Abstract
During ischemic nerve block of an extremity, the cortical representations of muscles proximal to the block are known to expand, increasing the overlap of different muscle representations. Such reorganization mimics that seen in actual amputees. We investigated whether such changes degrade voluntary control of muscles proximal to the block. Nine subjects produced brief, isometric flexion force selectively with each fingertip before, during, and after ischemic block at the wrist. We recorded the isometric force exerted at the distal phalanx of each digit, along with electromyographic (EMG) activity from intrinsic and extrinsic finger muscles. Despite paralysis of the intrinsic hand muscles, and associated decrements in the flexion forces exerted by the thumb, index, and little fingers, the selectivity of voluntary finger flexion forces and of EMG activity in the extrinsic finger muscles that generated these forces remained unchanged. Our observations indicate that during ischemic nerve block, reorganization does not eliminate or degrade motor representations of the temporarily deafferented and paralyzed fingers.
Keywords: amputation, ischemic nerve block, hand, finger, motor cortex, electromyography
INTRODUCTION
In both animals and humans, upper extremity amputation results in substantial reorganization in the somatosensory and motor systems. In the somatosensory system, for example, areas of cortex that responded to stimulation of the upper extremity before amputation become responsive to stimulation of the stump and face after amputation (Merzenich et al., 1983; Pons et al., 1991; Ramachandran, 1993; Elbert et al., 1997; Grusser et al., 2001). Similarly in the motor system, stimulation of cortical territory that evoked responses in distal muscles before amputation comes to evoke responses in the remaining stump muscles after amputation (Hall et al., 1990; Sanes et al., 1990; Cohen et al., 1991; Ridding & Rothwell, 1995; Pascual-Leone et al., 1996; Schieber & Deuel, 1997; Wu & Kaas, 1999). Movement and muscle representations of the remaining stump thus appear to invade the territory that previously had represented movements and muscles of the amputated distal part.
Does such invasion by proximal representation abolish the motor representation of the distal part that was amputated? Recently, transcranial magnetic stimulation (TMS) over the contralateral motor cortex of hand amputees was found to evoke perceptions of various movements of the phantom hand (Mercier et al., 2006). Furthermore, when amputees attempt various voluntary movements of their phantom fingers, different patterns of EMG activity are produced in proximal stump muscles; patterns distinct from those that characterize voluntary movements of the stump (Reilly et al., 2006). These observations suggest that amputees retain a motor representation of their amputated fingers. Invasion by proximal representations might nevertheless degrade the distal representation. Amputees often describe physical distortion of their phantom hand (e.g. telescoping), and a reduced ability to move the phantom dexterously (Ramachandran & Rogers-Ramachandran, 1996). Quantifying such degradation of movement representation is limited, however, by the investigators’ inability to observe the phantom hand.
Ischemic nerve block provides a reversible model of short-term amputation-induced reorganization. When ischemic nerve block renders the hand temporarily paralyzed and numb, TMS of the motor cortex elicits responses in muscles proximal to the block at lower stimulation intensities and from a greater cortical area than before (Brasil-Neto et al., 1992; Brasil-Neto et al., 1993; Ridding & Rothwell, 1995, 1997; Ziemann et al., 1998; Werhahn et al., 2002). The expansion of a muscle’s cortical representation is generally presumed to be of functional significance for the control of that muscle. For example, in both animals and humans, the cortical representation of a muscle enlarges when that muscle is recruited during a motor learning task (Pascual-Leone et al., 1994; Pascual-Leone et al., 1995; Plautz et al., 2000), suggesting that increased cortical excitability is associated with motor skill learning. Other studies have also shown the functional importance of changes in cortical muscle representations; ischemic nerve block of one hand can improve performance on finger tapping and tactile acuity tasks executed by the other hand (Werhahn et al., 2002b; Bjorkman et al., 2004; Floel et al., 2004). Cortical reorganisation is not only observed under conditions of improved performance, however. Expanded sensorimotor maps have also been observed under conditions in which motor performance did not change (Muellbacher et al., 2000), suggesting it can sometimes be related to no functional change, and under conditions in which subjects experience extreme phantom limb pain (Flor et al., 1995; Birbaumer et al., 1997), suggesting that it can be related to a maladaptive change.
The rapid expansion of cortical representations of muscles immediately proximal to an ischemic nerve block not only results in an apparent invasion of distal muscle representation territory similar to that seen in long-term amputees, but also results in increased overlap of representations of those muscles immediately proximal to the nerve block. We wondered whether the increased overlap of muscle representations might degrade the normal ability to provide selective motor output to the muscles that remain functional, just proximal to the block. We investigated this possibility in normal subjects who underwent ischemic nerve block at the wrist, temporarily denervating the intrinsic muscles and sensory receptors of the hand. Because the thumb and fingers are flexed by muscles both within the hand (intrinsics) and long extrinsic muscles—with bellies in the forearm and tendons that cross the wrist to reach the digits—the fingers and thumb can still be flexed voluntarily, while the hand itself is denervated. Moreover, not only can the extrinsic finger muscles flex the fingers and the thumb when the intrinsic muscles are paralyzed, but selective flexion of individual digits is also possible with extrinsic muscles only. We took advantage of this arrangement to examine whether temporary deafferentation and paralysis of the hand, without physical amputation, would change the selectivity with which subjects use their long extrinsic muscles to exert flexion forces individually with each digit.
MATERIALS & METHODS
Subjects
Nine right-handed subjects (6 female and 3 male; mean age 32 years; range 21 to 50 years) participated in 10 recording sessions (one subject participated in two separate recordings sessions) after giving written informed consent. The Research Subjects Review Board of the University of Rochester Medical Center approved the study protocol. Experiments were conducted in accordance with the Declaration of Helsinki. None of the participants had any history of trauma, degenerative, or neurological disease affecting the upper limbs.
Electromyographic Recordings
The extrinsic finger muscle, flexor digitorum profundus (FDP) flexes the distal phalanges of the fingers, and extensor digitorum communis (EDC) is coactivated during individuated isometric finger flexions (Valero-Cuevas, 2000). We therefore recorded intramuscular EMG activity from 4 to 6 bipolar fine-wire electrodes placed in different regions of the left FDP and EDC muscles, as well as surface EMG from the first dorsal interosseous (FDI), an intrinsic hand muscle that flexes and abducts the index finger. A detailed description of the techniques for making the intramuscular electrodes has been published previously (Reilly & Schieber, 2003). For placement within FDP, each sterilized electrode was inserted percutaneously from the medial aspect of the forearm at a proximodistal level roughly one third of the distance from the olecranon to the wrist crease. For placement within EDC, subjects placed their palm flat on the table with their arm outstretched and the elbow externally rotated, and each sterilized electrode was inserted percutaneously from the dorsal aspect of the forearm roughly one half of the distance from the elbow flexion crease to the styloid process of the ulna. EMG activity then was recorded and played over an audiomonitor as the subject flexed and extended each fingertip in turn, followed by the wrist, confirming that the electrode was positioned appropriately the extrinsic finger muscle, and not an adjacent wrist muscle. Thereafter the needle was removed, leaving only the wires in place in the muscle. To monitor the paralysis of intrinsic hand muscles during ischemic nerve block, we also placed 10 mm Ag/AgCl surface electrodes in a bipolar configuration (7–12 mm apart depending on the size of the subject’s hand) over FDI. EMG activity was amplified (5 – 20 K), band-pass filtered (0.3 – 3 kHz), and then sampled at 17 kHz per channel.
Behavioral Task, Force Recordings and Ischemia Protocol
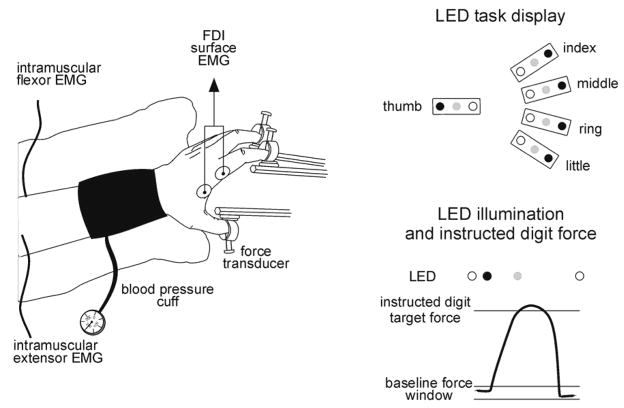
The behavioral task performed by the subjects has been described in detail previously (Reilly and Schieber 2003). In brief, after placement of all electrodes, subjects sat with their forearm in a semi-pronated position, the elbow flexed to ~ 50°, and the wrist extended to ~ 45°. A pediatric blood pressure cuff was placed around the forearm immediately proximal to the wrist, and a vacuum cast was used to stabilize the wrist, forearm, and elbow (Figure 1).
Figure 1. Experimental set-up and task protocol.
The drawing on the left shows the configuration of the hand and arm. Sites at which we tested sensory detection to assess whether the hand was deafferented were all on the ventral surface of the hand (not shown). The schematic on the top right illustrates the LED task display viewed by the subjects. Each row of LEDs represented one digit. Red LEDs (black circle) at the end of each row were illuminated one at a time, under computer control, instructing the participant with which digit to produce a criterion flexion force. The yellow LED (open circle) was illuminated when the force exerted by the digit was less than the criterion force and the green LED (grey circle) was lit whenever the force exerted by the digit exceeded the criterion flexion force. At the bottom on the right is a schematic single trial showing force in the instructed digit and the relative timing of LED task events.
The distal phalanx of each left-hand digit was secured in a small plastic ring with light pressure from a thumbscrew over the fingernail. Micropore tape was applied to prevent the fingertip from slipping out of the ring during paralysis of the intrinsic hand muscles. The 5 rings were arranged such that the shape of the hand was similar to that used to grasp a ball ~10 cm in diameter. Each ring was mounted on a stationary rod via a load cell (Omega LCL-010) that transduced flexion/extension forces. The force from each fingertip was amplified with a strain gauge amplifier (Omega DMD–465 WB) sampled at 0.5 kHz per channel.
Once the participant’s hand was arranged comfortably in the apparatus, the resting force at each fingertip was measured over one minute. A baseline force window for each digit then was established as the mean resting force ± 0.6 N. A criterion (target) force level was set at 1N (approximately 3% of maximum voluntary contraction force (Li et al., 1998)) beyond the upper limit of this window in the flexion direction. This criterion gave subjects the feeling of pressing similarly with each digit, and enabled them to perform our behavioral task accurately from the beginning of the experimental session, with little if any fatigue through an entire session.
Each digit was represented by a row of light-emitting diodes (LEDs). These were used to determine the timing of each trial and to cue subjects which digit to flex. A yellow LED in each row indicated that the force exerted by that digit was within the baseline force window, and a green LED in each row was lit whenever the force exerted by that digit exceeded the criterion flexion force. Once the forces in all fingers had been within their baseline force windows for 500 ms, an initial hold period randomly varied from 1000 to 1500 ms ensued, and then a red LED in one row was lit, instructing the subject with which digit to produce a criterion flexion force (i.e. the instructed digit), lighting the green LED in that row only. We did not ask subjects to produce individuated extension forces because paralysis of the intrinsic hand muscles during ischemic nerve block precluded production of extension forces at the fingertips.
Each trial was considered successful if the criterion force or greater was generated by the instructed digit within a 1000 ms allowed response time and maintained above criterion force for 500 ms, after which a 3000 Hz tone indicated to the subject that the trial was over. A trial was unsuccessful if i) the baseline fingertip forces moved outside their windows during the initial hold period; ii) the criterion force in the instructed digit was not produced within the allowed response time; or iii) the criterion force was produced within the allowed response time but was not maintained throughout the final hold period. The frequency of each of these three types of error did not appear to vary substantially throughout the experimental sessions. An unsuccessful trial was repeated until completed successfully.
Each of the five flexions — of the thumb, index, middle, ring, and little fingers, denoted as 1f, 2f, 3f, 4f, and 5f — was presented in randomized order within a sub-block of 5 trials, and 10 sub-blocks were accumulated to constitute a single block, for a total of 50 trials per block. Successive blocks were begun at 5 minutes intervals, providing 2–3 minutes rest for the subject between blocks. Four blocks were completed prior to cuff inflation (the control period).
Then as the subject continued to perform blocks of trials at 5 minute intervals, ischemia of the hand was produced by inflating the blood pressure cuff (positioned across the wrist) to 200 ± 10 mmHg (ischemic period). Progress of the ischemic nerve block was monitored by the decline in both voluntary activation of FDI and cutaneous sensation. As the subject rested between blocks of trials, sensory thresholds were measured on the palmar surface of the hand at i) the base of the index finger; ii) the tip of the index finger; and iii) immediately distal to the lateral border of the wrist crease (see Figure 1) using calibrated von Frey filaments (Semmes-Weinstein Aesthesiometers, Stoelting, IL, USA). During this sensory testing the subject was asked to close his/her eyes, and to say “touch” whenever a touch was felt on the fingers or hand. No verbal feedback about unappreciated touches was given to the subject during sensory testing. Time from the onset of ischemia to complete nerve block (no voluntary EMG in FDI and no appreciation of touch from the von Frey filaments) ranged from 39 to 73 minutes in different subjects with a mean of 56 minutes, similar to prior studies (e.g. Ziemann et al., 2001; McNulty et al., 2002; Werhahn et al., 2002). After complete nerve block had been achieved, the subject performed one more block of trials. Thereafter the blood pressure cuff was deflated, and the subject performed four more blocks of trials at 5 minute intervals (recovery period). The main discomfort subjects reported was intense paraesthesiae (“pins and needles”) that occurred during reperfusion following deflation of the cuff.
Throughout the control, ischemic, and recovery periods, the behavioral task was controlled by TEMPO software (Reflective Computing, St. Louis, USA) that also generated behavioral event markers. These markers were recorded simultaneously with continuous EMG and force data using a Micro1401 data acquisition interface hosted by a PC-compatible computer running Spike2 software (CED Ltd, Cambridge, UK). Each session lasted approximately 3 hours.
Data Analysis
Quantifying Fingertip Forces
On each trial the change in force at each digit (δforce) was quantified as the maximum (peak) force at that digit minus the average background force. The average background force for each digit was calculated as the mean force produced in that digit during two 250 ms periods; one before the trial began and one after the trial was finished. Each 250 ms period was taken while the force exerted by all digits remained within the baseline force window. The onset of force was calculated separately for each digit and was taken as the time at which the force in the digit was greater than 7.5 standard deviations above the mean background force level. This ensured that fluctuations in the baseline force exerted by non-instructed digits were not erroneously detected as the onset of the intended force production. We then measured the peak force in each digit between force onset and when the force in the instructed digit dropped to less than 7.5 standard deviations above the baseline force. Average peak force was also measured over 250 ms during the middle of the hold phase, but as there were no significant differences between this measure and absolute peak force, only the latter is reported. We quantified fingertip forces by calculating the average peak force exerted by each digit across the 10 trials of each instructed flexion performed in each block.
The average peak force produced by instructed digits during task performance was 3.26 ± 0.33 N (mean ± SEM), less than 10% of the average MVC force for digits (e.g. Li et al., 1998), but considerably more than the 1 N required. Participants consistently produced relatively low forces in non-instructed digits, averaging 0.35 ± 0.28 N (mean ± SEM). Thus, the finger forces produced were highly individuated, with forces exerted by the instructed digit an order of magnitude larger than forces exerted by non-instructed digits.
Quantifying EMG Activity
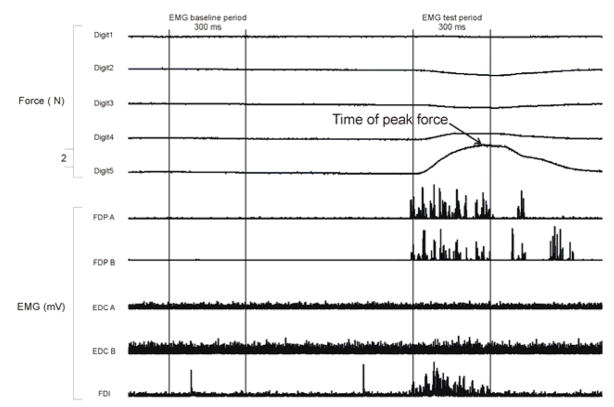
EMG data were quantified by calculating the root mean squared (RMS) value of the EMG waveform during both a baseline period (300 ms before illumination of the red LED instructing which digit to flex on that trial) and a test period (300 ms from 150 ms before to 150 ms after the force in the instructed digit reached the criterion force). We deliberately chose the test period to include both the dynamic phase of EMG activity during which the force in the digit was rapidly changing, as well as a more static phase of EMG activity during maintenance of the required force. Figure 2 shows a single trial example of the relationship between the time of peak force and the time period over which the EMG activity was analyzed. On each trial the change in EMG activity (δEMG) then was calculated as the RMS value from the test period minus the RMS value from the baseline period. For each instructed flexion the δEMG values were examined, and outliers greater than 2 standard deviations from the mean of the 10 trials from that block were excluded from further analysis. This exclusion process was performed separately for each subject, each instructed flexion, and each block of trials. For example, for subject X, if one of the 10 5f δEMG values for block 1 was greater than 2 standard deviations from the mean of those 10 values, then that δEMG value was excluded from further analyses. Applying this criterion rejected 7% of trials.
Figure 2. Relative timing of EMG and force analyses.
A continuous record of force and rectified EMG data recorded from one subject during a single trial in which the subject was instructed to flex the little finger. In this example the subject reached peak force 120 ms after reaching the criterion force level, which was always the midpoint of the 300 ms EMG test period. On average, subjects reached peak force 161 ms (SD = 29) after they attained the minimum criterion force level.
Quantifying the selectivity of force and EMG activity
Selectivity indices for the force produced by a given digit and for the EMG activity recorded by a particular electrode were derived as described previously (see Schieber et al., 2001; Reilly & Schieber, 2003). These indices quantify: i) the extent to which a given digit exerted force selectively, varying from SEL = 0 if equal peak force was exerted during instructed flexion of each digit to SEL = 1 if a peak force above baseline was exerted only when that digit was the instructed digit; or ii) the extent to which EMG activity recorded at a given electrode occurred selectively, varying from SEL = 0 if equal EMG activity changes were recorded during instructed flexion of all digits, to SEL = 1 if EMG activity changed only during instructed flexion of a single digit.
Different subjects required different durations of ischemia to develop characteristics indicative of nerve block as defined by our criteria. To permit averaging across subjects, we therefore selected 9 data blocks for each subject in which particular events occurred, and then averaged data across subjects in each of these 9 blocks. We selected: the second (a) and fourth (b) control blocks before cuff inflation; the first block after cuff inflation (c); the block in which cutaneous sensation at the index fingertip initially decreased (d); the block in which complete anesthesia was attained at the index fingertip (e); the block in which voluntary attempts to abduct the index finger produced no EMG in FDI (f); the last block before cuff deflation (g); and the second (h); and fourth (i) recovery blocks after the cuff was deflated. Differences between these nine periods then were examined using repeated measures ANOVA with Student-Newman-Keuls post hoc analysis, with differences considered significant when p < 0.05.
To exclude the possibility that any changes in fingertip forces and EMG might have resulted simply from the forearm remaining immobilized for the approximately 1 hour of data collection, in 3 subjects we measured forces and EMG during isometric finger flexions every 5 minutes for 1 hour (instead of the usual 20 minutes) before inflating the blood pressure cuff and continuing the experiment as usual. There were no systematic changes in force or EMG in these extended control periods, neither in comparison with shorter control periods, or when we compared between the first and last block of the one hour control period.
RESULTS
We analyzed the force produced at the distal phalanges and the EMG activity recorded from 49 electrodes in 10 recording sessions while healthy human subjects produced individuated flexion forces before, during, and after ischemic nerve block at the wrist. During the ischemic period, each subject experienced numbness of the hand that progressed to anesthesia, accompanied by paralysis of the intrinsic hand muscles as assessed with surface EMG recorded from FDI. Despite this anesthesia of the hand and paralysis of the intrinsic hand muscles, all subjects remained able to perform the behavioral task of producing individuated flexion forces at the tip of each digit throughout the ischemic period.
Single subject force and EMG data
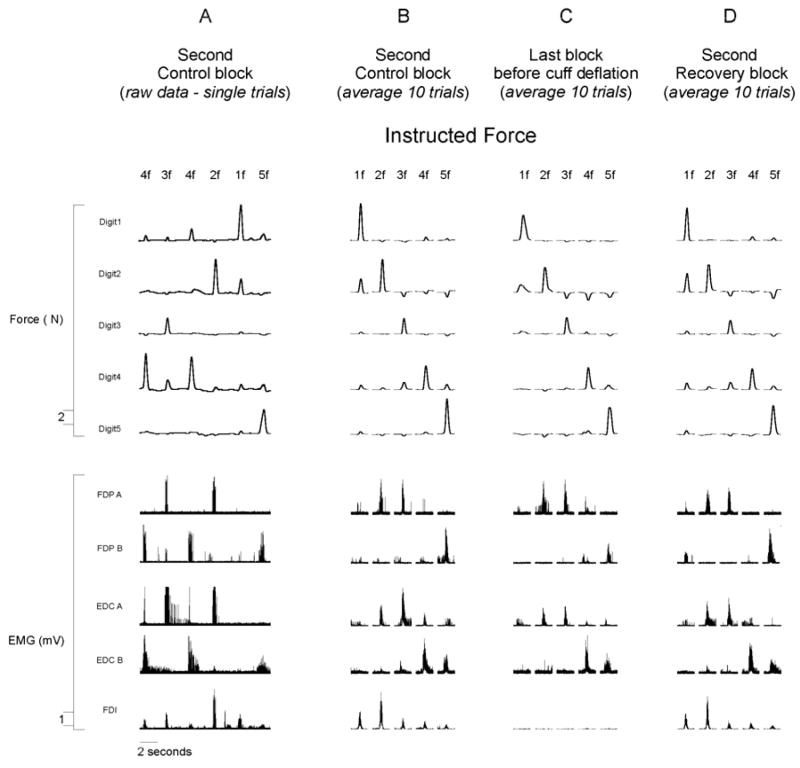
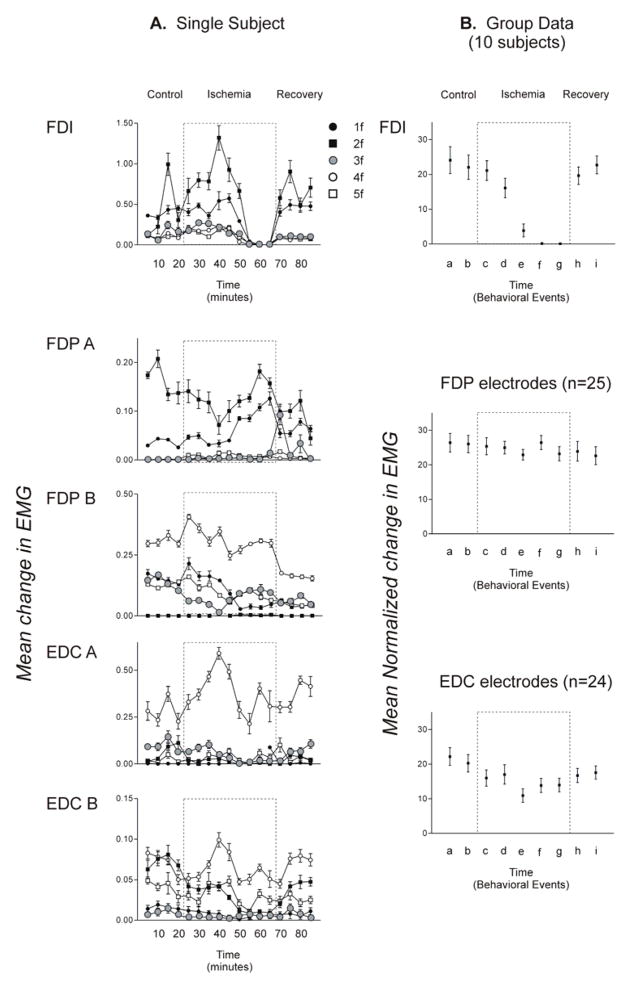
Continuous force from each fingertip and rectified EMG from five electrodes, recorded simultaneously during six sequential trials of the second control block (approximately 10 minutes after the recording session began) from a single subject (X) are illustrated in Figure 2A. The instructed digit on each trial is indicated at the top of the figure. Data from the same subject also are shown averaged over the 10 trials of each instructed finger flexion (1f through 5f) recorded during the second control block (Fig. 2B), the last block before cuff deflation (Fig. 2C), and the second recovery block (Fig. 2D). Both the raw and the averaged finger force traces in the top half of the figure show that the subject performed each trial by producing force relatively selectively with the instructed digit. The amplitude of EMG activity recorded from a given electrode varied depending on the instructed digit, consistent with the behavior of FDP described previously (Reilly & Schieber, 2003). Furthermore, electrodes at different positions in the muscle belly of FDP or EDC recorded different patterns of activity across the different finger flexions. Electrode FDP A recorded activity during 2f and 3f while electrode FDP B recorded activity primarily during 5f. Similarly, electrode EDC A recorded the most activity during 2f and 3f, whereas electrode EDC B recorded activity during 4f and some during 5f as well. Although our electrodes were not necessarily placed in highly selective subdivisions of FDP and EDC serving single digits, each electrode recorded a consistent pattern of EMG activity across the five flexions, which differed from the pattern recorded by other electrodes.
The bottom row of Figure 2 shows surface EMG recorded from FDI. The absence of EMG in FDI during the last block before cuff deflation (Fig. 2C) demonstrates that the ischemia resulted in nerve block, paralyzing FDI and presumably the other intrinsic hand muscles as well. Despite the paralysis of intrinsic muscles of the hand that resulted from the ischemic nerve block, the subject remained able to perform the behavioral task of flexing each instructed digit when cued.
Fingertip forces before, during, and after ischemic nerve block
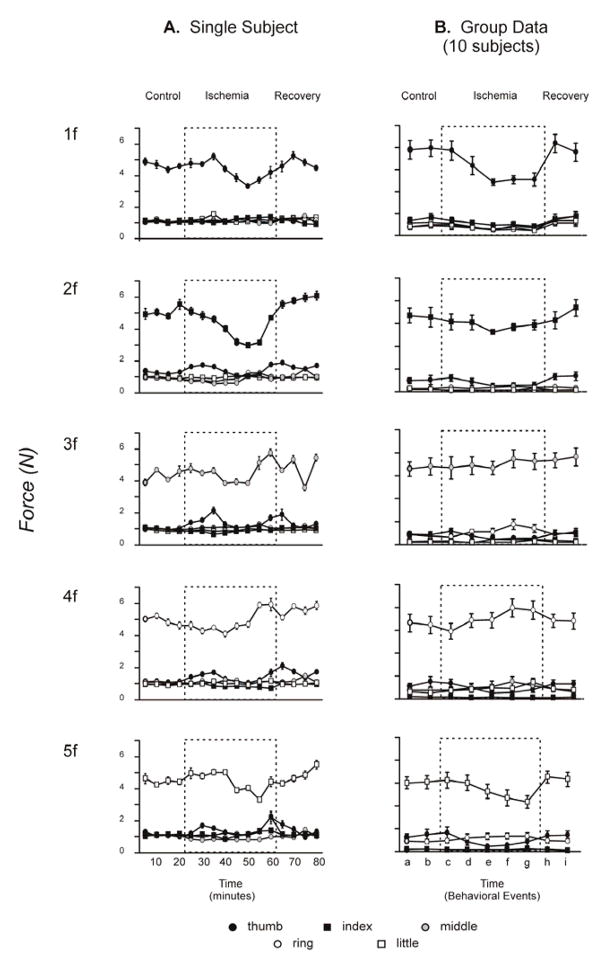
In the examples in Figure 2, the peak flexion forces exerted by digit 1 during 1f, by digit 2 during 2f, and by digit 5 during 5f, each appeared somewhat lower during ischemic nerve block (Fig. 2C) than during the preceding control (Fig. 2B) or following recovery (Fig. 2D) blocks. In contrast, the peak forces exerted by digit 3 during 3f and by digit 4 during 4f appeared unaffected by ischemic nerve block. To examine changes in flexion forces exerted by each digit, we averaged the peak flexion force in each digit across the 10 trials of each instructed digit flexion in each experimental block.
The mean (± SEM) peak fingertip forces in both the instructed and non-instructed digits for all 16 blocks performed by subject SE in one experimental session are shown in Figure 3A. For subject Y, as for subject X, the peak flexion forces exerted by the middle finger during 3f, and by the ring finger during 4f, remained stable during the ischemic period, while forces exerted by other instructed digits decreased. During the second half of the ischemic period, peak flexion forces exerted by the thumb during 1f, by the index during 2f, and by the little finger during 5f, all decreased. These decreases in peak force were not accompanied, however, by increases in the forces produced by the non-instructed digits. In subject Y, flexion force production by each instructed digit remained relatively selective throughout the ischemic nerve block.
Figure 3. Single trial average rectified EMG and force data from one participant.
(A) A continuous record of force and rectified EMG data recorded from one subject during six sequential trials of the second control block (approximately 10 minutes after the recording session began). (B–D) Averaged data from the same subject (average of 10 trials of each instructed finger flexion) recorded during the second control block (B), the last block before cuff deflation (C), and the second recovery block (D). The instructed finger flexion on each trial is indicated at the top of the figure.
Figure 3B shows peak fingertip forces averaged across all 10 subjects. Mean peak fingertip forces from each subject were averaged from the 9 blocks representing key points in an experimental session (described in the Methods). One-way repeated-measures ANOVAs performed on these data revealed that, across all subjects, peak flexion force decreased significantly towards the end of the ischemic period for the thumb [F(8,72)=8.07, p<0.05], index [F(8,72)=2.44, p<0.05], and little finger [F(8,72)=3.64, p<0.05] when each was the instructed digit. The middle finger showed no change in peak force [F(8,72)=1.13, p>0.05], and the ring finger showed significantly larger peak forces at the end of the ischemic period than immediately after cuff inflation [F(8,72)=3.60, p<0.05]. As for subject Y (Fig. 3A), however, these changes in peak forces produced by the instructed digits were not accompanied by substantial increases in the forces produced by the other, non-instructed digits. Across subjects, force production by each instructed digit remained relatively selective throughout the ischemic nerve block.
EMG before, during, and after ischemic nerve block
The average change in EMG activity recorded in subject Z from the surface electrode on FDI, from two intramuscular electrodes in FDP, and from two in EDC is illustrated in Figure 4A. During the control period, subject Z activated FDI during 1f and 2f. EMG activity in FDI during 1f and 2f did not decline substantially in the first 6 data blocks of the ischemic period, but FDI became virtually silent for the last 3 data blocks, indicating paralysis resulting from the ischemic nerve block at the wrist. Concurrently, the intramuscular recordings from the extrinsic finger muscles proximal to the nerve block showed a variety of trends, with no consistent pattern of changes in their EMG activity.
Figure 4. Average fingertip forces.
A. An example of mean peak fingertip forces (± standard error) is shown for each digit in all 16 blocks performed by a single subject in one session. For each instructed flexion (1f – 5f), mean peak force is shown for each digit (thumb – little finger, legend at bottom). Note that the instructed digit always produced the largest force, while smaller forces sometimes were produced by the other, non-instructed digits. B. Similar data averaged across all 10 sessions for the 9 trial blocks representing key behavioral events: the second (a) and fourth (b) control blocks before cuff inflation; the first block after cuff inflation (c); the blocks in which cutaneous sensation at the index fingertip initially decreased (d); in which complete anesthesia was attained at the index fingertip (e); in which voluntary attempts to abduct the index finger produced no EMG in FDI (f); the last block before cuff deflation (g); and the second (h); and fourth (i) recovery blocks after the cuff was deflated. Dashed boxes represent the ischemic period during which a blood pressure cuff placed at the wrist was inflated above arterial pressure.
To examine whether systematic changes in EMG activity occurred in the entire population of recordings obtained from all subjects, we normalized EMG activity on each trial in each recording to the maximum mean change in activity recorded during any of the five instructed flexions from any of the nine data blocks representing key points in the experimental session. Data from a particular instructed flexion then were included in further analyses only if the normalized mean change in EMG activity averaged across all trial blocks of that instructed flexion was greater than or equal to 10% of the maximum mean change in EMG activity. We thereby eliminated data in which little EMG activity occurred in a particular recording during a given instructed flexion. Taking the data from all subjects and all electrodes (surface and intramuscular) there were 50 EMG recording-instructed force combinations for FDI (10 surface recordings × 5 instructed flexion forces). Of these 50 recordings 40 were retained and 10 were rejected. Of the 125 EMG recording-instructed force combinations for FDP (25 intramuscular recordings × 5 instructed flexion forces), 75 were retained and 50 were rejected; and of the 120 EMG recording-instructed force combinations for EDC (24 intramuscular recordings × 5 instructed flexion forces), 96 were retained and 24 were rejected. We then pooled the retained data for all 5 instructed flexions separately for FDI, FDP and for EDC, and then averaged these values (across subjects and across electrodes) for each of the 9 data blocks representing key points in an experimental session.
The population means of the normalized δEMG activity for FDI, FDP, and EDC in each of the nine data blocks representing key points in the experimental session are shown in Figure 4B. On average, FDI EMG had started to decrease when cutaneous sensation in the index fingertip decreased (d), was almost gone by the time the index fingertip was anesthetic (e), and showed no activity in the last data block of the ischemic period (g) (Block f is the first experimental block in which FDI EMG was absent). A one-way repeated measures ANOVA revealed significant differences in the amount of FDI EMG across the nine data blocks [F(8,72)=5.63 p<0.05]. Post-hoc tests showed significantly less FDI EMG during the last three blocks of the ischemic period (e,f,g) than during any of the other blocks—the two control blocks (a,b), the first two blocks after cuff inflation (c,d) and the two recovery blocks (h,i). In contrast, FDP EMG activity showed no changes across the nine selected data blocks [one-way repeated measures ANOVA, F(8,192)=0.64, p>0.05]. EMG activity recorded in EDC appeared to decrease somewhat in the ischemic period, increasing slightly towards control levels during the recovery period. A one-way repeated measures ANOVA showed significant differences in EDC EMG across the nine selected data blocks [F(8,184)=4.37, p<0.05]. Post-hoc tests showed significantly less EMG but only for one comparison; that between the ischemic block when complete anesthesia was attained at the index fingertip (e) and the first control block (a). For the entire population, as FDI became paralyzed during ischemic nerve block, FDP showed no change in EMG activity levels, and EDC EMG decreased only slightly in one block.
Selectivity of fingertip forces and EMG activity
During the ischemic period (culminating in complete nerve block) slight decreases were found both in the force of some instructed flexions (1f, 2f, and 5f) and in the EMG activity recorded from EDC but not FDP. These changes appeared to be associated with parallel decreases in non-instructed forces, suggesting that ischemic nerve block did not affect the selectivity of instructed flexion force production or the underlying EMG activity. To assess this quantitatively, we calculated indices of selectivity for fingertip force production and EMG activity in each of the nine data blocks representing key points in an experimental session, and averaged these indices across instructed flexions and across subjects. As shown in Figure 5 (top) the average selectivity with which the instructed digits exerted forces did not change significantly throughout the experiment [F(8,72)=1.01, p>0.05], suggesting that any decreases in instructed flexion forces were accompanied by decreases in non-instructed forces. Similarly, the selectivity of EMG activity recorded from FDP electrodes [F(8,192)=1.57, p>0.05], EDC electrodes [F(8,184)=0.85, p>0.05], or from all electrodes combined [F(8,384)=0.70, p>0.05] did not change. EMG activity of the extrinsic finger muscles and the fingertip flexion forces they produced thus remained highly selective during ischemic nerve block of the hand.
Figure 5. Mean change in EMG activity in FDI, FDP, and EDC.
A. Average changes in EMG activity recorded simultaneously from surface electrodes on FDI, from two intramuscular electrodes in FDP, and from two in EDC in a single subject are shown for each instructed finger flexion force. Each point represents the trial block average (± standard error) δEMG recorded at that electrode during a particular instructed isometric finger flexion (1f – 5f). B. Similar data are shown, now averaged across all 10 sessions for the 9 trial blocks representing key behavioral events: the second (a) and fourth (b) control blocks before cuff inflation; the first block after cuff inflation (c); the blocks in which cutaneous sensation at the index fingertip initially decreased (d); in which complete anesthesia was attained at the index fingertip (e); in which voluntary attempts to abduct the index finger produced no EMG in FDI (f); the last block before cuff deflation (g); and the second (h); and fourth (i) recovery blocks after the cuff was deflated. These group averaged data have been collapsed over the five instructed forces, but include only data from instructed forces that showed EMG during the control period.
DISCUSSION
During ischemic nerve block at the wrist, healthy subjects readily produced voluntary flexion forces selectively with each digit. The extrinsic finger flexor muscles—FDP (which we recorded) along with flexor pollicis longus and flexor digitorum superficialis (which we did not record), apparently continued to produce these selective fingertip forces during total paralysis of the intrinsic hand muscles. Given that the EMG activity of FDP did not change during hand ischemia, the declines in flexion forces exerted by the thumb during 1f, by the index during 2f, and by the little finger during 5f probably reflect loss of forces normally contributed by intrinsic muscles—such as FDI, (which we recorded), as well as flexor pollicis brevis and flexor digiti minimi (which we did not record). While paralysis of the intrinsics does not prevent flexion of the fingertips, it does, however, render subjects unable to extend the fingertips effectively. Even though extrinsic extensor muscles remained active in the present study, extension forces could not be transmitted to the fingertips, as this requires co-contraction of the intrinsic interosseous muscles that tension the extensor hood. As such, we did not ask subjects to perform extensions. Moreover, since the intrinsic muscles also control abduction and adduction of the digits the loss of this control during ischemic nerve block of the hand necessitated that we tape the fingertips in the apparatus to prevent their slipping out. So although the instrinsic muscles of the hand are necessary for normal individuated control of the digits, subjects remained able to produce selective flexion forces with each digit during total paralysis of these intrinsic muscles.
Ischemic nerve block is known to result in rapid expansion of the cortical representations of muscles proximal to the block, increasing the mutual overlap of these representations and invading the representation of the temporarily deafferented and paralyzed distal muscles (Brasil-Neto et al., 1992; Brasil-Neto et al., 1993; Ridding & Rothwell, 1995; Ziemann et al., 1998). Given that this cortical representation is crucial for normal individuation of finger movements (Schieber & Poliakov, 1998; Lang & Schieber, 2003), we expected to observe a decline in the selectivity of EMG activation in FDP and EDC, and an associated decline in the selectivity of voluntary flexion forces during ischemic nerve block of the hand. While a decrease in force selectivity could be interpreted as evidence that the intrinsic muscles are important for digit individuation, an associated decrease in the selectivity of extrinsic muscle EMG activity would suggest that the increased overlap of cortical representations of extrinsic muscles decreases the ability to selectively activate these muscles during a digit individuation task. Although some quantitative decline in flexion forces was observed as noted above, no systematic changes were found in the selectivity of EMG activation in the extrinsic muscle FDP, nor in the flexion forces exerted at the fingertips. Even though these results show that normal control of muscle selectivity can be retained during ischemic nerve block they cannot be used as evidence that nerve block has no effect on motor control. Our task was relatively easy, with forces remaining well above criterion throughout the experimental session, but had we asked subjects to perform a different task, for example one which required precise tracking of a target force or learning of a motor behavior, nerve block might have affected control of muscles immediately proximal to the block.
In the context of the digit individuation task that we asked subjects to perform, we suggest that ischemic nerve block temporarily dampened representations of muscles distal to the block, but that it did not affect representations of finger flexion movements, and that representations of these movements are retained during temporary deafferentation and paralysis of the hand. This suggestion is consistent with other observations indicating that a representation of distal movements is retained during both ischemic nerve block and actual amputation, and that muscle representations (both proximal and distal to the block) might not be as radically altered as initially supposed.
First, amputees can perform specific voluntary movements with the amputated part, as many amputees have the perception that they can voluntarily move their phantom limb (Mercier et al., 2006; Reilly et al., 2006). In addition, hand transplant recipients learn to control their new hand to accomplish functional tasks (Dubernard et al., 2003; Lanzetta et al., 2005; Neugroschl et al., 2005; Schuind et al., 2006). The time required for nerve regeneration in these transplant recipients makes the acquisition of such control a long process that could entail substantial relearning as well as activation of their distal muscle and movement representations. In contrast, the speed and accuracy with which amputees learn to control a prosthetic limb after targeted reinnervation of remaining proximal muscles (Kuiken et al., 2004) supports the notion that amputees retain a substantial, voluntarily accessible motor representation of movements of the amputated body part.
Second, although stump muscle representations are expanded while the muscle is at rest, voluntary activation can reverse this expansion. Ridding and Rothwell (1995; 1997) found that when hand amputees were at rest, and each hemisphere was stimulated using TMS at the same intensity, the motor cortex map of the flexor carpi radialis (FCR) contralateral to the amputated hand was larger than the FCR map contralateral to the intact hand. When the amputee voluntarily contracted FCR and again each hemisphere was stimulated at the same intensity (though at a lower intensity than that used in the rest condition), the asymmetry of map size reversed: the FCR map in the motor cortex contralateral to the amputated hand now became smaller that the cortical FCR map contralateral to the intact hand. Thus, although at-rest measures suggest that muscles proximal to an amputation have expanded cortical representations, voluntary activation can reverse the expansion. Similarly during ischemic nerve block, although muscle representations expand, inputs to the motor cortex produced by voluntary effort may promptly restore muscle representations to normal.
Third, microneurographic recordings have demonstrated that during ischemic nerve block, expansion of representations is a generalized process, not limited to proximal muscles; cortical output to the motoneuron pools of distal, paralyzed muscles increases as well. Although EMG activity is eliminated distally, intra-neural recordings proximal to the block demonstrate increased TMS-evoked discharge of motoneurons that innervate the paralyzed distal muscles (McNulty et al., 2002). Hence the representations of distal muscles are not lost during nerve block, but presumably expand along with the representations of proximal muscles, as suggested by the increased descending motoneural volley to paralyzed distal muscles.
We speculate, then, that reorganization of the motor system observed during both ischemic nerve block and actual amputation results largely from loss of somatosensory input. The loss of somatosensory input could produce changes rapidly by altering patterns of intracortical inhibition (Jacobs & Donoghue, 1991; Levy et al., 2002). Decreased intracortical inhibition could enlarge representations of all muscles—those proximal to the block or amputation, as well as distal muscles that are temporarily denervated or actually amputated. Inputs received by the motor system with voluntary effort, however, might restore normal representations promptly, in part by normalizing patterns of intracortical inhibition. In the present study, such a motor representation normalized with voluntary effort presumably permitted selective flexion of each finger during ischemic nerve block. In actual amputation, the normalized representation evoked by voluntary effort (despite some degree of anatomical reorganization (Wu & Kaas, 1999)), may give the amputee the perception of movement in the phantom limb (Reilly et al., 2006).
Figure 6. Selectitivy of force and of EMG recordings.
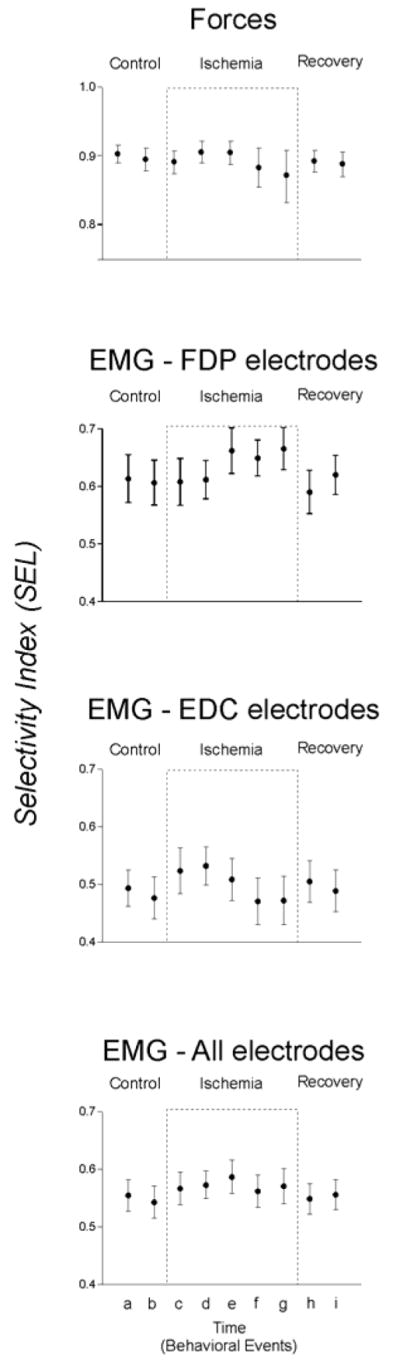
Selectivity of fingertip forces and of EMG activity recorded from FDP and from EDC averaged across all 10 sessions is shown for each of the 9 trial blocks representing key behavioral events; the second (a) and fourth (b) control blocks before cuff inflation; the first block after cuff inflation (c); the blocks in which cutaneous sensation at the index fingertip initially decreased (d); in which complete anesthesia was attained at the index fingertip (e); in which voluntary attempts to abduct the index finger produced no EMG in FDI (f); the last block before cuff deflation (g); and the second (h); and fourth (i) recovery blocks after the cuff was deflated.
Acknowledgments
This work was supported by BCS-022-5611 from the National Science Foundation (USA), R01/R37-NS27686 from the National Institute of Neurological Disorders and Stroke (USA) (MHS), and Fondation pour la Recherche Medicale (France) (KTR). PMc was a K.J. and C.F. Schmitt Foundation Visiting Fellow at the University of Rochester and was also supported by a SpinalCure Australia Kelly McCann Fellowship.
References
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Dubernard JM, Petruzzo P, Lanzetta M, Parmentier H, Martin X, Dawahra M, Hakim NS, Owen E. Functional results of the first human double-hand transplantation. Ann Surg. 2003;238:128–136. doi: 10.1097/01.SLA.0000078945.70869.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Sterr A, Flor H, Rockstroh B, Knecht S, Pantev C, Wienbruch C, Taub E. Input-increase and input-decrease types of cortical reorganization after upper extremity amputation in humans. Experimental Brain Research. 1997;117:161–164. doi: 10.1007/s002210050210. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Winter C, Schaefer M, Fritzsche K, Benhidjeb T, Tunn P, Schlag PM, Flor H. Perceptual phenomena after unilateral arm amputation: a pre-post-surgical comparison. Neuroscience Letters. 2001;302:13–16. doi: 10.1016/s0304-3940(01)01606-8. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Flament D, Fraser C, Lemon RN. Non-invasive brain stimulation reveals reorganized cortical outputs in amputees. Neuroscience Letters. 1990;116:379–386. doi: 10.1016/0304-3940(90)90105-i. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. Journal of Neurophysiology. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Lanzetta M, Pozzo M, Bottin A, Merletti R, Farina D. Reinnervation of motor units in intrinsic muscles of a transplanted hand. Neurosci Lett. 2005;373:138–143. doi: 10.1016/j.neulet.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Annals of Neurology. 2002;52:755–761. doi: 10.1002/ana.10372. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Newell KM, Zatsiorsky VM. Motor redundancy during maximal voluntary contraction in four-finger tasks. Experimental Brain Research. 1998;122:71–78. doi: 10.1007/s002210050492. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Macefield VG, Taylor JL, Hallett M. Cortically evoked neural volleys to the human hand are increased during ischaemic block of the forearm. Journal of Physiology. 2002;538:279–288. doi: 10.1113/jphysiol.2001.013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Reilly KT, Vargas CD, Aballea A, Sirigu A. Mapping phantom movement representations in the motor cortex of amputees. Brain. 2006;129:2202–2210. doi: 10.1093/brain/awl180. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Neugroschl C, Denolin V, Schuind F, Van Holder C, David P, Baleriaux D, Metens T. Functional MRI activation of somatosensory and motor cortices in a hand-grafted patient with early clinical sensorimotor recovery. Eur Radiol. 2005;15:1806–1814. doi: 10.1007/s00330-005-2763-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catala MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. NeuroReport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques.[see comment] Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Mercier C, Schieber MH, Sirigu A. Persistent hand motor commands in the amputees’ brain. Brain. 2006;129:2211–2223. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Schieber MH. Incomplete functional subdivision of the human multitendoned finger muscle flexor digitorum profundus: an electromyographic study. J Neurophysiol. 2003;90:2560–2570. doi: 10.1152/jn.00287.2003. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Reorganisation in human motor cortex. Canadian Journal of Physiology & Pharmacology. 1995;73:218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalography & Clinical Neurophysiology. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Experimental Brain Research. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Deuel RK. Primary motor cortex reorganization in a long-term monkey amputee. Somatosensory & Motor Research. 1997;14:157–167. doi: 10.1080/08990229771024. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Gardinier J, Liu J. Tension distribution to the five digits of the hand by neuromuscular compartments in the macaque flexor digitorum profundus. Journal of Neuroscience. 2001;21:2150–2158. doi: 10.1523/JNEUROSCI.21-06-02150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. Journal of Neuroscience. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuind F, Van Holder C, Mouraux D, Robert C, Meyer A, Salvia P, Vermeylen N, Abramowicz D. The first Belgian hand transplantation-37 month term results. J Hand Surg. 2006 doi: 10.1016/j.jhsb.2006.01.003. [Br] [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. Journal of Neurophysiology. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125:1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. Journal of Neuroscience. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. Journal of Neuroscience. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]