Abstract
Proton magnetic resonance spectroscopy was performed at 3 T using the echo time-averaged point-resolved spectroscopy method to determine the effects of age, gender and brain region on glutamate (Glu) concentrations in the healthy human brain. Thirty healthy men and 20 healthy women aged between 21 and 71 years were studied. Significant regional variations of Glu concentrations were observed. Glu concentration in the gray matter (GM) was approximately 25% higher than that in the white matter. Significant age-dependent decreases in Glu concentrations were observed in the basal ganglia (r=−0.75, P<.001) and parietal GM (r=−0.66, P<.001) of men but not those of women. Our findings demonstrate regional variations of Glu concentrations and suggest that the male brain may be more vulnerable to aging than the female brain. Our results also highlight the importance of brain region, age and gender matching in clinical studies.
Keywords: Proton MRS, Glutamate, Normal brain, 3 T
1. Introduction
Glutamate (Glu) is one of the most prevalent neurotransmitters in the brain and the primary excitatory neurotransmitter in the mammalian central nervous system (CNS). In the brain, Glu and glutamine (Gln) are compartmentalized primarily within the neurons and glia (astrocytes), respectively. Transient increases in extracellular Glu occur normally when glutamatergic neurons depolarize, but the extracellular Glu is typically taken up quickly by astrocytes. However, toxicity to brain cells can occur in pathological states during prolonged extracellular exposure to high Glu concentrations [1]. The neurotoxicity of Glu has been implicated in a wide range of neurodegenerative diseases, including multiple sclerosis (MS) [2], amyotrophic lateral sclerosis [3], Alzheimer’s disease (AD) [4–6] and schizophrenia [7]. Glu also plays key roles in pathological processes that occur as a result of CNS insults, such as stroke, head trauma and spinal injuries [8].
Major brain chemicals that are easily detectable with both short (30–45 ms) and long (135–270 ms) echo times (TEs) in conventional single-voxel proton magnetic resonance spectroscopy (MRS) include N-acetylaspartate (NAA; 2.02 ppm), total creatine (Cr; 3.03 ppm), choline (Cho)-containing compounds (3.22 ppm) and myo-inositol (mI; 3.7 ppm). At short TEs, the overlapping resonances of Glu plus Gln are also readily measured as total Glu+Gln (Glx). Glx concentrations appear to be abnormal in various brain disorders; for instance, increases in Glx levels have been reported in patients with epilepsy [9] and hepatic encephalopathy [10], while decreases in Glx have been found in those with AD [5], obsessive–compulsive disorder [11] and antiretroviral-naive HIV [12]. Unfortunately, Glx does not measure changes in Glu/Gln stasis and is less likely to be an adequate marker for changes in intracellular conditions that may precede or accompany conditions of Glu excitotoxicity. Therefore, measurement of Glu without contamination of Gln and the aspartyl group of NAA resonances would be very important for assessing excitotoxicity in brain disorders. However, direct detection of Glu using proton MRS in the brain is difficult at magnetic field strengths below 4 T due to the strong coupling of Glu, Gln and the aspartyl moiety of NAA resonances.
Recently, a method for measuring uncontaminated brain Glu concentrations using TE-averaged point-resolved spectroscopy (PRESS) has been applied successfully in MS [13] and AD [14] patients. However, it is unclear how Glu concentrations vary in different brain regions and whether age and gender affect the Glu concentration. The present study aimed to apply the TE-averaged PRESS method to determine if there are regional differences in Glu concentrations among four brain regions: frontal white matter (FWM); frontal gray matter (FGM); parietal GM (PGM); and basal ganglia (BG). In addition, we aimed to determine whether Glu concentrations vary with gender and age. Concentrations of other major brain metabolites (NAA, Cr and Cho), as determined by the TE-averaged PRESS method, are reported as well.
2. Methods
2.1. Subjects
Fifty healthy participants (30 men and 20 women) were recruited from the local community. The study participants were between 21 and 71 years old (mean±S.D.=40.4±14.9 years). Of this group, 16 participants were aged between 20 and 30 years, 11 were aged between 30 and 40 years, 6 were aged between 40 and 50 years, 11 were aged between 50 and 60 years and 6 were aged between 60 and 71 years. The men and women had 14.6±2.4 and 15.9±2.3 years of education, respectively. All subjects were in good health, as assessed by stable vital signs and a normal general physical examination, and none used medications or hormones, except for vitamins (1 participant) and birth control hormones (2 female participants). All participants had normal cognitive function, with Mini Mental State Examination scores being in the normal range (mean±S.D.= 29.0±1.5). The first 15 participants were scanned with both the TE-averaged PRESS technique and the regular PRESS technique. In addition, the first 10 participants were each scanned twice using the TE-averaged PRESS technique within a 6-month period to evaluate the reproducibility of the measurements. Prior to scanning, each participant signed an informed consent form as well as an MR imaging (MRI) safety screening form, approved by the local institutional review board.
2.2. Data acquisition
All studies were performed with a Siemens Trio 3-T scanner using an eight-channel phased-array head coil. Sagittal T1-weighted magnetization-prepared rapid gradient echo [15] localizer scans were acquired [repetition time (TR)=500 ms, TE=11 ms, slice thickness=4 mm, gap=1 mm, field of view=24 cm]. These localizer images were reconstructed into coronal and transverse planes that were used to prescribe voxels of interest (VOIs). Two sets of single-voxel data were acquired in each voxel at multiple TEs. The first set involved a single-voxel water-suppressed TE-averaged PRESS pulse sequence [16]. This sequence acquired multiple TE spectra in a single scan (TR=2 s), starting at TE 30 ms and ending at 195 ms with 32 increments of 5 ms (effective TE=82 ms). Voxel sizes were 8 cc for the FWM, FGM as well as PGM and 12 cc for the BG (Fig. 1). The number of averages for each TE step was 8 for the FWM, FGM as well as PGM and was 12 for the BG, resulting in a total acquisition time of 8 min per voxel location for the FWM, FGM as well as PGM and that of 12 min for the BG. The second data set involved measurement of the T2 decay of the fully relaxed unsuppressed water free induction decay using single-voxel PRESS at a TR of 10 s and nine TE values (30, 30, 45, 65, 85, 125, 200, 500 and 1000 ms), with one excitation per TE step and an acquisition time of 90 s.
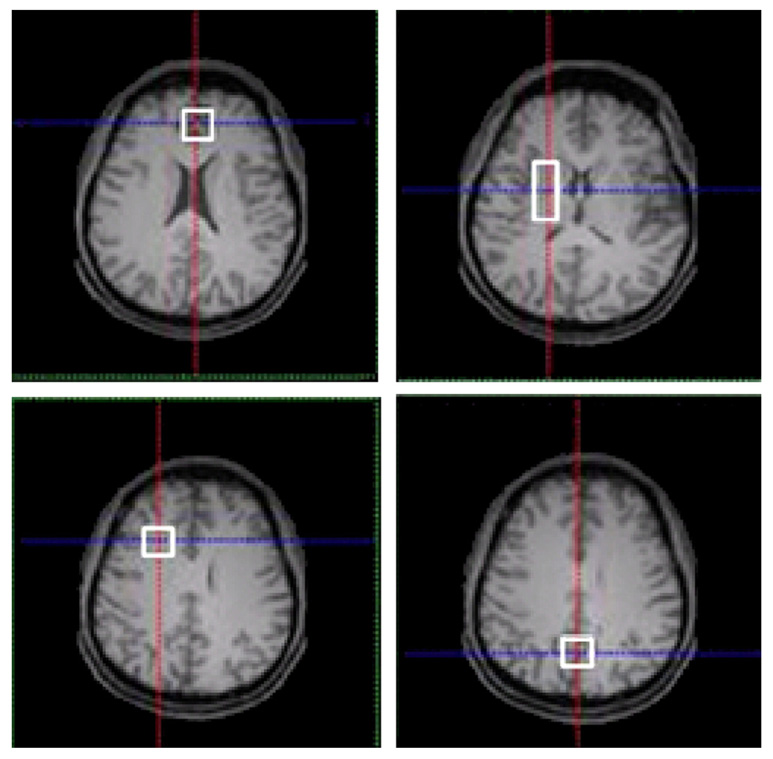
Fig. 1.
T1-weighted spin-echo MR images showing the MRS voxel locations: (A) FGM; (B) BG; (C) FWM; and (D) PGM.
2.3. Data analysis
In all, eight time domain data sets from each coil element of the eight-channel phased-array head coil were generated for each voxel location. The time domain data were corrected for eddy currents, zero-order phase, and averaged over the 32 TE values to generate a single TE-averaged spectrum from each coil. These eight data sets were then combined to generate a single data frame using the relative unsuppressed water signal intensity from each coil element as a scaling factor. Metabolite concentrations for NAA, Cr, Cho and Glu were estimated using a frequency domain fitting routine (LCModel) [17]. The goodness of fit was defined as satisfactory if the standard deviation (percentage of standard deviation) from the LCModel fitting was below 25%. The basis-set reference solutions used in TE-averaged fitting were acquired using the same data acquisition parameters as those in the in vivo acquisition, and the basis sets used in the single-TE (30 ms) fitting included metabolites nulled for baseline fitting [18]. Metabolite concentrations, corrected for differences in cerebrospinal fluid (CSF) in each voxel, were calculated using a biexponential T2 fit of the water data set [19].
Voxel segmentation was done to account for variation in tissue composition using the Functools workstation software (GEHC, Waukesha, WI, USA) by placing VOIs on the T1-weighted spoiled gradient-echo images of three healthy subjects. The software is an interactive tool that allows for visualization of image intensity pixel by pixel based on predefined pixel intensity values. GM intensity ranges were defined as intensity values below minimal white matter intensities and above maximal CSF intensities. From these intensity ranges, the ratios of GM to total VOI pixels were 0.82, 0.99, 0.11 and 0.60 for the FGM, BG, FWM and PGM, respectively.
2.4. Statistical considerations
Descriptive analyses were performed on each metabolite for the mean and standard error. An analysis of covariance (ANCOVA) was performed, with age as a covariate, in order to determine the interaction effect between age and gender on the metabolite concentrations. Regional differences of metabolite concentrations were assessed using a randomized block design with “patient” as a blocking factor. The method of least square means was used to generate adjusted values for the mean differences between groups. P values of .05 or lower were considered to be statistically significant. All statistical analyses were performed using the SAS software (SAS Institute, Cary, NC, USA).
3. Results
3.1. Regional variations of CSF content in the brain in relation to age
The ratios of CSF and brain water content in each voxel were calculated from the biexponential fitting of the T2 decay curve. Significant positive correlations of CSF partial volume and age were observed in the PGM (r=0.502, P<.001), BG (r=0.356, P=.01) and FGM (r=0.566, P<.001). However, only a trend for correlation between age and CSF was observed in the FWM (r=0.262, P=.09).
3.2. Precision and reproducibility of Glu measurements
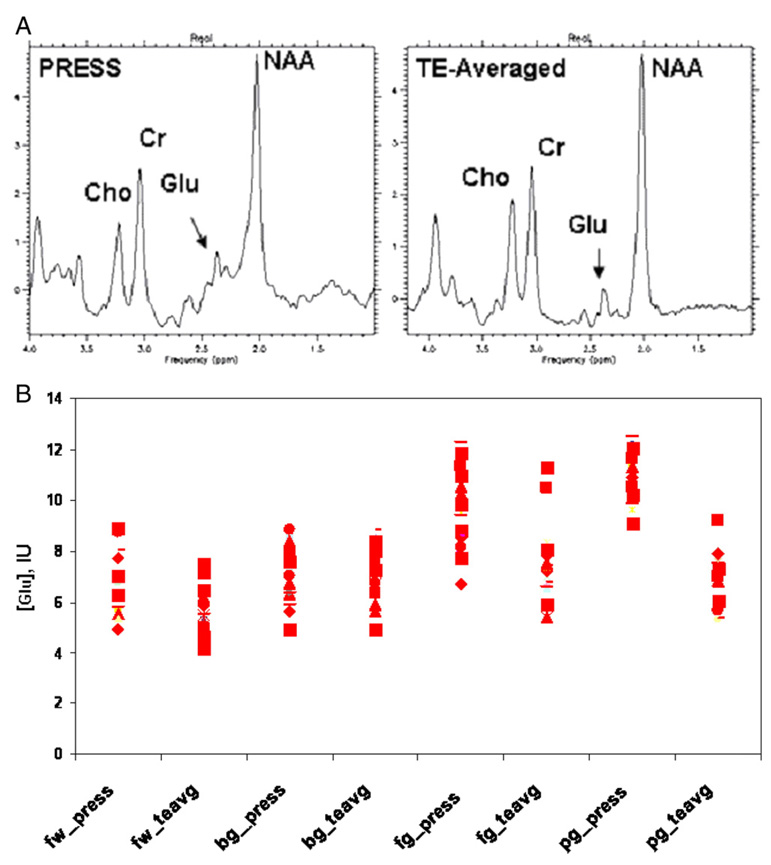
Fifteen participants were scanned with both conventional single-voxel PRESS at a TE of 30 ms and TE-averaged PRESS to evaluate the precision of Glu measurements. Fig. 2A shows spectra acquired from the white matter region using the two methods. Fig. 2B shows the scatter plots of Glu concentrations in the four brain regions using the two methods. Compared with conventional PRESS data, the TE-averaged PRESS data showed a smaller standard deviation for the Glu concentrations in the FWM (−10%), BG (−13%), FGM (−30%) and PGM (−19%). NAA and Cr resonances were also measured and showed a lower standard deviation of −30%, as compared with the short-TE PRESS (TE=30 ms) for the four brain regions. Additionally, we evaluated the reproducibility of Glu measurements; the first 10 participants were each scanned twice within a 6-month period. The metabolite concentrations measured using the TE-averaged PRESS method were highly reproducible, as shown in the scatter plots for the metabolite concentrations measured in the first and second scans (Fig. 3). The average difference between the first and second scans of the Glu concentrations was ±10% for the four brain regions.
Fig. 2.
(A) Spectra from the FWM region acquired using conventional PRESS at a TE of 30 ms (left) and TE-averaged PRESS (right). (B) Glu concentrations in the FWM, BG, FGM and PGM from the LCModel analysis using conventional PRESS and TE-averaged PRESS.
Fig. 3.
Scatter plots of the Glu concentrations in the four brain regions from the first and second scan sessions.
3.3. Regional variations of metabolite concentrations
Table 1 summarizes the concentrations of the Glu, total Cr (Cr+phosphocreatine), Cho-obtaining compounds and NAA in the four brain regions for the combined group of men and women. After corrections were carried out for voxel segmentation, the concentrations of NAA, Cr, Cho and Glu were 8.07, 4.72, 2.16 and 5.08 in the white matter and 7.59, 6.02, 1.96 and 7.83 in the GM. These corrected concentrations are within the standard deviation of the results in Table 1. The P values for the differences in the least square means of metabolite concentrations in the four brain regions are shown in Table 2. Significant regional differences (P<.001) in Glu concentrations were observed among the four regions, except between the FGM and the PGM (P=.84). The Glu concentration is approximately 25% higher in the GM than in the white matter. NAA concentrations in the four brain regions also showed significant regional differences, with P values lower than .001. However, no significant difference was observed for NAA between the BG and the FGM (P=.53) as well as between the FWM and the PGM (P=.11). Cr showed significant regional differences in all four brain regions, except between the BG and the FWM (P values between .007 and < .001). Cho showed significant differences between all four regions, except between the BG and the PGM (P=.96).
Table 1.
Metabolite concentrations (in institutional units) in the FWM, FGM, PGM and BG regions among all subjects
| Voxel location | NAA | Cr | Cho | Glu |
|---|---|---|---|---|
| FWM | 8.02±0.12 | 4.88±0.08 | 2.14±0.04 | 5.41±0.16 |
| FGM | 7.67±0.11 | 5.79±0.12 | 2.00±0.05 | 7.31±0.15 |
| PGM | 8.25±0.11 | 6.15±0.07 | 1.50±0.03 | 7.08±0.16 |
| BG | 7.63±0.09 | 4.60±0.07 | 1.52±0.04 | 3.95±0.16 |
Values are reported as mean±S.E.
Table 2.
P values for the least square mean analysis of NAA, Cr, Cho and Glu from the FWM, BG, FGM and PGM regions
| FWM | BG | FGM | PGM | |
|---|---|---|---|---|
| NAA | ||||
| FWM | .059 | .039 | .208 | |
| BG | .059 | .864 | .002 | |
| FGM | .039 | .864 | .001 | |
| PGM | .208 | .002 | .001 | |
| Cr | ||||
| FWM | .087 | < .001 | < .001 | |
| BG | .088 | < .001 | < .001 | |
| FGM | < .001 | < .001 | .092 | |
| PGM | < .001 | < .001 | .090 | |
| Cho | ||||
| FWM | < .001 | .0456 | < .001 | |
| BG | < .001 | < .001 | .384 | |
| FGM | .045 | < .001 | < .001 | |
| PGM | < .001 | .384 | < .001 | |
| Glu | ||||
| FWM | < .001 | < .001 | < .001 | |
| BG | < .001 | < .001 | < .001 | |
| FGM | < .001 | < .001 | .840 | |
| PGM | < .001 | < .001 | .840 |
3.4. Effect of gender
Table 3 shows the metabolite concentrations in the four brain regions of the men and women. The P values for age, gender as well as the interaction of gender and age and the ratios of variances (F) between age and gender in the four brain regions from the ANCOVA are shown in Table 4. Significant regional differences were observed for NAA, Cr and Glu concentrations in the men and for NAA, Cho and Glu in the women. In addition, there was a marginally significant gender difference in the Glu concentration in the PGM (9% higher in the men, P=.06). The Cr concentration showed a significant gender difference in the PGM (P=.05) and the BG (P=.04).
Table 3.
Metabolite concentrations (in institutional units) in the FWM, FGM, PGM and BG regions among the subjects by gender
| Voxel location | NAA | Cr | Cho | Glu |
|---|---|---|---|---|
| FWM | ||||
| Men (N=30) | 8.05±0.14 * | 4.82±0.11 ** | 2.16±0.05 | 5.21±0.17 *** |
| Women (N=20) | 7.92±0.19 a | 4.93±0.11 | 2.10±0.06 b | 5.71±0.23 c |
| FGM | ||||
| Men (N=30) | 7.85±0.12 | 5.83±0.15 | 1.99±0.06 | 7.16±0.18 |
| Women (n=19) | 7.49±0.16 | 5.85±0.21 | 2.02±0.08 | 7.54±0.25 |
| PGM | ||||
| Men (n=26) | 8.26±0.13 | 6.22±0.13 | 1.50±0.05 | 7.35±0.24 |
| Women (n=16) | 7.98±0.25 | 5.95±0.16 | 1.44±0.04 | 6.64±0.24 |
| BG | ||||
| Men (n=28) | 7.90±0.11 | 4.62±0.11 | 1.52±0.07 | 3.85±0.21 |
| Women (n=19) | 7.29±0.15 | 4.63±0.11 | 1.52±0.05 | 4.09±0.24 |
Values are reported as mean±S.E.
Effect of region is significant by analysis of variance (ANOVA), P<.0001.
Effect of region is significant by ANOVA, P=.0057.
Effect of region is significant by ANOVA, P<.0001.
Effect of region is significant by ANOVA, P=.024.
Effect of region is significant by ANOVA, P=.025.
Effect of region is significant by ANOVA, P=.033.
Table 4.
P and F values from the ANCOVA
| Voxel location |
NAA | Cr | Cho | Glu | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| FWM | ||||||||
| Gender | 1.89 | .17 | 0.89 | .35 | 1.06 | .30 | 2.64 | .11 |
| Age | 8.36 | .005 | 0.07 | .79 | 5.98 | .01 | 6.31 | .01 |
| Gender×Age | 2.07 | .15 | 0.44 | .51 | 0.51 | .47 | 1.14 | .29 |
| FGM | ||||||||
| Gender | 0.72 | .40 | 0.03 | .85 | 0.06 | .80 | 1.44 | .23 |
| Age | 1.16 | .28 | 3.09 | .08 | 1.27 | .26 | 0.34 | .56 |
| Gender×Age | 0.07 | .79 | 0.01 | .90 | 0.05 | .82 | 0.71 | .40 |
| PGM | ||||||||
| Gender | 1.88 | .17 | 3.76 | .05 | 0.79 | .37 | 3.65 | .06 |
| Age | 0.13 | .71 | 0.91 | .34 | 1.18 | .23 | 10.90 | .002 |
| Gender×Age | 1.74 | .19 | 2.87 | .09 | 0.42 | .52 | 2.58 | .11 |
| BG | ||||||||
| Gender | 2.44 | .12 | 4.43 | .04 | 2.43 | .12 | 2.59 | .11 |
| Age | 0.48 | .49 | 2.57 | .12 | 0.60 | .44 | 9.62 | .003 |
| Gender×Age | 0.38 | .53 | 5.67 | .02 | 2.84 | .09 | 4.93 | .03 |
3.5. Effect of age on brain Glu and other metabolites
Within the combined group of men and women, there were significant negative correlations with age for Glu concentrations in the BG (−3.7%/decade, r=−0.50, P<.001), PGM (−4.3%/decade, r=−0.56, P<.001) and FWM (−2.1%/decade, r=−0.267, P=.01). In the FWM, older age was associated with decreased NAA concentration (−2%/decade, r=−0.35, P<.01) and increased Cho concentration (+0.5%/decade, r=0.30, P=.01). Slight decreases in Cr concentration with aging were observed in the BG (−1.4%/decade, r=−0.37, P<.01).
3.6. Effect of gender on age-related changes in brain metabolites
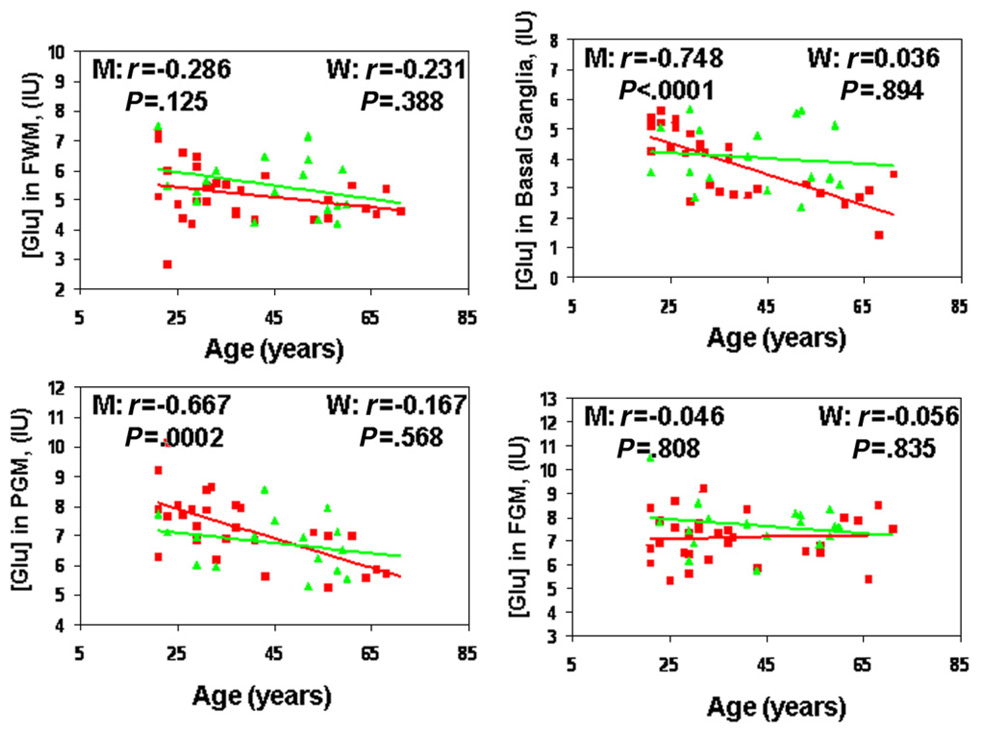
An ANCOVA showed a significant gender difference in Glu concentrations with aging in the PGM and BG (Fig. 4 and Table 4). Specifically, men, but not women, showed significant age-related decreases in Glu concentrations (< −5%/decade) in the BG (r=−0.75, P<.001) and PGM (r= −0.67, P<.001). Cr also showed a greater age-related decline in men than in women in the BG (P=.02). However, there was no significant gender-by-age interaction for NAA and Cho concentrations in any of the brain regions.
Fig. 4.
Age-related decreases in Glu concentrations in the FGM, PGM, FWM and BG. Squares represent men (M); triangles, women (W).
4. Discussion
The major findings of this study were regional variations of Glu concentrations (FGM>PGM>FWM>BG) and age-related decline in Glu concentrations, particularly in the BG and PGM of men, with fewer changes in women.
4.1. Regional variations of metabolite concentrations
Glu concentrations in cortical GM are significantly higher than those in white matter. However, the BG Glu concentration is approximately half that observed in the cortex. Similarly, the concentrations of Cr and, to a lesser extent, NAA in the cortical GM are higher than those in the white matter. In contrast, Cho was higher in the FWM than in either GM brain region. These findings are consistent with those from previous studies. Kassem and Bartha [20], using short-TE LASER spectroscopy at 4 T, reported a similar Glu concentration in the white matter region of normal human brain of 5.49 mM. Pan et al. [21] also reported a higher Glu concentration in the hippocampal and temporal GM as compared with the temporal white matter of healthy subjects (8.8 vs. 3.7 mM), measured using a three-dimensional localized spectroscopic imaging technique at 4 T. Regional differences in NAA, Cr and Cho concentrations have been the subject of many MRS studies, with varying results. One study that used a short-TE single-voxel MRS technique at 2 T found that NAA–Glu concentrations were higher in the white matter than in the GM, while Cr, mI, Glu and Gln were less concentrated in the cortical white matter than in the GM [22]. Similarly, another study that used a long-TE MRS imaging technique found that metabolite concentrations, particularly Cho, show strong regional variations, both within the cortical GM and between the genu and splenium of the corpus callosum [23]. Several MRS studies were performed in the parietal gray matter and parietal white matter, and variable ratios of NAA to Cr were reported, ranging from 1.9 to 2.8 [24–27]. Despite these discrepancies, there is general consensus of regional variations in NAA, Cho and Cr concentrations between white matter and GM. These regional variations could reflect differences in the structural and cellular compositions of different brain regions. Our current study extends prior findings that NAA, Cr and Cho vary between different brain regions and that Glu is also higher in the FGM than in other brain regions.
4.2. Effect of gender
Men showed trends for higher NAA concentrations as compared with women in both white matter and GM regions. Relatively few studies have evaluated gender differences in brain metabolite concentrations, with conflicting results. Our results are consistent with the findings of studies that used single-voxel MRS with a long TE (135 ms) [28] and multivoxel MRS with a TE of 277 ms [29] that NAA/Cho and Cho/Cr ratios are lower in the white matter region in men as compared with those in women. However, two other MRS studies found no significant gender difference in metabolite ratios from various brain regions [21,25]. Significant gender differences in Glu concentrations were observed in the BG (12% higher in women) and FGM (6% higher in women). To our knowledge, there is no previous study on the effect of gender on the Glu concentration in the normal brain. Our observations suggest that sex steroid hormones (e.g., estrogen) play an important role in regulating and interacting with Glu metabolism [30] and neurotransmitters [31].
4.3. Effect of age
An age-related decline in Glu concentrations was found in the BG, PGM and FWM. When men and women were evaluated separately, the age-related Glu decline in the PGM and BG was significant for men only. Furthermore, an age-related decline in the NAA concentration was also observed in the FWM. These findings are similar to the finding of a recent study that used short-TE single-voxel MRS at 4 T that the Glu concentration decreased with age in the medial motor cortex [32]. Our data also suggest a more rapid aging process in men as compared with women, especially in the parietal cortex and BG region. This interpretation is supported by a quantitative structural MRI study that reported that age-specific changes in brain size were significantly greater in men than in women [33]. Furthermore, several studies have suggested that sex-specific hormones, such as estrogen, confer neuroprotection against the effects of aging to the female brain [34,35].
Studies on rodents demonstrated age-related decreases in the Glu concentration in the frontal cortex and hippocampus that were consistent with neuronal loss in these areas [36]. In our study, however, age-dependent changes in Glu might account only partially for neuronal loss or damage since we observed corresponding age-related decreases in the concentration of NAA, a tentative marker of neuronal viability [37], only in the FWM. However, some recent data suggest that a decreased NAA concentration may not indicate neuronal loss, but neuronal dysfunction instead [38,39]. Along with the age-related decline in BG Glu, we also observed a corresponding age-related decline in total Cr, especially in men, which might reflect a disturbance in the energy metabolism of this brain region. Altogether, our findings of age-related decline in Glu and the corresponding changes in NAA or Cr in the same brain regions suggest that Glu may be an important marker for assessing the effects of aging.
4.4. Technical considerations
The TE-averaged PRESS method at 3 T allows for direct detection of Glu concentrations in the brain without contamination from the overlapping of Gln, NAA and mI resonances. In addition, the spectral simplification and flat baselines provided by the TE-averaging method appear to make the measurements of NAA, Cr and Cho more reliable. We consistently found lower percentages of standard deviation from the LCModel analysis for NAA, Cho and Cr, as compared with the conventional short-TE single-voxel PRESS.
The TE-averaged PRESS method measures the total MR-visible Glu signals in the brain. The ratio of intracellular (in the internal neuronal and astrocytic pools) to intercellular (synaptic cleft pool) Glu concentrations is about 10:1, with a Glu concentration in the synaptic cleft of 0.1–1 mM [40]. Therefore, intercellular Glu is at or below the detection limit for MRS and much of the Glu signal measured by our MRS technique was from the intracellular neuronal and astrocytic pools.
Several limitations are pertinent to our data acquisition. First, our results were not corrected for metabolite T1 and T2 relaxations. Since the total scan time of our protocol was already relatively long, adding scans to measure T1 values would have resulted in an unacceptably long scanning session. Regarding T2 relaxation correction, due to the strong coupling of the Glu and Gln resonances, the Glu signal intensity did not follow a simple exponential T2 decay model, which would have made fitting difficult. Therefore, we did not correct the metabolite concentrations for T1 and T2 values. Since the current study was performed at a TR (2 s) and multiple TEs (effective TE=82 ms), the metabolite concentrations were T1 and T2 weighted. From the reported T1 and T2 relaxation times of brain metabolites [41,42], our metabolite concentrations are underestimated by 10–20% and should be considered to reflect “apparent” concentrations. In addition, our metabolite concentration measurements cannot be compared directly with those from prior studies due to differences in TEs, regions of interest and pulse sequence timing.
In conclusion, brain Glu concentration is variable across brain regions and decreases with age in several brain regions, particularly in men. Glu may be a key neurometabolite for assessing neuronal health and may be a more useful surrogate marker to monitor disease processes as compared with NAA. Our finding of variable Glu concentrations in healthy subjects also highlights the importance of region, gender and age matching in clinical studies. It is important to recognize such variations when evaluating pathological conditions in different brain regions and with different ages among the patient population.
Acknowledgments
This study was supported by the National Institutes of Health (1K25DA021112 for N.S., 5K24DA016170 for L.C. and 5K02DA016991 for T.E.).
We thank Drs. Jimmy Efird and Jim Davis for their guidance on the statistical analyses of the data. We also thank L. Girton, R. Yakupov and Dr. K. Yue for their assistance in the MRS data acquisition as well as Dr. Christine Cloak, D. Ramones, K. Taketa, T. Chahil and T. Wu for their assistance in subject recruitment and evaluations.
Footnotes
Parts of this study were presented at the 14th ISMRM Scientific Meeting in Seattle (2006).
References
- 1.Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 2.Westall FC, Hawkins A, Ellison GW, Myers LW. Abnormal glutamic acid metabolism in multiple sclerosis. J Neurol Sci. 1980;47:353–364. doi: 10.1016/0022-510x(80)90088-x. [DOI] [PubMed] [Google Scholar]
- 3.Pioro EP, Majors AW, Mitsumoto H, et al. 1H-MRS evidence of neurodegeneration and excess glutamate+glutamine in ALS medulla. Neurology. 1999;53:71–79. doi: 10.1212/wnl.53.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Cowburn RF, Hardy JA, Roberts PJ. Glutamatergic neurotransmission in Alzheimer’s disease. Biochem Soc Trans. 1990;18:390–392. doi: 10.1042/bst0180390. [DOI] [PubMed] [Google Scholar]
- 5.Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate +glutamine in Alzheimer’s disease detected in vivo with (1)H-MRS at 0.5 T. Neurology. 2001;56:737–742. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- 6.Jones RS, Waldman AD. 1H-MRS evaluation of metabolism in Alzheimer’s disease and vascular dementia. Neurol Res. 2004;26:488–495. doi: 10.1179/016164104225017640. [DOI] [PubMed] [Google Scholar]
- 7.Theberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma. 2000;17:857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]
- 9.Helms G, Ciumas C, Kyaga S, Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binesh N, Huda A, Bugbee M, et al. Adding another spectral dimension to 1H magnetic resonance spectroscopy of hepatic encephalopathy. J Magn Reson Imaging. 2005;21:398–405. doi: 10.1002/jmri.20291. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg DR, MacMaster FP, Keshavan MS, et al. Decrease in caudate glutamatergic concentrations in pediatric obsessive–compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Ernst T, Witt MD, et al. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Sailasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- 14.Hancu I, Zimmerman EA, Sailasuta N, Hurd RE. 1H MR spectroscopy using TE averaged PRESS: a more sensitive technique to detect neurodegeneration associated with Alzheimer’s disease. Magn Reson Med. 2005;53:777–782. doi: 10.1002/mrm.20419. [DOI] [PubMed] [Google Scholar]
- 15.Mugler JP, III, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 16.Hurd R, Sailasuta N, Srinivasan R, et al. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 17.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 18.Seeger U, Klose U, Mader I, et al. Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magn Reson Med. 2003;49:19–28. doi: 10.1002/mrm.10332. [DOI] [PubMed] [Google Scholar]
- 19.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 20.Kassem MN, Bartha R. Quantitative proton short-echo-time LASER spectroscopy of normal human white matter and hippocampus at 4 Tesla incorporating macromolecule subtraction. Magn Reson Med. 2003;49:918–927. doi: 10.1002/mrm.10443. [DOI] [PubMed] [Google Scholar]
- 21.Pan JW, Mason GF, Pohost GM, Hetherington HP. Spectroscopic imaging of human brain glutamate by water-suppressed J-refocused coherence transfer at 4.1 T. Magn Reson Med. 1996;36:7–12. doi: 10.1002/mrm.1910360103. [DOI] [PubMed] [Google Scholar]
- 22.Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- 23.Degaonkar MN, Pomper MG, Barker PB. Quantitative proton magnetic resonance spectroscopic imaging: regional variations in the corpus callosum and cortical gray matter. J Magn Reson Imaging. 2005;22:175–179. doi: 10.1002/jmri.20353. [DOI] [PubMed] [Google Scholar]
- 24.Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Li SJ. Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1998;39:28–33. doi: 10.1002/mrm.1910390107. [DOI] [PubMed] [Google Scholar]
- 26.Komoroski RA, Heimberg C, Cardwell D, Karson CN. Effects of gender and region on proton MRS of normal human brain. Magn Reson Imaging. 1999;17:427–433. doi: 10.1016/s0730-725x(98)00186-6. [DOI] [PubMed] [Google Scholar]
- 27.Pfefferbaum A, Adalsteinsson E, Spielman D, et al. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999;41:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson ID, Paley MN, Miszkiel KA, et al. Cerebral volumes and spectroscopic proton metabolites on MR: is sex important? Magn Reson Imaging. 1997;15:243–248. doi: 10.1016/s0730-725x(96)00334-7. [DOI] [PubMed] [Google Scholar]
- 29.Kadota T, Horinouchi T, Kuroda C. Development and aging of the cerebrum: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2001;22:128–135. [PMC free article] [PubMed] [Google Scholar]
- 30.Haghighat N. Estrogen (17B-estradiol) enhances glutamine synthetase activity in C6-glioma cells. Neurochem Res. 2005;30:661–667. doi: 10.1007/s11064-005-2754-5. [DOI] [PubMed] [Google Scholar]
- 31.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey CE, Lucke JF, Saxton JA, et al. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 34.Marin R, Guerra B, Alonso R, et al. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287–301. doi: 10.2174/156720205774322629. [DOI] [PubMed] [Google Scholar]
- 35.Singh M, Dykens JA, Simpkins JW. Novel mechanisms for estrogen-induced neuroprotection. Exp Biol Med (Maywood) 2006;231:514–521. doi: 10.1177/153537020623100505. [DOI] [PubMed] [Google Scholar]
- 36.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 37.Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 38.Brooks JC, Roberts N, Kemp GJ, et al. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11:598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- 39.Arnold DL, De Stefano N, Narayanan S, Matthews PM. Proton MR spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2000;10:789–798. [ix–x] [PubMed] [Google Scholar]
- 40.Mark LP, Prost RW, Ulmer JL, et al. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am J Neuroradiol. 2001;22:1813–1824. [PMC free article] [PubMed] [Google Scholar]
- 41.Traber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 Tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 42.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45:765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]