development, prominin-1 is associated with the midbody and is excluded from both daughter cells, and is released into the extracellular fluid.35,36 Upon shifting to asymmetric stem cell division later in the neurogenic stage, the prominin-1-containing apical area is inherited by the neuroblast.37,38 Furthermore, since prominin-1 is a typical marker of many types of stem cells, including cancer stem cells, it is possible that asymmetric inheritance of stem cell markers is governed by the asymmetric nature of cell division. It would be interesting to examine whether the inheritance of prominin-1-positive midbody ring is somehow related to the mother-daughter centrosomes inheritance. Given that the midbody ring inheritance (cytokinetic abscission) pattern is tightly correlated with the mother-daughter centrosome difference as described above,10 it would not be surprising if this were the case. Then, it would be very interesting to examine if a failure in the mother-daughter centrosome inheritance leads to any developmental defects.
Summary
Asymmetric stem cell division is fundamental to homeostasis in many tissues. Although asymmetric division has been described primarily in the embryos of C. elegans, Drosophila GSCs and neuroblasts, where asymmetric fates are obvious, recent discoveries in the cell biology field have illuminated many asymmetric features of apparently symmetrically dividing cells. Future studies will extend our understanding of asymmetric cell division and how specific cell types such as stem cells utilize basic machinery of cellular asymmetry during division.
Acknowledgements
I thank members of the Yamashita laboratory for insightful discussions. This work is supported by the March of Dimes Basil O'Conner Starter Scholar Research Award, the Searle Scholar Program, and NIH RO1GM086481-01.
Footnotes
Previously published online as a Prion E-publication: http://www.landesbioscience.com/journals/prion/article/8821
References
- 1.Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Doxsey S, McCollum D, Theurkauf W. Centrosomes in Cellular Regulation. Annu Rev Cell Dev Biol. 2005 doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- 3.Pereira G, Schiebel E. The role of the yeast spindle pole body and the mammalian centrosome in regulating late mitotic events. Curr Opin Cell Biol. 2001;13:762–769. doi: 10.1016/s0955-0674(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 4.Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 5.Delattre M, Gonczy P. The arithmetic of centrosome biogenesis. J Cell Sci. 2004;117:1619–1630. doi: 10.1242/jcs.01128. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PG. Centriole inheritance. Prion. 2008;2:9–16. doi: 10.4161/pri.2.1.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbani L, Stearns T. The centrosome. Curr Biol. 1999;9:315–317. doi: 10.1016/s0960-9822(99)80201-2. [DOI] [PubMed] [Google Scholar]
- 8.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- 10.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesiclemediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11:65–70. doi: 10.1038/ncb1813. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180:261–266. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusan NM, Rogers GC. Centrosome function: Sometimes less is more. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JD, Nagy LM. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682–686. doi: 10.1038/nature01241. [DOI] [PubMed] [Google Scholar]
- 18.Rusan NM, Peifer M. A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol. 2007;177:13–20. doi: 10.1083/jcb.200612140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebollo E, Sampaio P, Januschke J, Llamazares S, Varmark H, Gonzalez C. Functionally Unequal Centrosomes Drive Spindle Orientation in Asymmetrically Dividing Drosophila Neural Stem Cells. Dev Cell. 2007;12:467–474. doi: 10.1016/j.devcel.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lew DJ, Burke DJ. The spindle assembly and spindle position checkpoints. Annu Rev Genet. 2003;37:251–282. doi: 10.1146/annurev.genet.37.042203.120656. [DOI] [PubMed] [Google Scholar]
- 23.Pereira G, Tanaka TU, Nasmyth K, Schiebel E. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 2001;20:6359–6370. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Aquino KE, Monje-Casas F, Paulson J, Reiser V, Charles GM, Lai L, et al. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell. 2005;19:223–234. doi: 10.1016/j.molcel.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Pereira G, Manson C, Grindlay J, Schiebel E. Regulation of the Bfa1p-Bub2p complex at spindle pole bodies by the cell cycle phosphatase Cdc14p. J Cell Biol. 2002;157:367–379. doi: 10.1083/jcb.200112085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira G, Schiebel E. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol Cell. 2005;19:209–221. doi: 10.1016/j.molcel.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Piatti S, Venturetti M, Chiroli E, Fraschini R. The spindle position checkpoint in budding yeast: the motherly care of MEN. Cell Div. 2006;1:2. doi: 10.1186/1747-1028-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huisman SM, Segal M. Cortical capture of microtubules and spindle polarity in budding yeast—where's the catch? J Cell Sci. 2005;118:463–471. doi: 10.1242/jcs.01650. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair DA, Guarente L. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 30.Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- 31.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 33.Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MA, van der Want JJ, et al. Polarised Asymmetric Inheritance of Accumulated Protein Damage in Higher Eukaryotes. PLoS Biol. 2006;4:417. doi: 10.1371/journal.pbio.0040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhvi A, Garriga G. Asymmetric divisions, aggresomes and apoptosis. Trends Cell Biol. 2009;19:1–7. doi: 10.1016/j.tcb.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 36.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol. 2008;20:707–715. doi: 10.1016/j.ceb.2008.09.008. [DOI] [PubMed] [Google Scholar]