Abstract
Background
A functional polymorphism in the promoter region of the monoamine oxidase A (MAO A) gene has two common alleles that are referred to as the high and low MAO A genotypes. We report the first in vivo human study to determine whether there is an association between MAO A genotype and brain MAO A activity in healthy male subjects.
Methods
Brain MAO A activity was measured with positron emission tomography and [11C]clorgyline in 38 healthy adult male nonsmokers genotyped for MAO A polymorphism.
Results
There was no significant difference in brain MAO A activity between the high (n = 26) and low (n = 12) MAO A genotypes.
Conclusions
The lack of an association between the high and low MAO A genotype and brain MAO A activity suggests that this polymorphism by itself does not contribute to differences in brain MAO A activity in healthy adult male subjects.
Keywords: Brain MAO activity, MAO A genotype
There is a well-characterized variable number tandem repeat (VNTR) functional polymorphism in the promoter region of the monoamine oxidase A (MAO A) gene that has two common alleles (4-repeat and 3-repeat) that occur in a ∼ 60:40 ratio in male humans (Sabol et al. 1998). These are referred to as high and low MAO A genotypes, defined by their significantly different transcriptional activities in human nonneuronal cell lines. Because of the importance of MAO in the regulation of monoamine levels and on mood and behavior, the MAO A genotype has been investigated as a variable in many clinical and behavioral phenotypes (Chen 2004; Shih et al. 1999). In particular, there appears to be a link between the low MAO A genotype and impulsive behavior (Manuck et al. 2000) through interaction with early environmental stressors (Caspi et al. 2002; Huang et al. 2004; Kim-Cohen et al. 2006). The relationship between MAO A genotype and brain MAO A activity has never been examined in vivo in healthy human subjects, however. The purpose of this study was to determine whether high and low MAO A genotypes correspond to high and low brain MAO A activity in normal healthy male subjects using the MAO A radiotracer, [11C]-clorgyline and positron emission tomography (PET; Fowler et al. 1996a, 2001).
Methods and Materials
Subjects
Thirty-eight healthy male nonsmokers were recruited specifically for this study. Nonsmoking status was ascertained by breath carbon monoxide measurement. Exclusion criteria included female gender due to a distribution of 9:1 of phenotypes (Caspi et al. 2002), current or past psychiatric or neurologic disease, history of drug or alcohol abuse, dependence on any substance other than caffeine, positive urine screen for drugs of abuse, history of head trauma with loss of consciousness, history of cardiovascular or endocrinologic disease, and current medical illness. Subjects were interviewed for age, education, and socioeconomic status (SES) and assessed for intelligence (Table 1). Written informed consent was obtained as approved by the local institutional review board.
Table 1.
Demographic Characteristics and Brain Monoamine Oxidase A (MAO A) Levels (λk3) for 38 Study Participants
| High MAO A Genotype (n = 26) |
Low MAO A Genotype (n = 12) |
|
|---|---|---|
| Age | 31.15 ± 6.7 | 32.9 ± 6.4 |
| Education | 14.96 ± 2.3 | 15.4 ± 1.3 |
| Socioeconomic Status (Hollingshead) |
44.04 ± 10.6 | 43.17 ± 14.04 |
| Intelligence | ||
| WRAT-3 Readinga | 102.76 ± 25 | 101.67 ± 13 |
| WASI MATRIX Reasoningb | 11.42 ± 2.02 | 11.4 ± 2.4 |
| Ethnicity | ||
| White | 18 | 4 |
| African American | 4 | 5 |
| Hispanic | 4 | 2 |
| Asian | 0 | 1 |
| MAO A (λk3) mL g− min−1 | ||
| Medial Frontal Cortex | .273 ± .037 | .273 ± .039 |
| Dorsolateral Prefrontal Cortex |
.279 ± .046 | .264 ± .049 |
| Anterior Cingulate Gyrus | .299 ± .044 | .289 ± .036 |
| Primary Visual Cortexc | .350 ± .055 | .307 ± .048 |
| Temporal Cortex | .293 ± .039 | .289 ± .034 |
| Precuneus | .319 ± .047 | .293 ± .046 |
| Caudate Nucleus | .228 ± .039 | .230 ± .045 |
| Putamen | .266 ± .045 | .262 ± .042 |
| Thalamus | .389 ± .053 | .376 ± .062 |
| Pons | .311 ± .057 | .312 ± .045 |
Wide Range Achievement Test III Reading subscale; estimate of verbal IQ.
Matrix Reasoning from the Wechsler Abbreviated Scale of Intelligence; estimates of nonverbal/fluid IQ.
p = .026 (high > low).
Procedures
Brain MAO A activity was measured in each subject with PET and [11C]clorgyline (average dose 6.2 ± .7 mCi; specific activity 250 mCi/μmol) using the scanning protocol reported previously (Fowler et al. 2001). Briefly, PET images were acquired using a whole body, high-resolution PET (Siemen’s HR+) in 3-dimensional dynamic acquisition mode.
From each subject, DNA for MAO A genotyping was obtained from cheek swab samples (Freeman et al. 2003). Polymerase chain reactions (PCRs) were performed as described by Sabol et al. (1998), and PCR products were analyzed on an Applied Biosystems 3100 Genetic analyzer. Alleles were seen in expected ranges using Genescan version 3.7 and Genotyper version 3.6 software.
Data Analysis
Ten regions of interest (ROIs; medial frontal cortex, dorsolateral prefrontal cortex, anterior cingulate gyrus, primary visual cortex, temporal cortex, precuneus, caudate nucleus, putamen, thalamus, and pons) were selected. Right and left regions were averaged.
PET time-activity data for [11C]clorgyline from different brain regions and time-activity data in arterial plasma were used to calculate the model term K1, the plasma to brain transfer constant which is related to blood flow and λk3, which is a function of the concentration of catalytically active MAO A molecules (Fowler et al. 2001). The model term k3 is related to the binding of [11C]clorgyline by MAO A; λis defined as K1/k2 and is independent of blood flow and k2 is related to the efflux of tracer from brain to blood.
In our statistical analysis, we first examined the normality assumption of the continuous variables using the Shapiro—Wilk test. Depending on the normality of the data, the parametric or nonparametric test was applied. We compared model terms K1 and λk3 for the high and low MAO A genotypes using the Wilcoxon Rank Sum Test for the 10 brain regions (because most of these regions are not normally distributed) setting the significance level at p = .005 (Bonferroni corrected). The nonparametric Kruskal—Wallis test was applied to examine whether ethnicity would influence K1 and λk3 among the high or low MAO genotype. All tests were two-tailed.
Results
The genotype distribution for the 38 male subjects recruited for this study was consistent with a previous study (Sabol et al. 1998) with 26 (68%) classified as the high and 12 (32%) as the low MAO A genotype. The two groups were well matched for age, socioeconomic status, education, and intelligence but not ethnicity (except for African Americans; Table 1).
For all subjects, [11C]clorgyline binding was highest in the thalamus and visual cortex and lowest uptake was in the caudate nucleus (Figure 1). We found no significant difference K1 or λk3 (which is proportional to MAO A activity) between the high and low MAO A genotypes in any brain region (Table 1) although there was a trend for a small difference for λk3 in the visual cortex (p = .026, high > low). There were no ethnic differences in absolute measures of λk3 for all subjects and between the MAO A genotyped groups.
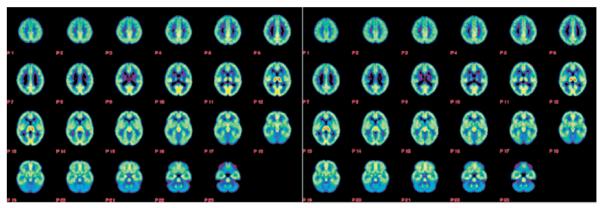
Figure 1.
Averaged parametric images (λk3) of the high (left panel) and the low (right panel) MAO A genotype groups showing 23 transaxial planes from the top of the head to the base of the skull and using a rainbow color scale where red indicates regions of highest monoamine oxidase A (MAO A) activity and blue indicates regions of lowest MAO A activity. Note the similarity in image intensity between the two groups, corroborating the findings of the region of interest analysis (Table 1).
Discussion
The prevalence of the high and low MAO A genotypes in the male population has stimulated many studies on the association of MAO A genotype with impulsivity, inhibitory control, and aggression (Huang et al. 2004; Manuck et al. 2000; Passamonti et al. 2006). Of particular interest are a number of studies showing that MAO A genotype influences vulnerability to environmental stress both in humans (Caspi et al. 2002) and animals (Newman et al. 2005) and that this biological process can be initiated early in life (Kim-Cohen et al. 2006). In addition, the low MAO A genotype was recently reported to be associated with pronounced limbic volume reductions (ventral cingulate and amygdala; Meyer-Lindenberg et al. 2006).
A possible biochemical link underlying genotype—phenotype associations is that high and low MAO A genotypes may be associated with high and low levels of brain MAO A. Monoamine oxidase A is an enzyme that regulates the concentration of the MAO A—specific substrates—serotonin and norepinephrine, as well as dopamine. A number of direct studies in nonneuronal cell lines and in postmortem human brain provide conflicting results. For example, normalized MAO A specific activity from the high MAO A genotype has been reported to be significantly higher than that for the low MAO A genotype in human fibroblast cultures (Denney et al. 1999). In contrast, a direct assay of A from cortical autopsy samples from 31 human subjects did not find a significant association of any single MAO A polymorphism with expression levels of MAO A (Balciuniene et al. 2002). Whether the high and low MAO A genotypes (which are defined in terms of their respective transcriptional activities) are associated with differences in MAO A (the gene product) has not been determined in vivo, however.
Here we report the first in vivo measurement of brain MAO A activity in 38 healthy adult male volunteers of high (n = 26) and low (n = 12) MAO A genotypes matched for age, SES, education, intelligence, and without smoking or drinking histories. We found no significant difference between MAO A genotype and brain MAO A activity in any brain region examined except for a trend for a small elevation in the visual cortex (high > low). The sample size in this study would guarantee a power of 76% for the Wilcoxon Rank Sum Test to detect the difference between the groups at the magnitude of effect size 1 (ratio between the mean difference and the pooled standard deviation) at the significance level of .05 (two-sided). In our study, the pooled standard deviation is about 13%–18% of the population mean. Thus, we have a power of 76% to detect a mean difference at the magnitude of 13%–18% of the individual population mean. This indicates that the effect of the MAO A genotype in MAO A activity in the healthy adult brain, if any, is smaller than the variability in MAO A brain concentration between adult subjects. This could reflect that either MAO A genotype has no effect on MAO A activity or that other factors (environmental, developmental, other genes) have a greater effect in modulating brain MAO activity in the adult brain. We note that other PET studies have also addressed the effect of genotype on brain levels of associated protein products such as the dopamine transporter, the dopamine D2 receptor, and the serotonin transporter. Overall, these studies, which used different tracers and different study populations, show that the effect of genotype on protein expression in the adult brain is inconsistent, and those that show differences report small differences (Martinez et al. 2001; Parsey et al. 2006; Shioe et al. 2003; van Dyck et al. 2004).
These findings suggest that variables other than baseline MAO A regional activity in the adult brain need to be considered in explaining gene behavior, gene—brain function, and gene—brain structure relationships in healthy individuals. One possibility is that the influence of the MAO A genotype may occur predominantly during brain development in the fetal and postnatal periods. The MAO A genotype may be the main variable regulating developmental catecholamine levels including those of serotonin, known to be crucial for brain development particularly because MAO B develops later than MAO A and would not be present to compensate for deficient MAO A (Shih et al. 1999). Compelling evidence suggests that the absence of MAO A during development results in an aggressive phenotype in both animals (Cases et al. 1995; Mejia et al. 2002; Whitaker-Azmitia et al. 1994) and humans (Brunner et al. 1993). In contrast there is evidence of aggression as a side effect in adults treated with MAO A—inhibiting drugs, and in rodents MAO A inhibition in adulthood reduces stress-induced aggression (Ossowska et al. 1999). Stress-induced monoamine surges could be particularly damaging during fetal and childhood development. Thus, the determination of brain MAO A levels corresponding to high and low MAO A genotype at different developmental stages as well as their interaction with environmental factors such as smoking that can further inhibit brain MAO (Fowler et al. 1996a, 1996b) pregnancy merits further investigation. Similarly, future studies to MAO evaluate the relationship between MAO A genotype and brain MAO A levels in diverse neuropsychiatric populations will allow determination of whether MAO A in the adult brain is differentially regulated in some neuropsychiatric diseases.
Acknowledgments
This work was carried out at Brookhaven National Laboratory under contract DE-AC02-98CH10886 with the U.S. Department of Energy and supported by its Office of Biological and Environmental Research and by the National Institute for Biomedical Imaging and Bioengineering (Grant No. EB002630), National Institutes of Health (NIH) General Clinical Research Centers (GCRC) (Grant No. MO1RR10710), NIH K05DA020001, GCRC Grant No. MO1RR10710, and the Office of National Drug Control Policy.
Footnotes
This work was presented at the 52nd Annual Meeting of the Society of Nuclear Medicine, Toronto Ontario, June 18–23, 2005. The authors thank Donald Warner, Pauline Carter, Barbara Hubbard, Noelwah Netusil, Millard Jayne, Payton King, Hai-Dee Lee, Richard Ferrieri, Colleen Shea, Youwen Xu, Victor Garza, David Schlyer, and Michael Schueller for advice and assistance in various aspects of these studies. We also thank the subjects who volunteered for these studies and Fritz Henn for helpful discussions.
References
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin EE. Investigation of functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet. 2002;110:1–7. doi: 10.1007/s00439-001-0652-8. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAO A. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chen K. Organization of MAO A and MAO B promoters and regulation of gene expression. Neurotoxicol. 2004;25:31–36. doi: 10.1016/S0161-813X(03)00113-X. [DOI] [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Ding YS, Franceschi D, Wang GJ, Volkow ND, et al. Non—MAO A binding of clorgyline in white matter in human brain. J Neurochemistry. 2001;79:1039–1046. doi: 10.1046/j.1471-4159.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996b;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad SciUSA. 1996a;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA obtained from buccal swabs recruited by mail: An evaluation of the effects of storage on long-term stability and suitability for multiplex PCR genotyping. Behav Genet. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. John Wiley & Sons; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase A gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene—environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006 Jun 27; doi: 10.1038/sj.mp.4001851. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase—A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Laruelle M. Imaging neurochemical endophenotypes: Promises and pitfalls. Pharmacogenomics. 2001;2:223–237. doi: 10.1517/14622416.2.3.223. [review] [DOI] [PubMed] [Google Scholar]
- Mejia JM, Ervin FR, Baker GB, Palmour RM. Monoamine oxidase inhibition during brain development induces pathological aggressive behavior in mice. Biol Psychiatry. 2002;52:811–821. doi: 10.1016/s0006-3223(02)01418-x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Klenk-Majewska B, Danilczuk Z, Wrobel A, Zebrowska-Lupina I. Reversal of stress-induced deficit in aggression by monoamine oxidase inhibitors. Pol J Pharmacol. 1999;51:391–397. [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, et al. Monoamine oxidase—A genetic variations influence brain activity associated with inhibitory control: New insight into the neural correlates of impulsivity. Biol Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: From genes to behavior. Ann Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, et al. Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161(3):525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Zhang X, Clarke C. Effects of gestational exposure to monoamine oxidase inhibitors in rats: Preliminary behavioral and neurochemical studies. Neuropsychopharmacology. 1994;11:125–132. doi: 10.1038/npp.1994.42. [DOI] [PubMed] [Google Scholar]