Abstract
The functions of most RNA molecules are critically dependent on the distinct local dynamics that characterize secondary structure and tertiary interactions and on structural changes that occur upon binding by proteins and small molecule ligands. Measurements of RNA dynamics at nucleotide resolution set the foundation for understanding the roles of individual residues in folding, catalysis, and ligand recognition. In favorable cases, local order in small RNAs can be quantitatively analyzed by NMR in terms of a generalized order parameter, S2. Alternatively, SHAPE (selective 2'-hydroxyl acylation analyzed by primer extension) chemistry measures local nucleotide flexibility in RNAs of any size using structure-sensitive reagents that acylate the 2'-hydroxyl position. In this work, we compare per-residue RNA dynamics, analyzed by both S2 and SHAPE, for three RNAs: the HIV-1 TAR element, the U1A protein binding site, and the Tetrahymena telomerase stem loop 4. We find a very strong correlation between the two measurements: nucleotides with high SHAPE reactivities consistently have low S2 values. We conclude that SHAPE chemistry quantitatively reports local nucleotide dynamics and can be used with confidence to analyze dynamics in large RNAs, RNA-protein complexes, and RNAs in vivo.
RNA molecules perform important cellular functions that depend on the ability to form both rigid and dynamic structural elements and that often require large changes in conformation and motion. Critical examples include ribosomal protein synthesis, ribonucleoprotein assembly, and riboswitches.1 The intrinsic RNA motions that underlie these processes occur over a wide range of time scales, from very fast ps motions to global conformational changes that require minutes.2
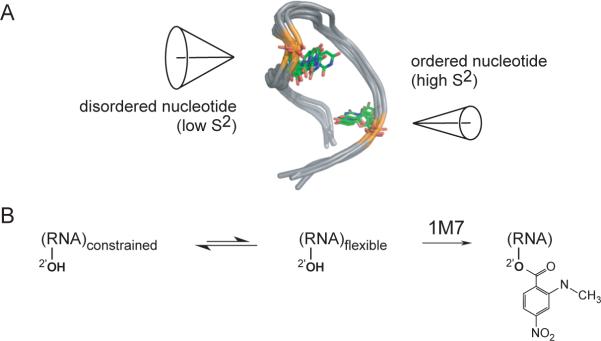
A number of NMR methods have been devised to measure RNA dynamics at single nucleotide resolution.2a,3 One of the most useful is the model-free framework, originally developed for protein motions,4 that interprets T1, T2 and NOE measurements in terms of two physically intuitive parameters: an effective correlation time for molecular tumbling, and the generalized order parameter, S2, which describes the spatial restriction of motion on a per-residue basis. S2 can adopt values ranging from 0 (completely disordered) to 1 (fully ordered) (Figure 1A).4
Figure 1.
Schemes for interpreting (A) the generalized order parameter S2, and (B) RNA SHAPE chemistry.
Detailed motions at nucleotide resolution have been described for paradigmatic RNAs,5,6 including the TAR element from HIV,5a–5c a regulatory element that binds the U1A protein,6 a stem loop derived from the U6 RNA,5d the lead-dependent ribozyme,5e and tetraloop-containing RNAs.5f–5i These studies have yielded important information regarding RNA dynamics both alone and in complex with small molecule ligands5a and proteins.6b However, analysis of RNA dynamics by NMR is limited to small and spectroscopically well-behaved RNA motifs, of ∼45 nucleotides or less. In order to analyze single-nucleotide resolution dynamics for large RNAs alone, as part of ribonucleoproteins, or in vivo, a different and more versatile approach is required.
Local motion in nearly any RNA can be easily measured at nucleotide resolution using the simple and rapid SHAPE (selective 2'-hydroxyl acylation analyzed by primer extension) experiment.7 SHAPE chemistry is based on the discovery that conformationally flexible nucleotides react preferentially towards acylating agents such as 1-methyl-7-nitroisatoic anhydride (1M7) (Figure 1B) to form a 2'-O-adduct. In contrast, nucleotides that are constrained by base pairing or tertiary interactions are unreactive. Sites of 2'-O-adduct formation are then detected as stops to primer extension.7
SHAPE is proving to be a powerful approach for addressing a wide variety of structure-function relationships in RNA, from short oligonucleotides to RNAs thousands of nucleotides long.7,8 To date, the correlation between SHAPE reactivity and local RNA motion, while clearly plausible, has not been rigorously established. In contrast, S2 is derived from a well-understood quantitative framework4 and is strongly correlated with local molecular motions and structure.9
We therefore sought to test whether SHAPE chemistry captures local nucleotide dynamics in a way that correlates with S2, a parameter that possesses clear physical meaning. SHAPE and NMR analyses were conducted in parallel for three RNAs: (1) the TAR RNA from HIV-1 which activates transcriptional elongation in concert with the Tat protein; (2) the U1A protein binding site RNA that autoregulates mRNA processing; and (3) the Tetrahymena telomerase stem loop 4 RNA (T-SL4) which promotes folding of a pseudoknot essential for enzyme activity (Figure 2A).6,10
Figure 2.
Local nucleotide structure in RNA analyzed by SHAPE and S2. (A) Secondary structures for the TAR, U1A target, and T-SL4 RNAs. Nucleotides with SHAPE reactivities greater than 0.5 for TAR and U1A, or greater than 0.6 for T-SL4 are red. Positions for which S2 was not obtained are shown as red or black spheres. (B). Histograms of SHAPE reactivities as a function of nucleotide position (columns) compared to 1-S2 measurements (blue spheres).
S2 values based on 13C relaxation at the C1' ribose position were calculated4,6a for all well-resolved nucleotides in the U1A and TAR RNAs and for C and A nucleotides in the T-SL4 RNA (the results are represented as black and red nucleotides in Figure 2A and blue spheres, Figure 2B). We also measured SHAPE reactivities for these RNAs under conditions similar to those used in the NMR experiments. SHAPE reactivities are normalized to a scale from 0 to ∼1.5, in which 1.0 is defined as the average intensity of highly reactive positions8b (bars, Figure 2B).
We then compared SHAPE reactivities with S2, for all positions where both measurements could be made (compare blue spheres with columns, Figure 2B). Because high SHAPE reactivities and low S2 values both correspond to a disordered site, we plot the generalized order parameter as 1-S2. For the TAR RNA, the three-nucleotide UCU bulge and the apical loop are both reactive by SHAPE and are also disordered, as indicated by high 1-S2 values. For the U1A RNA, the 39−45 loop is clearly identifiable by both SHAPE and S2 values. Importantly, three of four nucleotides in the 29−34 loop are both unreactive by SHAPE and have S2 values that reflect a high level of order, in agreement with previous observations that UUCG tetraloops are unusually stable, reflecting intramolecular base stacking and hydrogen bonding interactions.11 SHAPE experiments with T-SL4 were performed at 40 °C, as required by the NMR analysis of this RNA. Nucleotides in the T-SL4 RNA are more reactive than in the TAR and U1A target RNAs, consistent with a temperature-induced increase in RNA dynamics (compare panels, Figure 2B). For T-SL4, both SHAPE and S2 detect increased nucleotide dynamics in the apical loop and at each of the three small bulges in this RNA.
SHAPE and 1-S2 do not correlate well at three nucleotides: A24 and C33 of U1A and A20 of T-SL4. However, all three are single stranded or adjacent to unpaired positions. Therefore, the high observed SHAPE reactivity likely better represents the local structure in these regions than does the S2 measurement.
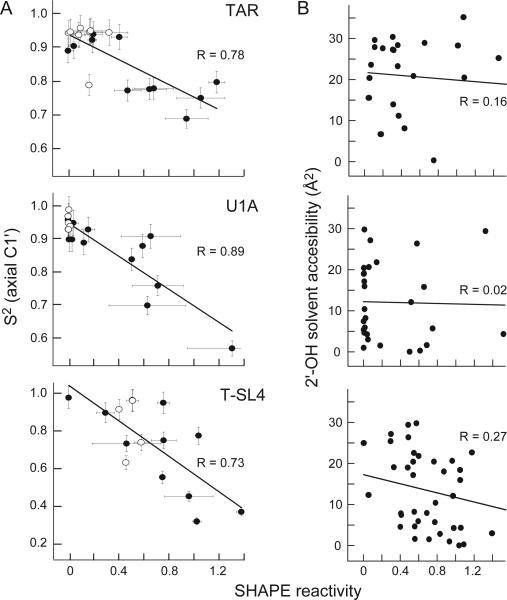
We quantified the correlation between SHAPE reactivities and S2 by plotting these values on a linear scale and calculating Pearson correlation coefficients, R (Figure 3A). We analyzed correlation coefficients in two ways. First, we determined R using all measured values and observed a strong correlation between SHAPE reactivities and S2 in all cases: R values are 0.78, 0.89 and 0.73 for the TAR, U1A and T-SL4 RNAs, respectively (all points, Figure 3A). We also calculated R values after excluding nucleotides that form canonical base pairs, which always have near-zero SHAPE reactivities and high S2 values. Correlation coefficients from this more stringent calculation (filled spheres, Figure 3A) had similar correlations: R values are 0.79, 0.86 and 0.75 for the TAR, U1A and T-SL4 RNAs, respectively.
Figure 3.
Quantitative correlation between SHAPE reactivity and (A) S2, measured at C1', or (B) solvent accessibility of the 2'-hydroxyl group. Pearson's linear R-values are shown.
S2 is derived primarily from NMR measurements sensitive to ps-ns motions while SHAPE chemistry is likely to be additionally influenced by dynamics on slower timescales. Nucleotides that are disordered on these slower timescales, but whose motions are not detected in the NMR experiments, would fall above the line in our correlation plots (Figure 3A).
Finally, we assessed whether SHAPE chemistry is influenced by the solvent accessibility of the 2'-hydroxyl position. This is a critical control to establish whether SHAPE might also report a reactive spatial orientation of the 2'-OH group in addition to measuring RNA dynamics. There is essentially no correlation between SHAPE reactivity and solvent accessibility at the 2'-hydroxyl group for any of the three RNAs (Figure 3B).
We conclude that local disorder at individual RNA nucleotides as quantified by S2 correlate strongly with SHAPE reactivities but not with solvent accessibility. SHAPE thus measures spatial disorder and structural dynamics at single nucleotide resolution in RNA. We anticipate that SHAPE chemistry will create many opportunities for understanding the roles of individual nucleotide dynamics in the structure of large RNAs, during ribonucleoprotein assembly, and upon RNA binding by proteins and other ligands and drugs, both in vitro and in vivo.
Supplementary Material
Acknowledgement
This work was supported by grants from the NSF (MCB-0416941) and NIH (AI068462) to K.M.W. and from the NSF (MCB-0642253) and NIH (EB003152) to G.V. We are indebted to Mike Jarstfer and Gary Pielak for thoughtful discussions.
Footnotes
Supporting Information Available: Experimental procedures and additional information regarding the NMR experiments with T-SL4. This material is available free of charge at http://pubs.acs.org.
References
- 1.a Korostelev A, Noller HF. Trends Biochem. Sci. 2007;32:434–441. doi: 10.1016/j.tibs.2007.08.002. [DOI] [PubMed] [Google Scholar]; b Buchmueller KL, Webb AE, Richardson DA, Weeks KM. Nature Struct. Biol. 2000;7:362–366. doi: 10.1038/75125. [DOI] [PubMed] [Google Scholar]; c Maity TS, Weeks KM. J. Mol. Biol. 2007;369:512–524. doi: 10.1016/j.jmb.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Bokinsky G, Nivón LG, Liu S, Chai G, Hong M, Weeks KM, Zhuang X. J. Mol. Biol. 2006;361:771–784. doi: 10.1016/j.jmb.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kim JN, Breaker RR. Biol. Cell. 2008;100:1–11. doi: 10.1042/BC20070088. [DOI] [PubMed] [Google Scholar]
- 2.a Shajani Z, Varani G. Biopolymers. 2007;86:348–359. doi: 10.1002/bip.20650. [DOI] [PubMed] [Google Scholar]; b Buchmueller KL, Hill BT, Platz MS, Weeks KM. J. Am. Chem. Soc. 2003;125:10850–10861. doi: 10.1021/ja035743+. [DOI] [PubMed] [Google Scholar]; c Al-Hashimi HM, Walter NG. Curr. Opin. Struct. Biol. 2008;18:321–329. doi: 10.1016/j.sbi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guéron M, Leroy JL. Methods Enzymol. 1995;261:383–413. doi: 10.1016/s0076-6879(95)61018-9. [DOI] [PubMed] [Google Scholar]
- 4.a Lipari G, Szabo A. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]; b Lipari G, Szabo A. J. Am. Chem. Soc. 1982;104:4559–4570. [Google Scholar]
- 5.a Dayie KT, Brodsky AS, Williamson JR. J. Mol. Biol. 2002;317:263–278. doi: 10.1006/jmbi.2001.5424. [DOI] [PubMed] [Google Scholar]; b Vallurupalli P, Scott L, Hennig M, Williamson JR, Kay LE. J. Am. Chem. Soc. 2006;128:9346–9347. doi: 10.1021/ja0632512. [DOI] [PubMed] [Google Scholar]; c Hansen AL, Al-Hashimi HM. J. Am. Chem. Soc. 2007;129:16072–16082. doi: 10.1021/ja0757982. [DOI] [PubMed] [Google Scholar]; d Blad H, Reiter NJ, Abildgaard F, Markley JL, Butcher SE. J. Mol. Biol. 2005;353:540–555. doi: 10.1016/j.jmb.2005.08.030. [DOI] [PubMed] [Google Scholar]; e Hoogstraten CG, Wank JR, Pardi A. Biochemistry. 2000;39:9951–9958. doi: 10.1021/bi0007627. [DOI] [PubMed] [Google Scholar]; f Hall KB, Tang C. Biochemistry. 1998;37:9323–9332. doi: 10.1021/bi9805285. [DOI] [PubMed] [Google Scholar]; g D'Souza V, Dey A, Habib D, Summers MF. J. Mol. Biol. 2004;337:427–442. doi: 10.1016/j.jmb.2004.01.037. [DOI] [PubMed] [Google Scholar]; h Duchardt E, Schwalbe H. J. Biomol. NMR. 2005;32:295–308. doi: 10.1007/s10858-005-0659-x. [DOI] [PubMed] [Google Scholar]; i Ferner J, Villa A, Duchardt E, Widjajakusuma E, Wöhnert J, Stock G, Schwalbe H. Nucl. Acids Res. 2008;36:1928–1940. doi: 10.1093/nar/gkm1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Shajani Z, Varani G. J. Mol. Biol. 2005;349:699–715. doi: 10.1016/j.jmb.2005.04.012. [DOI] [PubMed] [Google Scholar]; b Shajani Z, Drobny G, Varani G. Biochemistry. 2007;46:5875–5883. doi: 10.1021/bi602658x. [DOI] [PubMed] [Google Scholar]
- 7.a Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. J. Am. Chem. Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]; b Wilkinson KA, Merino EJ, Weeks KM. J. Am. Chem. Soc. 2005;127:4659–4667. doi: 10.1021/ja0436749. [DOI] [PubMed] [Google Scholar]; c Wilkinson KA, Merino EJ, Weeks KM. Nature Protocols. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- 8.a Mortimer SA, Weeks KM. J. Am. Chem. Soc. 2007;129:4144–4145. doi: 10.1021/ja0704028. [DOI] [PubMed] [Google Scholar]; b Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, Giddings MC, Weeks KM. PLoS Biology. 2008;6:e96. doi: 10.1371/journal.pbio.0060096. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang B, Wilkinson KA, Weeks KM. Biochemistry. 2008;47:3454–3461. doi: 10.1021/bi702372x. [DOI] [PubMed] [Google Scholar]; d Duncan CDS, Weeks KM. Biochemistry. 2008;47 doi: 10.1021/bi800207b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Gherghe CM, Mortimer SA, Krahn JM, Thompson NL, Weeks KM. J. Am. Chem. Soc. 2008;130:8884–8885. doi: 10.1021/ja802691e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Palmer AG. Chem. Rev. 2004;104:3623–3640. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]; b Jarymowycz VA, Stone MJ. Chem. Rev. 2006;106:1624–1671. doi: 10.1021/cr040421p. [DOI] [PubMed] [Google Scholar]
- 10.a Sharp PA, Marciniak RA. Cell. 1989;59:229–230. doi: 10.1016/0092-8674(89)90279-1. [DOI] [PubMed] [Google Scholar]; b Hall TM. Curr. Opin. Struct. Biol. 2002;12:82–88. doi: 10.1016/s0959-440x(02)00293-2. [DOI] [PubMed] [Google Scholar]; c Sperger JM, Cech TR. Biochemistry. 2001;40:7005–7016. doi: 10.1021/bi0103359. [DOI] [PubMed] [Google Scholar]; d Chen Y, Fender J, Legassie JD, Jarstfer MB, Bryan TM, Varani G. EMBO J. 2006;25:3156–3166. doi: 10.1038/sj.emboj.7601195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheong C, Varani G, Tinoco I. Nature. 1990;346:680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.