Abstract
Individuated finger movements of the human hand require selective activation of particular sets of muscles. Such selective activation is controlled primarily by the motor cortex via the corticospinal tract. Is this selectivity therefore lost when lesions damage the corticospinal tract? Or when the motor cortex reorganizes after amputation?
We studied finger movements in normal human subjects and in patients who had recovered substantially from pure motor hemiparesis caused by lacunar strokes, which damage the corticospinal tract without affecting other pathways. Even after substantial recovery from these strokes, individuation of finger movements remained reduced— both for flexion/extension and for adduction/abduction motion of the fingers. Stroke subjects regained the ability to move the instructed digit through a normal range, but unintentional motion of other digits was increased. This increase did not result from a change in the passive biomechanical coupling of the fingers. Rather, voluntary contractions of muscles that move the intended digit were accompanied by inappropriate contractions in muscles acting on additional digits. These observations suggest that the normal corticospinal system produces individuated finger movements not only by selectively activating certain muscles, but also by suppressing activation of other muscles during voluntary effort to move a given digit.
In a separate experiment, reversible amputation of the hand was produced in normal subjects by ischemic nerve block at the wrist. Motor output to the intrinsic muscles and sensory input both become blocked under these conditions, effectively amputating the hand from the nervous system. But the long extrinsic muscles that flex and extend the digits remain normally innervated, and thus flexion forces still can be generated at the fingertips. During reversible amputation of the hand produced by ischemic nerve block, the ability of subjects to activate subdivisions of extrinsic muscles and to exert flexion force at individual fingertips continued to show essentially normal selectivity. Voluntary activation of the remaining muscles thus continues to be selective after amputation, in spite of both the loss of sensory input from the amputated hand, and reorganization within the primary motor cortex.
During cortical reorganization after amputation, then, voluntary patterns of motor output intended for finger muscles may not be lost. We therefore examined activity in the stump muscles of above-elbow amputees, who have no remaining hand muscles. Different movements of the phantom hand were accompanied by different patterns of EMG in remaining proximal muscles, distinct from the EMG patterns associated with movement of the phantom elbow. We infer that voluntary motor output patterns that normally control finger movements after amputation may become diverted to remaining proximal muscles.
Introduction
Individuated finger movements require selective activation of particular sets of muscles (Long et al., 1970; Maier and Hepp-Reymond, 1995a; Maier and Hepp-Reymond, 1995b; Reilly and Schieber, 2003; Schieber, 1995; Valero-Cuevas, 2000; Valero-Cuevas et al., 2000). Crucial control of these movements is transmitted to the spinal cord from the primary motor cortex (M1) via the corticospinal tract. Lesions of the motor cortex or its corticospinal projection result in weakness of these muscles, often accompanied by increased reflexes and changes in tone, all of which impair hand function. Here we review recent studies showing that weakness, reflex changes and altered tone are not the only factors that impair hand function in patients with corticospinal lesions. When strength is adequate to move the fingers, muscles still cannot be activated selectively enough to move fingers individually. This deficit of selective activation cannot be attributed simply to changes in reflexes or tone, and therefore reflects loss of the control from M1 that normally produces the selective patterns of muscle activation needed to individuate finger movements.
The finger movement representations in M1 classically were viewed as separate regions controlling each digit. But experimental evidence developed over the past few decades indicates that control of different fingers involves extensively overlapping regions in M1 (Schieber, 2001; Schieber and Santello, 2004). In contrast to a static, somatotopically organized keyboard that plays finger movements, the distributed control of the hand from M1 provides a substrate that can be reorganized. One circumstance in which such reorganization occurs is peripheral amputation. After amputation of the distal upper extremity, stimulation of the cortical territory that previously had represented the fingers rapidly comes to evoke contractions of the proximal muscles remaining in the stump. This previously has been viewed as an invasion of the cortical finger territory by representation of the remaining proximal musculature, improving control of that musculature to compensate in part for loss of the amputated hand. Here we review recent evidence supporting an alternative view: that the re-routing of output from the M1 finger representation to more proximal musculature may serve instead to maintain distinct representations of selective muscle activation for different hand and finger movements.
Finger movements after stroke
Damage to the motor cortex or corticospinal tract has long been known to produce weakness of the affected body parts. Because most of the descending corticospinal axons cross sides at the cervico-medullary junction, weakness appears in the limbs ipsilateral to a lesion in the spinal cord but contralateral to a lesion in the brain. This weakness is more profound in distal than in proximal muscles, and finger movements become particularly weak (Colebatch and Gandevia, 1989). Above and beyond the degree of weakness, however, affected patients show a deficit of movement individuation. Voluntary movement of a particular digit is accompanied by more movement of adjacent digits, and the wrist, elbow and shoulder may move as well (Brunstrom, 1970) (Zackowski et al., 2004). Even when substantial strength recovers, a deficit of relatively independent finger movements that impairs tasks requiring fine manipulation typically persists, often permanently (Lawrence and Kuypers, 1968).
We have investigated factors underlying this loss of individuation in human subjects with pure motor hemiparesis, a stroke syndrome in which weakness of the face, arm and leg occurs contralateral to a circumscribed infarction relatively restricted to corticospinal fibers in the posterior limb of the internal capsule, or in the basis pontis (Fisher, 1982; Fisher and Curry, 1965). These lesions typically produce no sensory, language or cognitive deficits. Changes in motor performance therefore can be attributed by and large to disruption of the corticospinal pathway and any subsequent reorganization. We evaluated 10 subjects selected for recovery sufficient to hold a pen with a precision grip and to walk short distances with an assistive device. Subjects were studied 2 to 34 months after stroke.
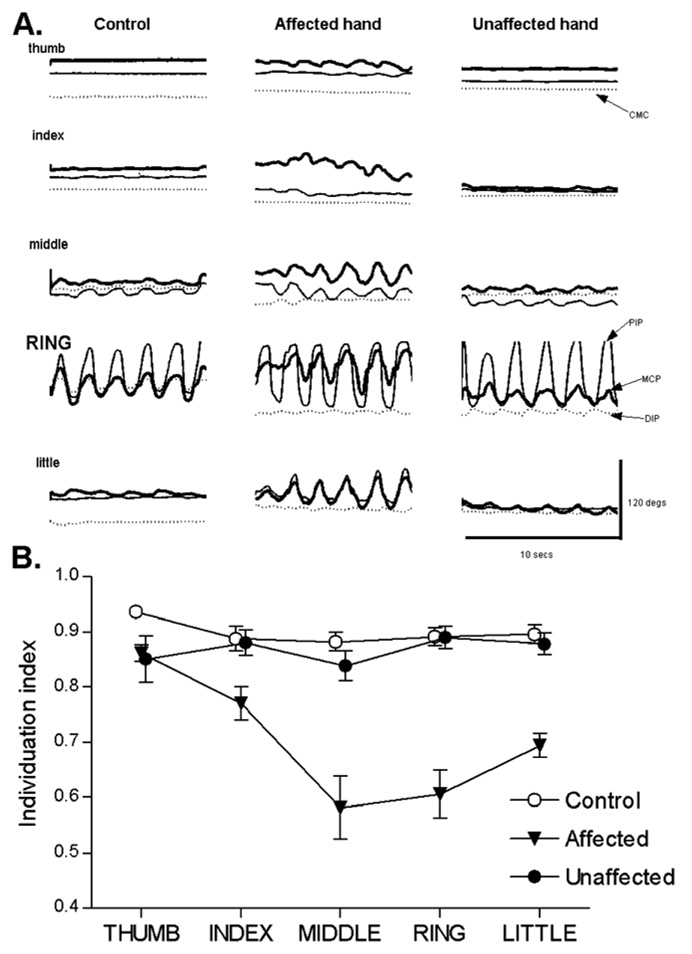
An instrumented glove recorded the motion of all the digits simultaneously as subjects performed cyclical flexion/extension or abduction/adduction movements of each digit. Figure 1A shows typical data from flexion/extension movements of the ring finger performed by a normal age-matched control subject, the affected hand of a subject with pure motor hemiparesis, and the unaffected hand of the same subject. In the control hand and in the patient’s unaffected hand, intentional movement of the ring finger was accompanied by low amplitude movement in the joints of the middle and little fingers. But in the affected hand, the accompanying movement of the middle and little fingers was much larger, and low amplitude movement was present as well in the thumb and index finger.
Figure 1.
A. Single trials of joint position versus time for a control (left column), and the affected (middle column) and unaffected hand (right column) of a hemiparetic subject. In each case, the subject was instructed to flex and extend the ring finger cyclically at a comfortable rate. Joint traces from the thumb are on top, followed by the index, middle, ring, and little (bottom) fingers in descending order. The thick line shows movement at the metacarpophalangeal joint (MCP), the thin line shows movement at the proximal interphalangeal joint (PIP), and the dotted line shows movement at the distal interphalangeal joint (DIP). For the thumb, the dotted line shows movement at the carpo-metacarpal joint (CMC). B. Data like that in A were used to calculate an individuation index for each digit in control (open circles), affected (filled inverted triangles) and unaffected (filled circles) hands. Each point represents a group mean ± standard error. Reproduced with permission from figure 3 and figure 5 of (Lang and Schieber, 2003).
We quantified the motion of the non-instructed digits using a previously described individuation index, which varies from 1 when there was no motion of non-instructed digits to 0 when all non-instructed digits moved as much as the instructed digit (Schieber, 1991). As shown in Figure 1B, the individuation index of affected fingers was lower than that of fingers in normal controls or the unaffected hands of subject with pure motor hemiparesis (Lang and Schieber, 2003). Similar deficits have been observed in the selectivity of isometric flexion force production at the fingertips following stroke (Li et al., 2003). Abduction/adduction movements showed a greater loss of individuation than flexion/extension movements (Lang and Schieber, 2004a).
Given that the motor cortex and corticospinal tract have a relatively magnified representation of the thumb compared to that of the other digits (Penfield and Rasmussen, 1950) (Woolsey et al., 1952), one might have expected that in patients with pure motor hemiparesis the thumb would show the most profound impairment. But instead the thumb showed the highest individuation index, both for flexion/extension and abduction/adduction movements. The higher degree of individuation of the thumb as compared to other digits in patients recovering from pure motor hemiparesis might reflect i) a larger reserve of thumb control in a partially damaged system, ii) greater reorganization of thumb control because of more emphasis on tasks that require use of the thumb during rehabilitation, and/or iii) greater biomechanical independence of the thumb and the muscles that move it.
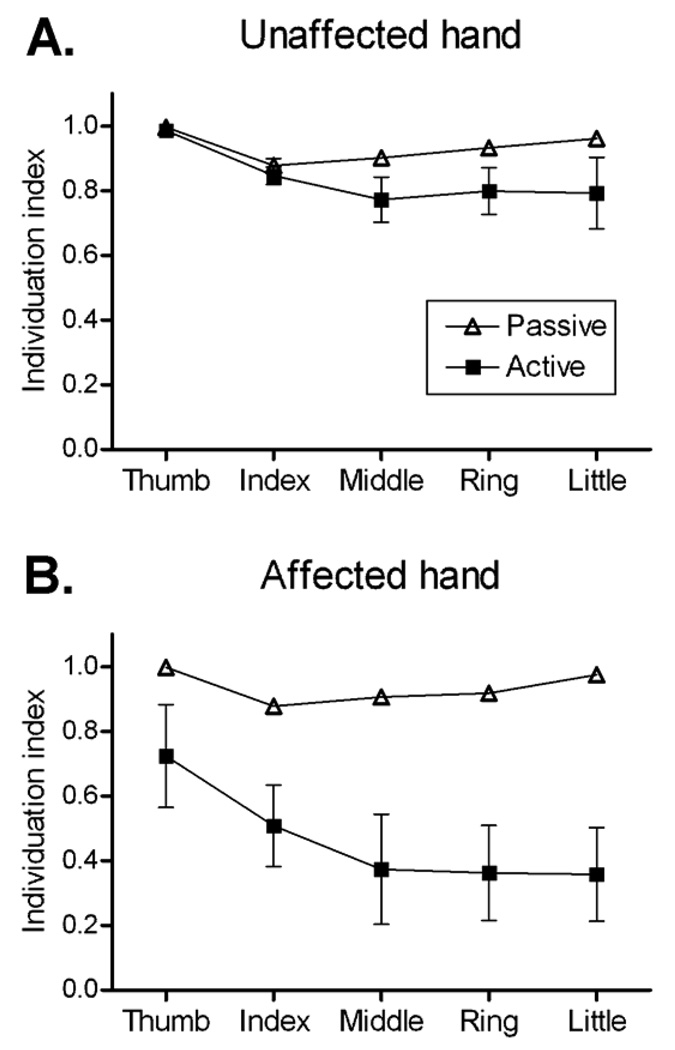
Both passive (mechanical and involuntary) and active (voluntary) factors might contribute to the deficits in individuation that result from lesions of the motor cortex or corticospinal tract. To distinguish the contributions of passive and active factors, we splinted the proximal and distal interphalangeal joints (one finger at a time) to permit movement only at the metacarpophalangeal joint, and then asked subjects to perform flexion/extension movements of the splinted finger actively. Next, a motor rotated each splinted finger passively at the metacarpophalangeal joint, using a rate and range of motion similar to that produced by the subject actively. During both passive and active movements, the simultaneous motion of all finger joints was recorded with an instrumented glove (Lang and Schieber, 2004a).
Figure 2 shows group data for the unaffected (A) and affected hands (B) of subjects with pure motor hemiparesis. In both unaffected and affected hands individuation indexes were higher during passive movement of the digits than during active movement. This indicates that even in normal hands, active, voluntary muscle contractions contribute to the concurrent low-amplitude movement of non-instructed digits along with the instructed digit, particularly in the more ulnar fingers (Lang and Schieber, 2004a). In hands affected by corticospinal lesions, we thought it possible that the hyperreflexia, abnormal spread of reflexes among muscles, increased tone, and muscle contracture, all of which can accompany the weakness in hemiparesis (Given et al., 1995; Kamper and Rymer, 2000; O'Dwyer et al., 1996; Vattanasilp et al., 2000), might contribute to increased motion of the non-instructed digits in the passive condition. Such increased coupling produced by these involuntary factors would have been evident as a decrease in passive individuation indexes in the affected hands. But as shown in Figure 2, the individuation indexes during passive motion of affected hands in our pure motor hemiparesis subjects were just as high as those of control hands. In contrast, during active movement the individuation indexes of the affected hands were significantly lower than those of the control hands. This active/passive difference indicates that the deficit of individuation reflects a change in active control of finger movements following corticospinal lesions.
Figure 2.
Individuation indexes are shown for passive (open triangles) and active (filled squares) movements of each digit in unaffected (A) and affected (B) hands of patients with pure motor hemiparesis. Points represent group means ± standard error. For most passive points the standard error bars fall within the symbol representing the mean.
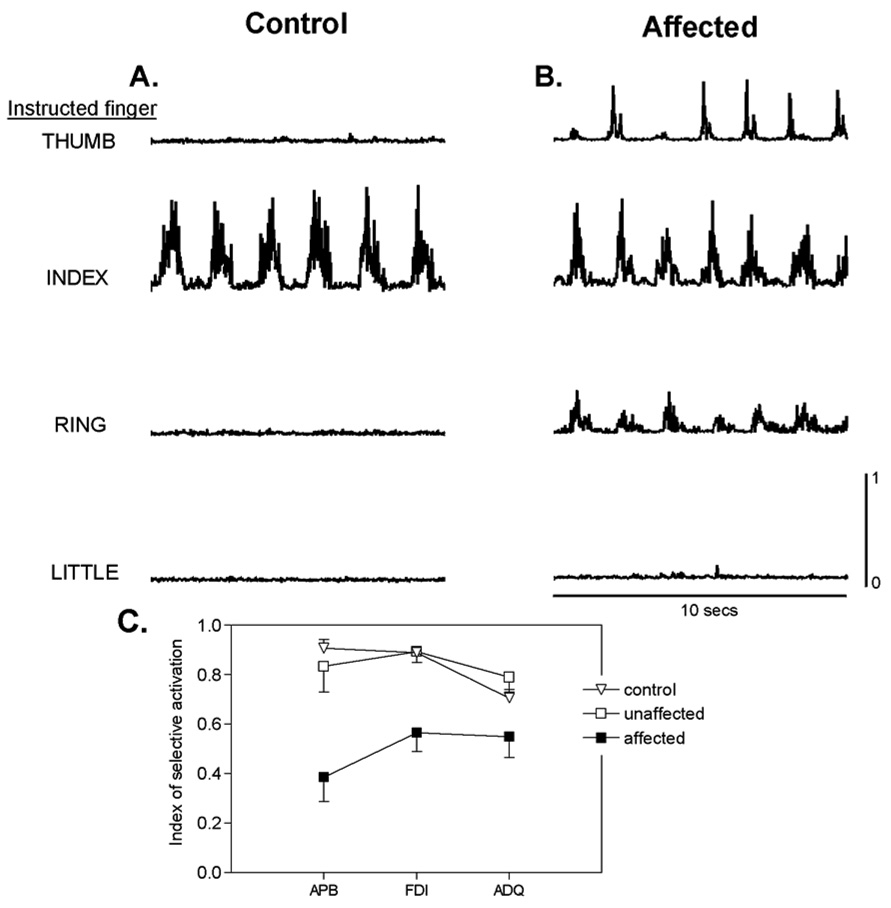
To examine this change in active control, we recorded surface EMG activity from three intrinsic muscles of the hand as subjects made abduction/adduction movements (Lang and Schieber, 2004b). We recorded from the abductor pollicis brevis (APB), first dorsal interosseous (FDI) and abductor digiti quinti (ADQ), which act on the thumb, index finger and little finger, respectively. Figure 3A shows example recordings from the FDI of a control and of an affected hand during cyclical abduction/adduction movements of the thumb, index finger, ring finger and little finger. (Middle finger movements were not studied here because our instrumented glove did not transduce abduction/adduction movement of the middle finger independently.) Whereas FDI in the control hand was active only when the index finger was abducting, FDI in the affected hand was active as well during movement of the thumb or the ring finger. These inappropriate contractions of FDI in the affected hand would cause the index finger to move when the subject attempted to move only the thumb or the ring finger. The resulting inappropriate movement of the non-instructed index finger would lower the individuation index of thumb and ring finger movements.
Figure 3.
Rectified surface EMG activity recorded from the first dorsal interosseous (FDI) muscle in the hand of a normal control subject (A) and the affected hand of a subject with pure motor hemiparesis (B) is shown from single trials in which the subjects were asked to perform cyclical abduction/adduction movements of the thumb, index, ring and little fingers at a comfortable rate. (C) shows the index of selective activation for three instrinsic hand muscles, abductor pollicis brevis (APB), FDI, and abductor digiti quinti (ADQ), that act on the thumb, index and little finger, respectively, in control (open inverted triangles), unaffected (open squares) and affected (filled squares) hands. Points represent group means ± standard error. The selectivity of activation with intended movement of particular digits was reduced significantly for all three intrinsic muscles in hands affected by pure motor hemiparesis. Reproduced with permission from figures 4 and 6 of (Lang and Schieber, 2004b).
We quantified the degree to which each muscle was active selectively during movement of a single finger using an index of selective activation that could vary from 1 (contraction for only one movement) to 0 (equal contraction for all movements). Figure 3B presents the index of selective activation for each muscle averaged over the control, unaffected and affected hands. In subjects affected by pure motor hemiparesis, the index of selective activation was reduced in all three muscles. Hence when patients with corticospinal tract lesions attempt to move a particular digit, inappropriate contractions occur in muscles that move other digits. These other digits therefore move along with the intended digit to an abnormal degree. Such observations suggest that i) the corticospinal system normally acts to minimize muscle contractions that would move other digits, and/or ii) other descending systems (rubrospinal, reticulospinal) which may participate in compensating for the corticospinal lesion are unable to activate muscles as selectively as the normal corticospinal system.
Representation of finger movements during ischemic nerve block
Although the representation of movements in the motor cortex of adults classically was thought to be static, studies in recent decades have shown that this representation is capable of considerable plastic reorganization (Chen et al., 2002; Nudo et al., 2001; Sanes and Donoghue, 2000). After practicing a movement, stimulation at foci where contraction of certain muscles was evoked previously now evokes contraction of different muscles. Similarly after amputation, stimulation of the cortical territory from which movement of the amputated part previously would have been evoked, now evokes contraction of the more proximal muscles remaining in the stump (Chen et al., 1998; Cohen et al., 1991; Dettmers et al., 1999; Karl et al., 2001; Pascual-Leone et al., 1996; Röricht et al., 1999; Schieber and Deuel, 1997; Wu and Kaas, 1999). The cortical territory that previously represented the amputated part thus appears to have been “invaded” by representation of the more proximal stump muscles.
What effect does this “takeover” have on the existing representations of movements of the amputated part? Do these representations remain latent in the cortex? Or are they eliminated so that cortical machinery that has been cut off from its normal effector can be put to use controlling movements of the remaining proximal parts (Chen et al., 2002)? The hand provides an interesting model in which to approach these questions because the digits are moved by two sets of muscles. The intrinsic muscles of the hand lie within the palm. The extrinsic finger muscles have bellies situated in the forearm, and tendons that cross the wrist and palm to reach the digits. Ischemic nerve block at the wrist therefore can denervate the hand, temporarily cutting it off from the nervous system, while motor and sensory innervation of the extrinsic muscles that flex and extend the digits remains intact.
Previous studies have shown that ischemic nerve block in either the upper or the lower extremity is followed rapidly by expansion of the cortical representation of muscles proximal to the block (Brasil-Neto et al., 1992; Brasil-Neto et al., 1993; Ziemann et al., 1998). Given that the cortical territories controlling both intrinsic and extrinsic finger muscles normally are intermingled and overlapping (Andersen et al., 1975; Schieber, 2001; Wassermann et al., 1992), ischemic nerve block at the wrist can be expected to produce rapid expansion of extrinsic muscle representations into territory that normally represents intrinsic muscles. We therefore investigated whether ischemic nerve block at the wrist, temporarily denervating the intrinsic muscles and blocking cutaneous afference from the hand, would alter the ability of the nervous system to selectively activate the extrinsic finger muscles as needed to exert force selectively at each fingertip.
The extrinsic muscle, flexor digitorum profundus (FDP), sends a separate tendon to the distal phalanx of each of the four fingers. In normal human subjects, motor units in FDP are recruited at lowest force threshold during flexion of a particular finger, but many FDP motor units are recruited at a slightly higher force during flexion of adjacent fingers (Kilbreath and Gandevia, 1994), and exert some force on adjacent fingers as well (Kilbreath et al., 2002). Furthermore, motor units in regions of FDP acting on different fingers often show short-term synchronization, indicating that motoneurons acting on different fingers nevertheless receive common input from the same corticospinal axons (Bremner et al., 1991a; Bremner et al., 1991c; Bremner et al., 1991b; Datta et al., 1991; Reilly et al., 2004). Functionally, FDP contains core regions that are activated selectively during flexion of a particular finger, and other regions that are activated to some degree during flexion of adjacent fingers as well (Fleckenstein et al., 1992; Reilly and Schieber, 2003). Another extrinsic finger muscle, the extensor digitorum communis (EDC), has somewhat more selective motor units, although some tension from EDC motor units may be distributed to adjacent fingers through interconnections between the tendons (Keen and Fuglevand, 2003b). As in FDP, many EDC motor units acting on different fingers receive common inputs (Keen and Fuglevand, 2003a). We therefore asked whether the selective activation of regions in these extrinsic muscles acting on different finger might change when their cortical representations expand into the territory of intrinsic hand muscles.
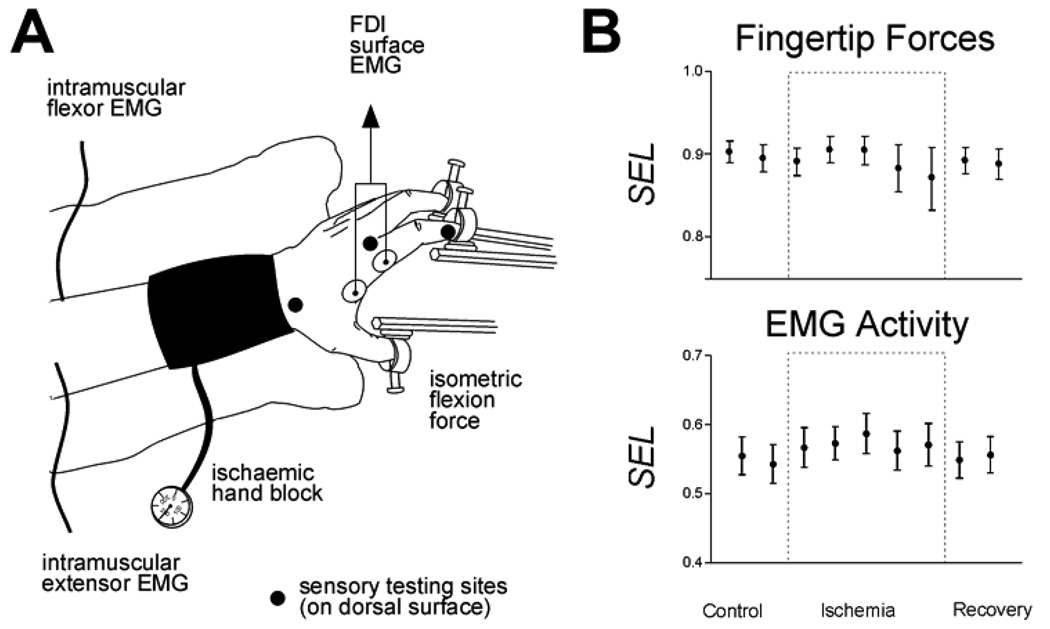
To address this question, in 10 experimental sessions we recorded fingertip forces and intramuscular EMG activity from FDP and EDC as 9 normal human subjects produced flexion forces with the tip of each finger and the thumb in blocks of 50 trials (10 trials per digit presented in randomized order cued by an LED display). After collecting blocks of data every 5 minutes for 20 minutes, a pediatric blood pressure cuff placed about the wrist was inflated to 200 mm Hg, rendering the hand and fingers ischemic. The subject continued to perform blocks of finger flexions every 5 minutes until von Frey filaments no longer could be felt on the digits, and the first dorsal interosseous muscle (FDI, a hand intrinsic muscle) showed no EMG activity during vigorous efforts to abduct the index finger. Then the subject performed a final block of flexion movements with the hand still ischemic. Thereafter the cuff was deflated, and 4 more blocks of finger flexions were performed at 5 minute intervals during recovery from ischemic nerve block.
For each block of data we calculated an index of the selectivity with which the subject exerted flexion force on each instructed digit, and an index of the selectivity of each EMG recording for flexion of one of the five digits (Figure 4). None of the subjects had difficulty performing the task prior to the onset of ischemia, and the data collected during the control period therefore reflects a normal level of variability. Although individual examples of fluctuation were observed during ischemic nerve block, no consistent changes either in the selectivity of force exerted at the fingertips or in the selectivity of EMG activity for particular digits were produced by ischemic nerve block. These observations suggest that the M1 representations of individuated finger movements remain in place during temporary amputation of the hand produced by ischemic nerve block at the wrist.
Figure 4.
Ischemic nerve block at the wrist. A. The subject exerted flexion forces with each digit in turn against stationary load cells as intramuscular EMG was recorded from different regions of the extrinsic flexor muscle, flexor digitorum profundus, and the extrinsic extensor muscle, extensor digitorum communis. After performing control blocks, the hand was rendered ischemic by inflation of a pediatric blood pressure cuff about the wrist. The subject continued to perform finger flexions while the progress of the ischemic nerve block was monitored with sensory testing at the three sites marked and with surface EMG recorded from the first dorsal interosseous muscle (FDI). After cutaneous sensation and EMG were lost in the hand, the cuff was deflated and the subject continued to perform fingertip flexion forces during recovery from ischemic nerve block. B. Selectivity indexes calculated for the force exerted by each instructed digit averaged across fingers and across subjects are shown above, and selectivity indexes calculated for the EMG activity recorded from intramuscular electrodes averaged across recordings and across subjects are shown below. Neither the selectivity of fingertip forces nor that of EMG activity changed while the hand was denervated.
Any expansion of the M1 representation of muscles proximal to the block thus did not reduce the ability of the nervous system to activate different regions within FDP and EDC voluntarily with a normal degree of selectivity. Nor was the selectivity of voluntarily produced fingertip forces reduced. EMG activity during ischemic nerve block can be assessed only in muscles proximal to the block, however. Previous studies using microneuronography to record the efferent outflow in the axons of motoneurons have shown that cortical output to muscles distal to an ischemic nerve block increases along with the output to proximal muscles (McNulty et al., 2002). Taken together, these results suggest that even though the cortical output to both innervated and denervated muscles increases during ischemic nerve block, this change is not accompanied by loss of the specific representations used voluntarily to activate muscles selectively in producing individuated finger movements.
Representation of finger movements after amputation
Reorganization during ischemic nerve block occurs quickly, but also reverses rapidly when blood flow resumes and the nerve block is reversed. More profound reorganization of output to muscles that remain innervated therefore might be found in subjects who have sustained actual amputations. In such amputees, however, determining which fingers previously had been acted upon by any given region of the remaining extrinsic muscles is precluded by our inability to observe finger movements. Microneuronography likewise is unable to specify which intrinsic muscle had been innervated by the remaining proximal portion of motor axons. These pitfalls preclude studies in amputees like those described above in normal subjects during ischemic nerve block.
Many amputees, however, have the sensory perception that the amputated body part still is present, a phantom limb (Kooijman et al., 2000; Ramachandran and Hirstein, 1998; Ramachandran and Rogers-Ramachandran, 2000). The amputee may experience ongoing pain perceived as arising in the phantom limb. The phantom limb often is perceived as being reduced in size and foreshortened, as if telescoped into the remaining stump. When the skin of the stump or face is touched, upper extremity amputees often perceive the touch as having occurred on the phantom limb.
Although these sensory phenomena of the phantom limb have been studied most extensively, some amputees also report that they can perform specific voluntary movements of all or part of their phantom limb. Indeed, performing such phantom movements may reduce phantom pain (Giraux and Sirigu, 2003). Furthermore, functional magnetic resonance imaging shows activation of the sensorimotor cortex during phantom limb movements. This activation appears in the sensorimotor region that normally would have been activated during movement of the amputated part, and is different from the region activated by movement of the stump (Roux et al., 2003)
We therefore investigated whether such phantom limb movements are accompanied by specific patterns of EMG activity in the remaining muscles of the stump (Reilly et al., 2006). While surface EMG activity was recorded from selected stump muscles, as well as more proximal upper extremity muscles, amputees were asked to perform different voluntary movements of their phantom hand. Amputees were asked to make only those phantom limb movements which they perceived their phantom was able to make. Because these movements could not be monitored directly, the amputee was asked to make simultaneous cyclical movements of the intact hand in parallel with the phantom hand, and movement of the intact hand was monitored with an instrumented glove. Alternatively, an investigator wore an instrumented glove and the subject watched the investigator’s hand while making parallel cyclical phantom hand movements. For elbow and shoulder movements, movement phases were marked manually based on the verbal report of the amputee
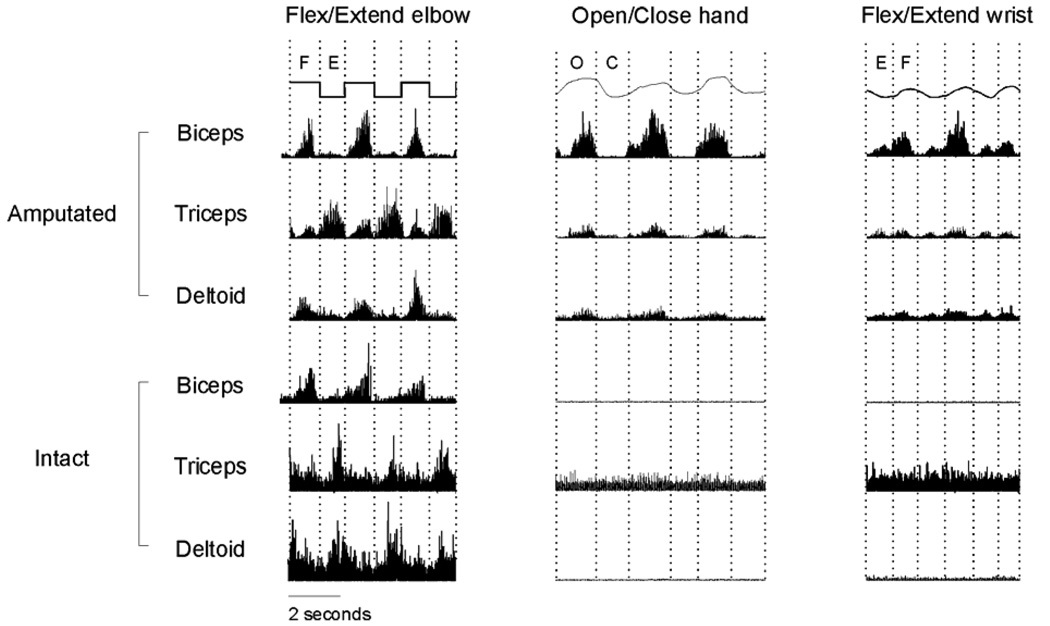
Subject RR sustained an above-the-elbow amputation of the left forearm and hand 15 years prior to recording. All intrinsic hand muscles, extrinsic finger muscles and wrist muscles therefore were amputated. Biceps and triceps remained in the stump although their distal insertions were amputated, and deltoid remained intact at the shoulder. Figure 5 shows rectified EMG activity recorded from the biceps, triceps and deltoid on both the amputated and intact sides of RR during 3 different cyclical movements performed simultaneously with the intact arm and the phantom. As RR made cyclical movements of the phantom elbow, alternating contractions of the biceps and triceps appeared on the amputated side during flexion and extension, respectively, similar to the contralateral muscle contractions moving the intact elbow. The EMG activity patterns were not entirely normal on the amputated side, however. Triceps and deltoid both showed small bursts during phantom elbow flexion.
Figure 5.
Raw EMG and kinematic profiles for patient RR executing three distinct cyclic bilateral movements. Instructed movement is noted above each column and movement phase is indicated as F (flexion), E (extension), O (opening) or C (closing). EMG has been scaled to maximize clarity, with EMG from a single muscle on a constant scale, but with different scales among the muscles. Reproduced with permission from Figure 2 of Reilly et al., 2006.
Distinct patterns of EMG activity also appeared on the amputated side during opening/closing of the phantom hand or flexion/extension of the phantom wrist, distal movements for which biceps, triceps and deltoid normally would not be active. Opening and closing of the phantom hand was accompanied, however, by simultaneous bursts of EMG in biceps, triceps and deltoid during opening, followed by little if any EMG activity during closing. In contrast, flexion and extension of the phantom wrist was accompanied by bursts from all three muscles during both the flexion and extension phases. Similar phasic contractions of biceps, triceps and deltoid did not occur on the intact side during synchronous movements of the intact hand or wrist. Distinct phantom movements of the amputated body parts thus were accompanied by distinct EMG patterns in remaining proximal muscles which normally would not have been activated. Similar observations have been obtained in two other amputees.
Tracing studies in experimental animals have shown that some spinal motoneurons axotomized by amputation can successfully innervate remaining stump muscles (Wu and Kaas, 2000). Innervation of the biceps, triceps and deltoid by motor axons that previously had innervated hand muscles may account in part for our observations that these muscles were activated during movements of the phantom hand. Alternatively, during voluntary attempts to move the phantom hand, motor output from the cortical hand representation may have been redirected to the remaining proximal musculature. In either case, rather than the representation of hand movements having been eliminated through “invasion” by proximal movement representations (Dettmers et al., 2001; Irlbacher et al., 2002; Schieber and Deuel, 1997), our amputated subjects still produced distinct patterns of motor output during voluntary efforts to perform distinct phantom hand movements. The “invasion” thus may reflect, not expanded control of proximal movements, but rather re-routing of output from representations of distal voluntary movements to activate proximal musculature.
Two additional observations suggest that motor output alone is not entirely responsible for the phenomenon of phantom movement. In one patient with a vivid but immobile phantom, attempts to perform different phantom movements all were associated with EMG activity in stump muscles, but for each attempted movement the subject produced the same EMG pattern. Thus the subject’s sensation that the phantom moves may require not only the ability to access remaining proximal muscles, but also the ability to produce multiple distinct patterns of EMG activity in these remaining proximal muscles. Second, in patients with below-the-elbow amputations and mobile phantom hands, ischemic nerve block at the elbow renders the phantom immobile. Hence sensory feedback from the remaining muscles may be needed to have the sensation that the phantom has moved.
Conclusions
The output of the motor cortex via its corticospinal projection is crucial for the production of individuated finger movements. Our studies in patients with lacunar strokes that interrupted the corticospinal projection show that the remaining descending pathways mediating voluntary movement are unable to produce the necessary patterns of selective muscle activation. When subjects with pure motor hemiparesis attempt to contract the muscles that move a given finger, additional contractions occur in other muscles, producing concurrent motion of other fingers.
In contrast, the selectivity of finger muscle activation was unaffected by ischemic nerve block at the wrist. Blocking both the cutaneous sensory input from the fingers and the proprioceptive input from the intrinsic hand muscles produced little if any change in the ability to exert selective flexion forces at different fingertips using only the extrinsic finger muscles. Any reorganization of the cortical representation of the extrinsic muscles, which remained innervated proximal to the block, thus did not change the ability of the cortex to activate them selectively under voluntary control.
Long-term amputees likewise showed normal patterns of EMG activity in remaining stump muscles during phantom movements that normally would have utilized those muscles. But when performing phantom movements that normally would have utilized muscles lost with the amputation, distinct patterns of EMG activity appeared in the remaining proximal muscles for different phantom movements. Although the output of these distal muscle/movement representations is re-routed to remaining proximal musculature after amputation, we infer that the representations of the amputated muscles and the movements they produced remain latent in the cortex.
The cortical representation of muscles and movement reorganizes rapidly after ischemic nerve block or amputation, but such reorganization does not eliminate the ability of voluntary activation of the motor cortex to elicit different selective patterns of muscle activity. In contrast, lesions damaging the corticospinal pathway, effectively cutting off the spinal cord from direct cortical control, result in long-lasting impairment of the ability to produce selective patterns of EMG activity.
Acknowledgements
This work was supported by NINDS grants NS44584 to CEL and NS27686 to MHS, NSF grant BCS0225611 to MHS and AS, PM was supported by SpinalCure Australia
References
- Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon's hand. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1975;188:31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Correlation between the discharges of motor units recorded from the same and from different finger muscles in man. J Physiol (Lond ) 1991a;432:355–380. doi: 10.1113/jphysiol.1991.sp018389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. J Neurophysiol. 1991b;66:2072–2083. doi: 10.1152/jn.1991.66.6.2072. [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol (Lond ) 1991c;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom S. New York: Harper and Row; 1970. Movement Therapy in Hemiplegia: A Neurophysiological Approach. [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci. 1998;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Datta AK, Farmer SF, Stephens JA. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol (Lond ) 1991;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers C, Adler T, Rzanny R, Van Schayck R, Gaser C, Weiss T, Miltner WH, Bruckner L, Weiller C. Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci Lett. 2001;307:109–112. doi: 10.1016/s0304-3940(01)01953-x. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Liepert J, Adler T, Rzanny R, Rijntjes M, Van Schayck R, Kaiser W, Brückner L, Weiller C. Abnormal motor cortex organization contralateral to early upper limb amputation in humans. Neurosci Lett. 1999;263:41–44. doi: 10.1016/s0304-3940(99)00105-6. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Curry HB. Pure motor hemiplegia of vascular origin. Arch Neurol. 1965;13:30–44. doi: 10.1001/archneur.1965.00470010034005. [DOI] [PubMed] [Google Scholar]
- Fleckenstein JL, Watumull D, Bertocci LA, Parkey RW, Peshock RM. Finger-specific flexor recruitment in humans: depiction by exercise-enhanced MRI. J Appl Physiol. 1992;72:1974–1977. doi: 10.1152/jappl.1992.72.5.1974. [DOI] [PubMed] [Google Scholar]
- Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage. 2003;20(Suppl 1):S107–S111. doi: 10.1016/j.neuroimage.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Given JD, Dewald JP, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Neurol Neurosurg Psychiatry. 1995;59:271–279. doi: 10.1136/jnnp.59.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irlbacher K, Meyer BU, Voss M, Brandt SA, Roricht S. Spatial reorganization of cortical motor output maps of stump muscles in human upper-limb amputees. Neurosci Lett. 2002;321:129–132. doi: 10.1016/s0304-3940(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Rymer WZ. Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle Nerve. 2000;23:954–961. doi: 10.1002/(sici)1097-4598(200006)23:6<954::aid-mus17>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609–3618. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol. 2003a doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Role of intertendinous connections in distribution of force in the human extensor digitorum muscle. Muscle Nerve. 2003b;28:614–622. doi: 10.1002/mus.10481. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbreath SL, Gorman RB, Raymond J, Gandevia SC. Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J Physiol (Lond ) 2002;543:289–296. doi: 10.1113/jphysiol.2002.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol. 2004a;92:2802–2810. doi: 10.1152/jn.00480.2004. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004b;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin Neurophysiol. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Long C2, Conrad PW, Hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. An electromyographic study. Journal of Bone & Joint Surgery - American. 1970;Volume 52:853–867. [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip I. Contribution of 15 finger muscles to isometric force. Exp Brain Res. 1995a;103:108–122. doi: 10.1007/BF00241969. [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res. 1995b;103:123–136. doi: 10.1007/BF00241970. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Macefield VG, Taylor JL, Hallett M. Cortically evoked neural volleys to the human hand are increased during ischaemic block of the forearm. J Physiol. 2002;538:279–288. doi: 10.1113/jphysiol.2001.013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119(Pt 5):1737–1749. doi: 10.1093/brain/119.5.1737. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual APL, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. New York: MacMillan; 1950. The Cerebral Cortex of Man. [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121(Pt 9):1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. 2000;57:317–320. doi: 10.1001/archneur.57.3.317. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Mercier C, Schieber MH, Sirigu A. Persistent hand motor commands in the amputees' brain. Brain. 2006;129:2211–2223. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle. J Neurophysiol. 2004;92:734–742. doi: 10.1152/jn.00027.2004. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Schieber MH. Incomplete functional subdivision of the human multitendoned finger muscle flexor digitorum profundus: An electromyographic study. J Neurophysiol. 2003;90:2560–2570. doi: 10.1152/jn.00287.2003. [DOI] [PubMed] [Google Scholar]
- Roux FE, Lotterie JA, Cassol E, Lazorthes Y, Sol JC, Berry I. Cortical areas involved in virtual movement of phantom limbs: comparison with normal subjects. Neurosurgery. 2003;53:1342–1352. doi: 10.1227/01.neu.0000093424.71086.8f. [DOI] [PubMed] [Google Scholar]
- Röricht S, Meyer BU, Niehaus L, Brandt SA. Long-term reorganization of motor cortex outputs after arm amputation. Neurology. 1999;53:106–111. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. J Neurophysiol. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: The roles of extrinsic finger muscles. J Neurosci. 1995;15:284–297. doi: 10.1523/JNEUROSCI.15-01-00284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Deuel RK. Primary motor cortex reorganization in a long-term monkey amputee. Somatosensory & Motor Research. 1997;14:157–167. doi: 10.1080/08990229771024. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Towles JD, Hentz VR. Quantification of fingertip force reduction in the forefinger following simulated paralysis of extensor and intrinsic muscles. J Biomech. 2000;33:1601–1609. doi: 10.1016/s0021-9290(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Vattanasilp W, Ada L, Crosbie J. Contribution of thixotropy, spasticity, and contracture to ankle stiffness after stroke. J Neurol Neurosurg Psychiatry. 2000;69:34–39. doi: 10.1136/jnnp.69.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalography & Clinical Neurophysiology. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Pub Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Wu CWH, Kaas JH. Reorganization in primary motor cortex of primates with longstanding therapeutic amputations. J Neurosci. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CWH, Kaas JH. Spinal cord atrophy and reorganization of motoneuron connections following long-standing limb loss in primates. Neuron. 2000;28:967–978. doi: 10.1016/s0896-6273(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]