Abstract
Acute inflammatory diseases are a major cause of death in the world, and effective treatments are greatly needed. Macrophages play a central role in causing acute inflammatory diseases, and there is currently great interest in developing drug delivery vehicles that can target therapeutics to macrophages. Microparticles formulated from aliphatic polyketals have great potential to enhance the treatment of acute inflammatory diseases, due to their ability to passively target therapeutics to macrophages, their acid sensitivity, and their biocompatible degradation products. However, existing aliphatic polyketals are unsuitable for treating acute inflammatory diseases because they require weeks to hydrolyze, and strategies for accelerating their hydrolysis kinetics are greatly needed. In this report we demonstrate that the hydrolysis kinetics of aliphatic polyketals can be accelerated by increasing their hydrophilic/hydrophobic balance. Aliphatic polyketals of varying hydrophobicity were synthesized, via the acetal exchange reaction, and their hydrolysis kinetics were investigated at the pH values of 4.5 and 7.4. A polyketal termed PK3 was developed, which had the hydrolysis kinetics suitable for treating acute inflammatory diseases. PK3 has a hydrolysis half-life of 2 days at pH 4.5, but requires several weeks to hydrolyze at pH 7.4. Microparticles were formulated with PK3, which encapsulated the anti-inflammatory drug, imatinib. In vivo experiments demonstrated that PK3 microparticles were able to significantly improve the efficacy of imatinib in treating acute liver failure. We anticipate that aliphatic polyketals will have numerous applications for the treatment of acute inflammatory diseases, given their pH sensitivity, tunable hydrolysis kinetics, and biocompatible degradation products.
INTRODUCTION
Acute inflammatory diseases such as acute lung injury and acute liver failure cause millions of death each year, and effective treatments are greatly needed (1, 2). Pro-inflammatory cytokines secreted by macrophages play a central role in mediating acute inflammatory diseases, and drug delivery vehicles that can target therapeutics to macrophages have great clinical potential (3). A key drug delivery requirement for the treatment of many acute inflammatory diseases is fast release of drugs to diseased organs, within several hours. This is because at the time of patient diagnosis, significant tissue damage has already occurred, and organ function is rapidly deteriorating (4). It has been challenging to develop clinically acceptable drug delivery vehicles that can target therapeutics to macrophages and release them rapidly. Liposomes are a potential delivery vehicle for treating acute inflammatory diseases, due to their ability to target macrophages (5). However, their serum instability and poor storage properties have slowed their progress in clinical trials. Microparticles, based on biodegradable polymers, also have potential to enhance the treatment of acute inflammatory diseases. Microparticles can be freeze-dried, have an excellent shelf-life, and can also passively target therapeutics to macrophages (6, 7). However, currently used biomaterials for drug delivery are predominantly based on polyesters, which are potentially problematic for treating acute inflammatory diseases because of their slow hydrolysis kinetics and acidic degradation products, which themselves frequently cause inflammation (8, 9).
Microparticles formulated from polyketals are a new drug delivery vehicle, which degrade into neutral compounds comprised of acetone and diols, and should therefore avoid the inflammatory problems associated with polyester-based materials (9–13). At present, only two polyketals have been synthesized for drug delivery, poly-(1,4-phenyleneacetone dimethylene ketal) (PPADK) and poly(cyclohexane-1,4-diyl acetone dimethylene ketal) (PCADK) (12, 13). PPADK has excellent hydrolysis kinetics for treating acute inflammatory diseases, having a half-life of 35 hours at pH 5.0, but degrades into benzene dimethanol, a compound with potential toxicity, due to its aromatic ring. PCADK is an aliphatic polyketal, which degrades into acetone and 1,4-cyclohexanedimethanol, both of which have excellent biocompatibility. However, PCADK has a hydrolysis half-life of 24 days at pH 4.5, which is too slow for applications involving the treatment of acute inflammatory diseases, and therefore strategies that can accelerate its hydrolysis kinetics are greatly needed.
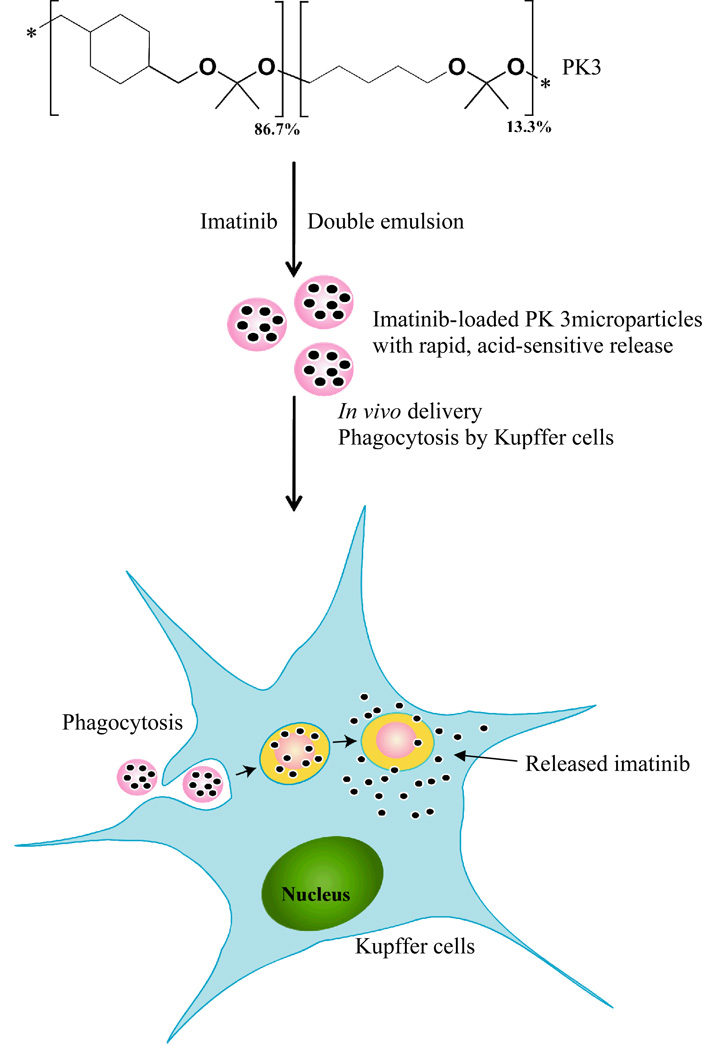
In this report we demonstrate that the hydrolysis kinetics of PCADK derived aliphatic polyketals can be accelerated by increasing their hydrophilic/hydrophobic balance. Using this principle, we were able to generate a polyketal copolymer, termed PK3, which had a hydrolysis half-life of 2 days at pH 4.5 but several weeks at pH 7.4. Microparticles formulated from PK3 should be suitable for treating acute inflammatory diseases because they should hydrolyze and release therapeutics rapidly in the phagolysosomes of macrophages, but remain stable at physiological pH. PK3 was used to generate microparticles 1–3 microns in size, which encapsulated the NFκB inhibitor imatinib, using a solvent evaporation procedure. The therapeutic efficacy of these PK3-imatinib microparticles was investigated in mice, using a Concavalin A (Con A) model of acute liver failure (Scheme 1). The results from these experiments demonstrate that imatinib-loaded PK3 microparticles significantly enhanced the therapeutic efficacy of imatinib, presumably due to their accumulation in liver macrophages (Kupffer cells). Based on these results, we anticipate that aliphatic copolyketals will have numerous applications for the treatment of acute inflammatory diseases.
Scheme 1.
PK3, a new biomaterial for treating acute inflammatory diseases. PK3 is a new polyketal copolymer, which hydrolyzes rapidly at the acidic pH of the macrophage phagosome. PK3 is designed to deliver therapeutics to liver macrophages and enhance the treatment of acute liver failure. PK3 microparticles were capable of enhancing the delivery of imatinib in mice suffering from acute liver failure.
EXPERIMENTAL SECTION
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were used as received unless otherwise specified. Benzene and 2,2-dimethoxypropane were purified by distillation. Imatinib was a gift from Novartis.
Animals
Male C57/BL6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Rochester Medical Center.
Synthesis of polyketal copolymers
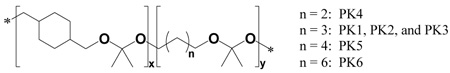
Polyketal copolymers were synthesized in a 25 mL two-necked flask, connected to a short-path distilling head. The diols, 1,4-cyclohexanedimethanol (1.04 g, 7.25 mmol) and, either 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol, or 1,8-octanediol were dissolved in 20 mL of distilled benzene and kept at 100 °C. Re-crystallized p-toluenesulfonic acid (5.5 mg, 0.029 mmol) was dissolved in ethyl acetate (500 µL) and added to the benzene solution. The ethyl acetate was distilled off, and the polymerization reaction was initiated by the addition of 2,2-dimethoxypropane (equal molar to the two diols combined). Additional doses of 2,2-dimethoxypropane (500 µL) and benzene (2 mL) were subsequently added to the reaction, every hour for six hours, via a metering funnel, to compensate for 2,2-dimethoxypropane and benzene that had distilled off. After 24 hours, the reaction was stopped with triethylamine (100 µL). The copolymers were isolated by precipitation into cold hexanes and analyzed by 1H-NMR and GPC. In general the resulting polymers had number average molecular weights between 2000 to 3000 Da. Table 1 lists the compositions and molecular weights of the polyketal copolymers synthesized. 1H NMR spectra were obtained from a Varian Mercury VX 400 MHz NMR spectrometer (Palo Alto, CA) using CDCl3 as the solvent. PK1 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.66 (s, 1.9H, CH), 1.85 – 0.93 (m, 8H, CH2), and 1.32 (s, 6H, CH3); PK2 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.66 (s, 1.8H, CH), 1.85 – 0.93 (m. 8H, CH2), and 1.32 (s, 6H, CH3); PK3 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.64 (s, 1.7H, CH), 1.85 – 0.93 (m, 8.2H, CH2), and 1.32 (s, 6H, CH3); PK4 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.68 (s, 2H, CH), 1.85 – 0.93 (m, 8H, CH2), and 1.32 (s, 6H, CH3); PK5 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.67 (s, 1.8H, CH), 1.85 – 0.93 (m, 8H, CH2), and 1.32 (s, 6H, CH3); PK6 1H NMR (400 MHz, CDCl3, δ): 3.4 – 3.18 (m, 4H, CH2), 1.68 (s, 1.8H, CH), 1.85 – 0.93 (m, 8H, CH2), and 1.32 (s, 6H, CH3).
Table 1.
Compositions and molecular weight of polyketal copolymers synthesized.
 | ||||
|---|---|---|---|---|
| Polymer ID |
Polymer composition | |||
| Monomer diol A (x) | Monomer diol 2 (y) | Mn | PDI* | |
| PK1 | 1,4-cyclohexanedimethanol (98.03%) |
1,5-pentanediol (1.93%) |
2149 | 1.742 |
| PK2 | 1,4-cyclohexanedimethanol (92.46%) |
1,5-pentanediol (7.56%) |
2530 | 1.629 |
| PK3 | 1,4-cyclohexanedimethanol (86.70%) |
1,5-pentanediol (13.30%) |
2596 | 1.432 |
| PK4 | 1,4-cyclohexanedimethanol (96.75%) |
1,4-butanediol (3.25%) |
2637 | 1.553 |
| PK5 | 1,4-cyclohexanedimethanol (85.32%) |
1,6-hexanediol (14.68%) |
2122 | 1.538 |
| PK6 | 1,4-cyclohexanedimethanol (87.31%) |
1,8-octanediol (12.69%) |
2181 | 1.786 |
PDI: polydispersity index.
Gel permeation chromatography
The molecular weights of the polyketal copolymers were determined by gel permeation chromatography (GPC) using a Shimadzu system (Kyoto, Japan) equipped with a UV detector. Tetrahydrofuran was used as the mobile phase at a flow rate of 1 mL/min. Polystyrene standards (Peak Mw = 1060, 2970, and 10680) from Polymer Laboratories (Amherst, MA) were used to establish a molecular weight calibration curve.
Hydrolysis of polyketal copolymers
The hydrolysis of the polyketal copolymers was measured according to the procedures of Lee et al (13). Briefly polymer samples (20 mg) were placed in buffered water (1 mL) at the pH values of 4.5 (100 mM AcOH) and 7.4 (100 mM Na2HPO4) at 37 °C. The polymer samples were mixed by gentle shaking, and at specific time points, were extracted into CDCl3 (1 mL). The CDCl3 phase was isolated and analyzed by 1H NMR, to determine the percent hydrolysis.
Release kinetics of rhodamine B from PK3 microparticles
Rhodamine B was encapsulated in PK3 microparticles using single emulsion procedures. Rhodamine B-loaded PK3 microparticles (10 mg) were suspended in pH 4.5 and pH 7.4 buffer solutions (10 mL). The suspensions were kept at 37°C under gentle shaking. At specific time points, the suspensions (100 µL) was centrifuged at 10,000 × g for 2 minutes to remove unhydrolyzed particles. The supernatant (3 µL) was then diluted in pH 7.4 buffer (3 mL), which was then analyzed by a Shimadzu spectrofluorophotometer (Kyoto, Japan) to quantify the relative concentration of rhodamine B released form the PK3 microparticles (excitation wavelength = 556 nm, emission wavelength = 573 nm).
Formulation of imatinib-loaded PK3 microparticles
Imatinib-loaded microparticles were formulated from PK3 using a modified water/oil/water emulsion method. Briefly, PK3 (100mg) was dissolved in dichloromethane (1 mL), and in a separate vial imatinib (40 mg) was dissolved in deionized water (400 µL). The aqueous solution of imatinib was mixed with the PK3 solution, and sonicated for 60 seconds (Misonix Incorporated, Farmigdale, NY). The sonicated mixture was then immersed in liquid nitrogen for 15 seconds, and a 5% w/w PVA solution (pH 7.45, 12 mL) was added to it. This mixture was homogenized for 120 seconds with a Powergen 500 homogenizer (Fisher Scientific, Waltham, MA), and then transferred to a beaker containing 1% w/w PVA (pH 7.4, 40 mL). This solution was stirred for 3 hours with a magnetic stir bar to evaporate the organic solvent. The particles were isolated by centrifuging at 10,000 × g for 15 min, washed twice with PBS buffer (15 mL) and freeze-dried.
Scanning Electron Microscopy (SEM)
SEM images were taken to analyze the morphology of the polyketal microparticles. Briefly, SEM samples were prepared by attaching lyophilized particles onto 12.7 mm diameter aluminum sample mounting stubs (Electron Microscopy Sciences, Hatifield, PA), using conductive double sided carbon discs (SPI Supplies, West Chester, PA). The samples were coated with a gold sputter coater (International Scientific Instruments, Prahran, Australia) for 2 minutes under an argon atmosphere. The SEM samples were subsequently analyzed using a Hitachi S-800 scanning electron microscope (Tokyo, Japan).
Determination of imatinib loading in PK3 microparticles
The loading of imatinib in PK3 microparticles was determined by U.V. spectrometry. A U.V. calibration curve for imatinib was established at 268 nm, with a Shimadzu UV-1700 spectrometer (Kyoto, Japan) in pH 7.4 buffer. Imatinib-loaded PK3 particles were dissolved in a small amount of methylene chloride, and the imatinib was extracted into pH 7.4 buffer (100 mM Na2HPO4). The absorbance of both the aqueous and organic phase was measured to verify that all the imatinib was partitioned into the aqueous phase. The concentration of imatinib loaded into the PK3 microparticles was determined against the established calibration curve as mentioned above.
Treatment of acute liver failure in mice with imatinib-loaded PK3 microparticles
Male C57/BL6 mice, 6–8 weeks old, were used in these studies. Appropriate doses of imatinib-PK3 particles (containing between 5 µg/kg to 500 µg/kg of imatinib), or an equal quantity of free imatinib, was suspended/dissolved in PBS (200 µL) and was injected intravenously using a 26 G5/8 sterilized needle. One hour later Con A (15 mg/kg), dissolved in PBS (200 µL), was injected into the intraperitoneal cavity using a 26 G5/8 sterilized needle. Between 4 and 8 mice were used per experimental group. The mice were euthanized 8 hours after the Con A injection, by cervical dislocation. Blood was then withdrawn from the heart using a 26 G5/8 sterilized needle, and kept at 4°C overnight. The serum was isolated by centrifuging at 600 × g for 10 minutes and sent to the core facility at the University of Rochester for measurement of ALT levels.
RESULTS AND DISCUSSION
There is currently great interest in developing microparticle-based delivery vehicles that have the biocompatibility and hydrolysis kinetics needed to treat acute inflammatory diseases. Microparticles formulated from the aliphatic polyketal PCADK have excellent biocompatibility and degrade under the acidic conditions of the phagolysosome. However, the slow hydrolysis kinetics of PCADK make it unsuitable for treating acute inflammatory diseases, and therefore strategies for accelerating its hydrolysis kinetics are greatly needed. We previously hypothesized that the slow hydrolysis kinetics of PCADK were due to its hydrophobicity, making the diffusion of water into the polymer matrix the rate-limiting step in ketal hydrolysis (13). This hypothesis was based on the fact that the hydrolysis half-life of a water-soluble dimethylacetone-based ketal is only 2 minutes at pH 5.0, which is 3 to 4 orders of magnitude faster than the hydrolysis of the ketal linkages in PCADK (14). Additionally, the hydrolysis kinetics of other water insoluble polymers, such as polyanhydrides, also scale with their hydrophobicity (10). In this report, the role of hydrophobicity in governing the hydrolysis kinetics of polyketals was investigated. This was accomplished by synthesizing polyketal copolymers of varying hydrophobicity, and measuring their hydrolysis kinetics.
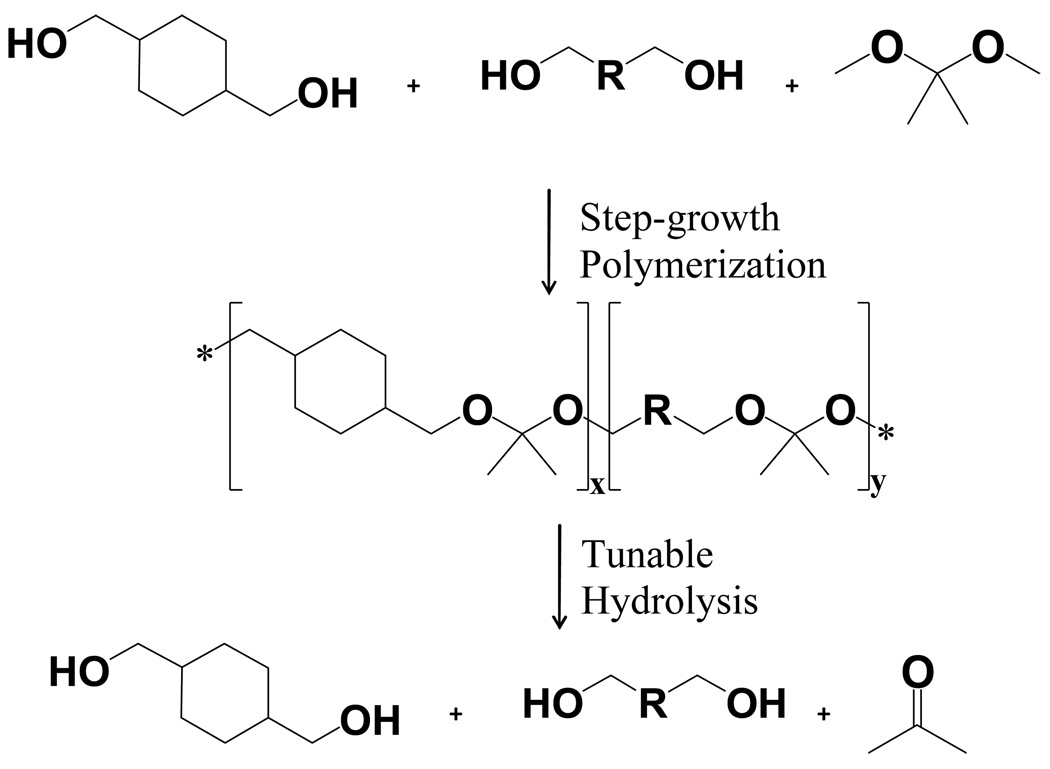
Six polyketal copolymers were synthesized in this report (PK1 – PK6, Table 1), by copolymerizing 1,4-cyclohexanedimethanol with either 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol, or 1,8-octanediol. The hydrophobicity of these diols is different from that of 1,4-cyclohexanedimethanol (log P = 1.46, where P = water/octanol partition coefficient), as evidenced by their respective log P values (Table 2). The synthesis of all the polyketal copolymers reported in this study was accomplished using the acetal exchange reaction, and was performed on a multi-gram scale with yields of 50 – 60% (Figure 1). In general, the introduction of additional diols other than 1,4-cyclohexanedimethanol did not cause any synthetic complications, and procedures developed for the synthesis of PCADK were suitable for the synthesis of all the copolymers. Importantly, all the polyketal copolymers reported in Table 1 were solid, and therefore have the potential for formulation into microparticles.
Table 2.
Hydrolysis half-lives of polyketal copolymers at pH 4.5 and pH 7.4, at 37 °C.
| PK ID | Polymer composition | Half life at pH 4.5 |
Estimated half life at pH 7.4 |
log P of diol B* |
|||
|---|---|---|---|---|---|---|---|
| Diol A | Percent diol A |
Diol B | Percent diol B |
||||
| PK4 | 1,4-cyclohexane dimethanol |
96.75% | 1,4-butanediol | 3.25% | 1.0 day | 54 days | −0.83 |
| PK3 | 86.70% | 1,5-pentanediol | 13.30% | 1.8 days | 39 days | 0.27 | |
| PK5 | 85.32% | 1,6-hexanediol | 14.68% | 4.4 days | 53 days | 0.76 | |
| PK6 | 87.31% | 1,8-octanediol | 12.69% | 18.6 days | 360 days | 1.75 | |
log P is the logarithm of the water/octanol partition coefficient; the log P data were obtained from the Syracuse Research Corporation’s PhysProp Database.
Figure 1.
Synthesis of polyketal copolymers from 1,4-cyclohexanedimethanol, a second diol, and 2,2-dimethoxypropane. Hydrolysis kinetics of polyketals can be controlled by manipulating the hydrophilicity of the polyketals through copolymerizing 1,4-cyclohexandimethanol with a more hydrophilic diol.
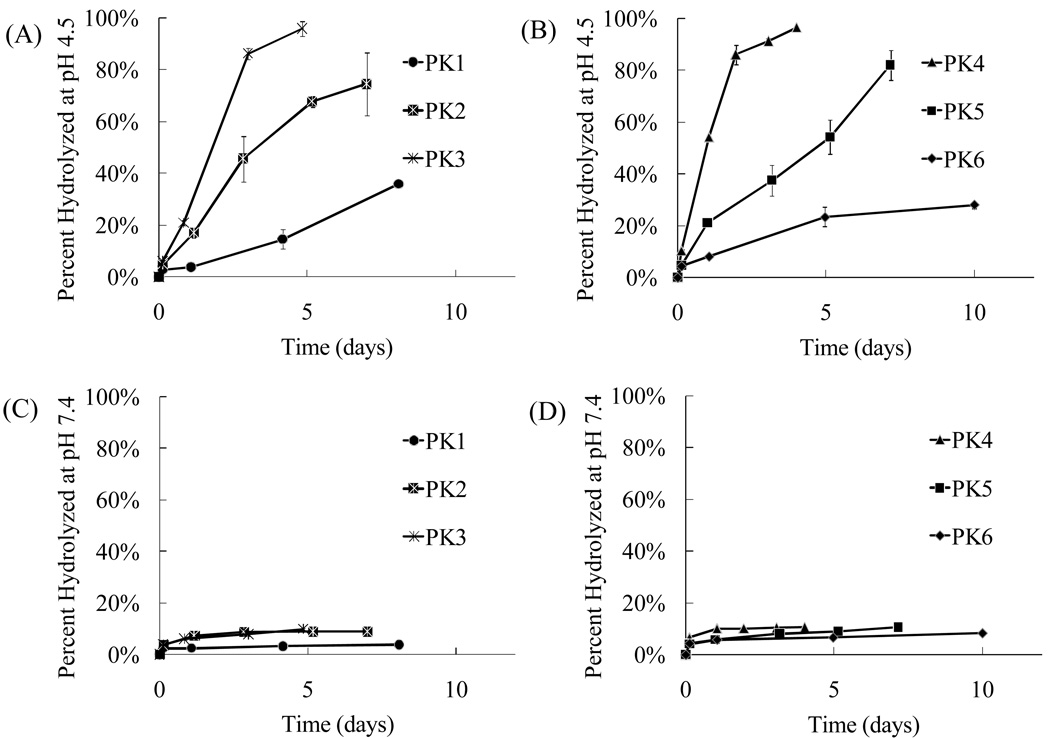
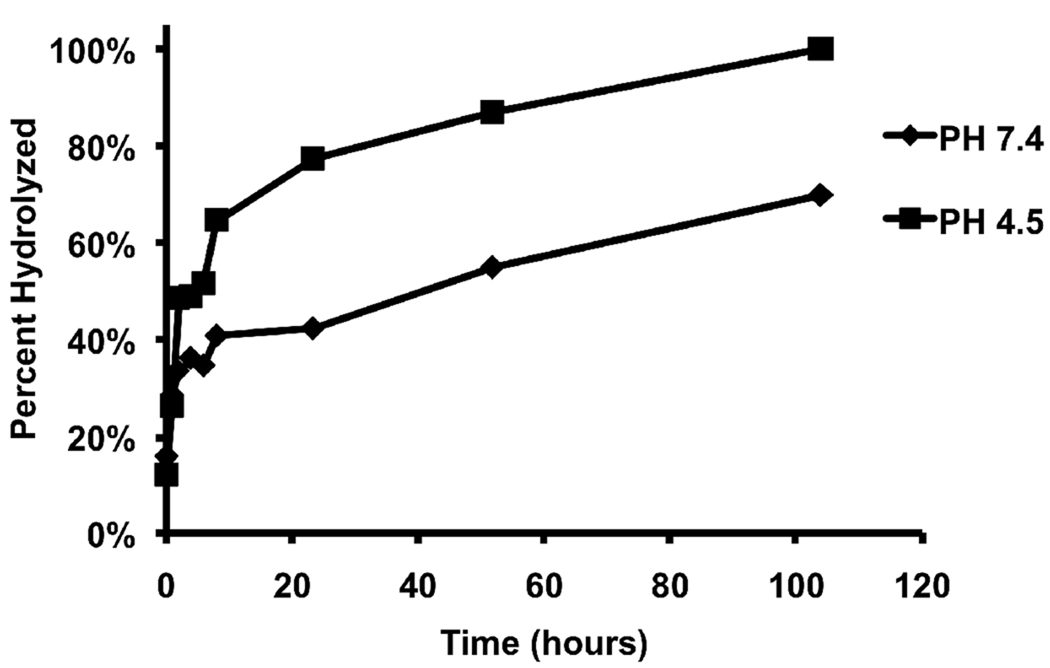
The hydrolysis kinetics of PK1 to PK6 were measured at the pH values of 4.5 and 7.4 to determine their behavior in the acidic environment of phagolysosomes and in the blood. Figure 2 demonstrates that all the polyketal copolymers (PK1 – PK6) undergo acid-catalyzed hydrolysis, and that their hydrolysis kinetics scale with their hydrophilicity. PK1, PK2, and PK3 are copolymers synthesized from 1,4-cyclohexanedimethanol and 1,5-pentanediol. Their hydrophilicity scales with the amount of 1,5-pentanediol incorporated into the copolymer. This is due to the large difference in hydrophilicity between 1,5-pentanediol and 1,4-cyclohexanedimethanol, as evidenced by their respective log P values of 0.27 and 1.46. Figure 2A demonstrates that 1,5-pentanediol significantly accelerates the pH 4.5 hydrolysis kinetics of 1,4-cyclohexanedimethanol-based polyketals. For example, the hydrolysis half-life of PCADK, a homo-polyketal synthesized from 1,4-cyclohexanedimethanol, is 24 days at pH 4.5 (13). On the other hand, PK3, a copolymer that incorporates 13 mole % 1,5-pentanediol and 87 mole % 1,4-cyclohexanedimethanol, had a hydrolysis half-life of only 2 days at pH 4.5. Similarly, PK2, another PCADK-derived copolymer, incorporating 7.5 mole % of 1,5-pentanediol, had a hydrolysis half-life of 3 days at pH 4.5, which is faster than PCADK but slower than PK3. PK1, the third copolymer derived from PCADK, incorporating 2 mole % 1,5-pentanediol, was only 30% hydrolyzed after 10 days at pH 4.5, which was expected based on its low incorporation of 1,5-pentanediol1.
Figure 2.
Hydrolysis kinetics of polyketals can be tuned by copolymerization. (A) Hydrolysis profiles of PK1, PK2, and PK3 at pH 4.5, (B) hydrolysis profiles of PK4, PK5, and PK6 at pH 4.5, (C) hydrolysis profiles of PK1, PK2, and PK3 at pH 7.4, and (D) hydrolysis profiles of PK4, PK5, and PK6 at pH 7.4. Data are presented as mean ± standard deviation. All experiments were conducted in triplicate at 37 °C.
To determine if diols other than 1,5-pentanediol could similarly influence the hydrolysis kinetics of PCADK-derived copolymers, the hydrolysis kinetics of PK4, PK5, and PK6 were also investigated. These polyketals are PCADK-derived copolymers synthesized from 1,4-cyclohexanedimethanol and either 1,4-butanediol, 1,6-hexanediol, or 1,8-octanediol. Figure 2 (B and D) and Table 2 demonstrate that this set of copolymers also has an inverse relationship between hydrophobicity and hydrolysis kinetics. For example, PK4, a copolymer synthesized from 1,4-cyclohexanedimethanol and 1,4-butanediol, has the fastest hydrolysis kinetics of all the copolyketals, with a hydrolysis half-life of 1 day at pH 4.5. This is predicted based on the more hydrophilic nature of 1,4-butanediol in comparison to the other diols. On the other hand, PK6, a copolymer composed of 1,4-cyclohexanedimethanol and 1,8-octanediol, had a pH 4.5 hydrolysis half-life of 18.6 days. In summary, these data demonstrate that the hydrolysis kinetics of polyketals can be tuned by varying their hydrophilicity and further support the hypothesis that diffusion of water into the polyketals is the rate-determining step governing their hydrolysis. Importantly, the hydrolysis kinetics of all the polyketal copolymers, PK 1–6, were pH-sensitive; in general they hydrolyzed at least one order of magnitude faster at pH 4.5 than at pH 7.4.
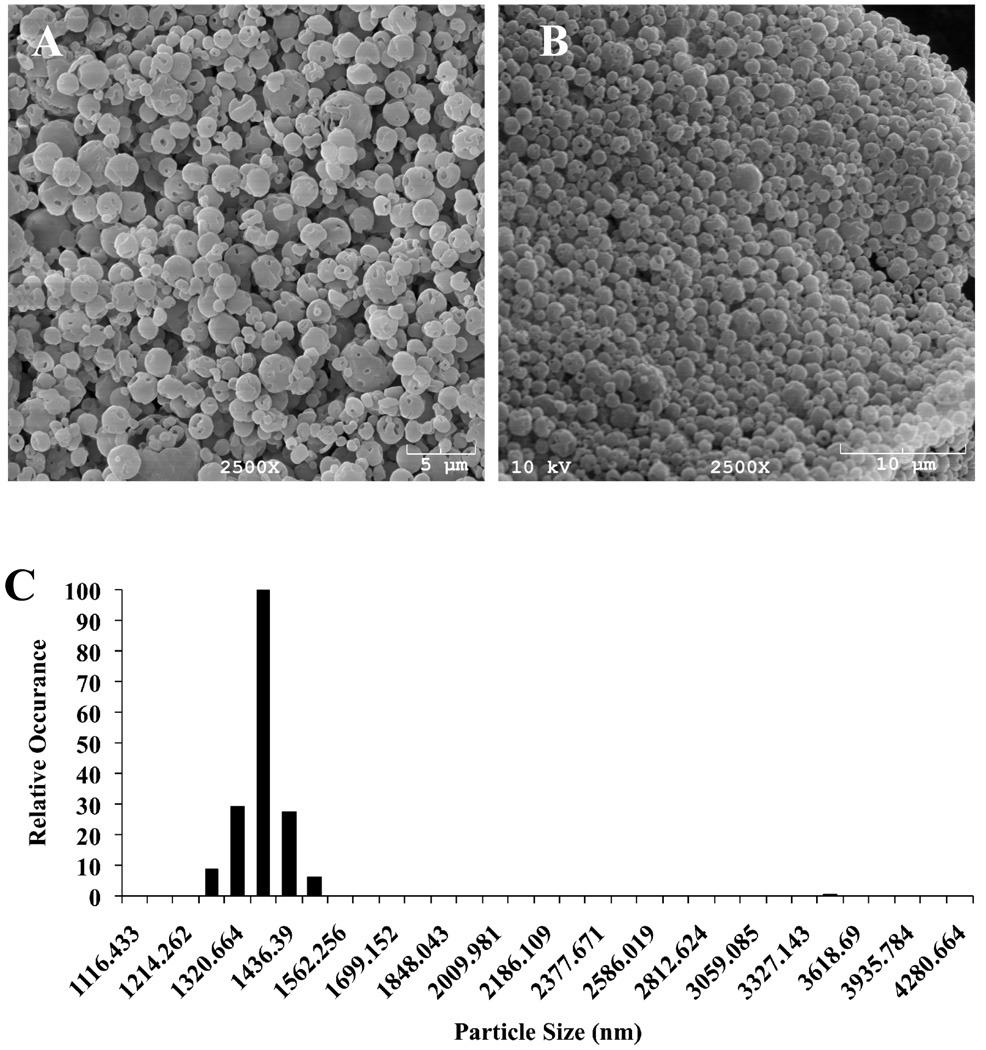
We chose PK3 for further investigation as a drug carrier for the treatment of acute inflammatory diseases because of its suitable hydrolysis kinetics and biocompatible degradation products. The copolymer PK4 has faster hydrolysis kinetics than PK3; however it degrades into 1,4-butanediol, which is converted into γ-hydroxybutyrate in vivo, and subsequently causes toxicity to the central nervous system (15). Microparticles were formulated from PK3, using a solvent evaporation procedure. Figure 4A demonstrates that the size range of PK3 microparticles is between 1 to 5 microns, which are suitable for phagocytosis by macrophages.
Figure 4.
SEM images of particles formulated with PK3. (A) SEM image of empty particles formulated via double emulsion. (B) SEM images of imatinib-loaded PK3 microparticles formulated via double emulsion. (C) Size Distribution of imatinib-loaded PK3 microparticles as determined by dynamic light scattering (DLS).
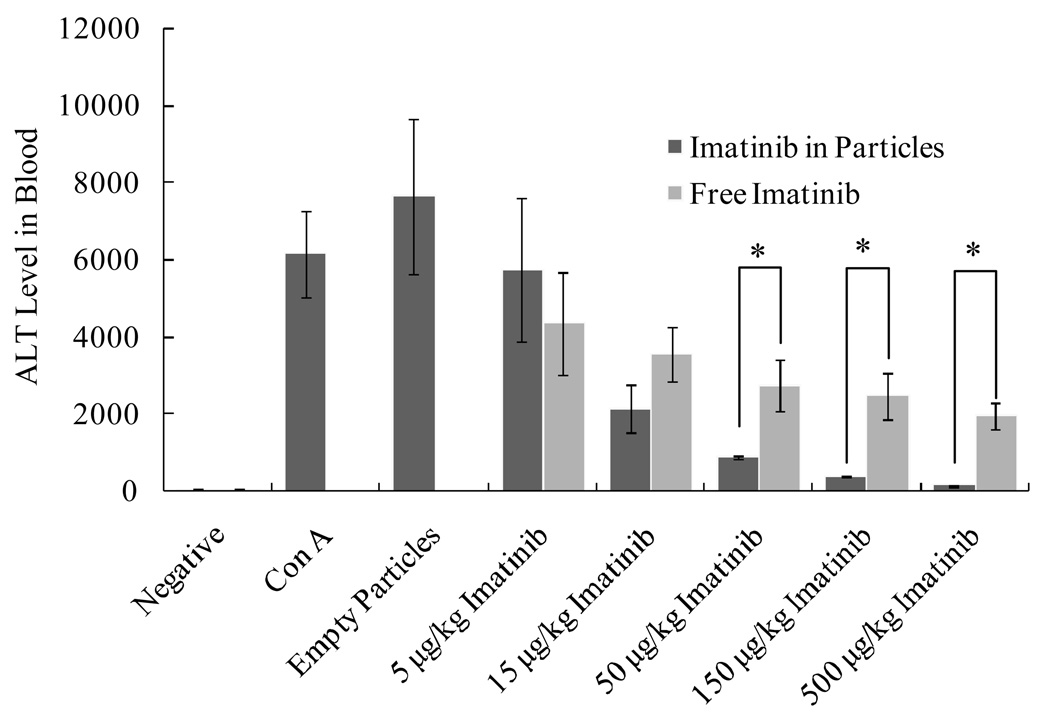
PK3 microparticles improve the efficacy of imatinib in treating acute liver failure in mice. Mice were injected intravenously with either imatinib or imatinib in PK3 microparticles, acute liver failure was then induced by intraperitoneal injection of Con A (15 mg/kg). 8 hours later, ALT levels in the blood were analyzed. * indicates statistical significance (Student’s t test, P < 0.05, n = 4 to 8).
In order to determine whether or not PK3 microparticles would have suitable drug-release kinetics for treating acute inflammatory diseases, a release study was conducted on PK3 microparticles which encapsulated the fluorescent dye, rhodamine B. Figure 3 demonstrates that the release half-life of rhodamine B from PK3 microparticles is approximately 6 hours at pH 4.5 and 40 hours at pH 7.4, which is suitable for treating acute liver failure. The drug-release kinetics of PK3 microparticles is 20 times faster than that of PCADK microparticles, which had a release half-life of 5 days at pH 4.5 and over 15 days at pH 7.4 (data not shown).
Figure 3.
Release kinetics of rhodamine B encapsulated in PK3 microparticles.
PK3 microparticles were used to enhance the delivery of imatinib in treating acute liver failure. Imatinib is a kinase inhibitor that inhibits NF-κB activation and has great potential for treating acute inflammatory diseases (16, 17). The activation of NF-κB in macrophages leads to the production of cytokines and reactive oxygen species, the central causes of tissue damage during acute inflammatory diseases. Although imatinib has shown promise for treating acute inflammatory diseases in mouse models, there are also numerous side effects associated with imatinib, including heart failure and hepatic toxicity (18, 19). PK3 microparticles should enhance the efficacy of imatinib by targeting it to macrophages, thereby increasing its concentration at the target site, and also by reducing the toxic side effects of imatinib on the heart and liver. Imatinib was encapsulated into PK3 microparticles through double emulsion procedures. These macroparticles were approximately 1.5 microns in size on average, as determined by SEM and DLS, which is suitable for delivering drugs to phagocytic cells such as Kupffer cells (Figure 4B, C). The loading of imatinib in PK3 microparticles was determined to be 0.90 ± 0.10 µg imatinib per 1 mg of particles (n = 3), suggesting that the encapsulation efficiency of imatinib in PK3 microparticles was approximately 0.32%.
The ability of PK3 microparticles to enhance the delivery of imatinib was investigated in mice suffering from Con A-induced acute liver failure. Mice were injected with either imatinib in solution (0.1 µg to 10 µg of imatinib dissolved in 200 µL of saline) or an equivalent amount of imatinib loaded in PK3 microparticles (0.11 mg to 11 mg of imatinib-loaded PK3 microparticles suspended in 200 µL of saline). Acute liver failure was subsequently induced by an intraperitoneal injection of Con A. The severity of liver injury in these mice was determined by measuring the alanine aminotransaminase (ALT) level in their blood, which is a clinical surrogate marker for hepatocyte injury. Figure 4 demonstrates that PK3 microparticles enhanced the treatment efficacy of imatinib in preventing Con A-induced liver damage. For example, a dose of 500 µg/kg of free imatinib resulted in an ALT value of approximately 2,000 U, whereas 500 µg/kg of imatinib encapsulated in PK3 microparticles reduced the ALT values to baseline levels of only 50 U. The therapeutic efficacy of 15 µg/kg of imatinib loaded in PK3 microparticles was similar to that of 500 µg/kg of free imatinib. These experiments demonstrate that imatinib-loaded PK3 microparticles significantly enhanced the therapeutic efficacy of imatinib, presumably due to their accumulation in liver macrophages. No noticeable toxicity was observed by injection of empty PK3 microparticles (11 mg suspended in 200 µL of saline).
CONCULSIONS
In this report we demonstrate that the hydrolysis rates of polyketals can be tuned by varying their hydrophilicity. Using this strategy, an aliphatic polyketal copolymer, PK3, was identified, which had a hydrolysis half-life of 2 days at pH 4.5, but was stable for several weeks at pH 7.4. Microparticles were formulated from PK3, which encapsulated the kinase inhibitor, imatinib, and these PK3 microparticles significantly improved the efficacy of imatinib in treating Con A-induced liver damage in vivo. Based on these findings, we anticipate numerous applications of copolymers of polyketals for treating acute inflammatory diseases, given their pH sensitivity, rapid and tunable hydrolysis kinetics, and biocompatible degradation products.
ACKNOWLEDGMENT
This work was funded in part by the Georgia Tech/Emory Center for the Engineering of Living Tissues (funded by NSF-EEC-9731643), NSF-BES-0546962 CAREER AWARD, NIH UO1 HL80711-01, NIH R21 EB006418, R01AI064463, and J&J/GT Health Care Innovation Seed Grant Proposal. S. C. Yang is supported by an NSF IGERT Fellowship.
Footnotes
Similar copolymers with higher incorporation of 1,5-pentanediol were also generated; however those copolymers were viscous liquids, which are not suitable for formulation into microparticles for drug delivery. Therefore we only focused our hydrolysis analysis on the solid polyketal copolymers, PK1, PK2, and PK3.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hudson LD, Steinberg KP. Epidemiology of Acute Lung Injury and ARDS. Chest 116. 1999:74S-a-82. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 3.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 4.O'Grady JG, Schalm SW. Acute liver failure: Redefining the syndromes. Lancet. 1993;342:273. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi Y, Kawakami S, Yamashita F, Hashida M. The potential role of fucosylated cationic liposome/NF[kappa]B decoy complexes in the treatment of cytokine-related liver disease. Biomaterials. 2007;28:532–539. doi: 10.1016/j.biomaterials.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 6.Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic Tumor Necrosis Factor Signaling and Nuclear Factor-{kappa}B: Effects on Liver Homeostasis and Beyond. Endocr Rev. 2007:er.2006–0031. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 7.Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Caspase 8 small interfering RNA prevents acute liver failure in mice. PNAS. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 1997;28:5. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 9.Fu K, Pack DW, Klibanov AM, Langer R. Visual Evidence of Acidic Environment Within Degrading Poly(lactic-co-glycolic acid) (PLGA) Microspheres. Pharmaceutical Research. 2000;17:100. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 10.Gopferich A, Tessmar J. Polyanhydride degradation and erosion. Advanced Drug Delivery Reviews. 2002;54:911. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 11.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Advanced Drug Delivery Reviews. 2002;54:889. doi: 10.1016/s0169-409x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 12.Heffernan MJ, Murthy N. Polyketal Nanoparticles: A New pH-Sensitive Biodegradable Drug Delivery Vehicle. Bioconjugate Chem. 2005;16:1340. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal Microparticles: A New Delivery Vehicle for Superoxide Dismutase. Bioconjugate Chem. 2007;18:4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 14.Kwon YJ, Standley SM, Goodwin AP, Gillies ER, Frechet JMJ. Directed Antigen Presentation Using Polymeric Microparticulate Carriers Degradable at Lysosomal pH for Controlled Immune Responses. Mol. Pharmaceutics. 2005;2:83–91. doi: 10.1021/mp0498953. [DOI] [PubMed] [Google Scholar]
- 15.Zvosec DL, Smith SW, McCutcheon JR, Spillane J, Hall BJ, Peacock EA. Adverse Events, Including Death, Associated with the Use of 1,4-Butanediol. N Engl J Med. 2001;344:87–94. doi: 10.1056/NEJM200101113440202. [DOI] [PubMed] [Google Scholar]
- 16.Deininger MWN, Druker BJ. Specific Targeted Therapy of Chronic Myelogenous Leukemia with Imatinib. Pharmacol Rev. 2003;55:401–423. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- 17.Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, Weiss G, Tilg H. The kinase inhibitor imatinib mesylate inhibits TNF-α production in vitro and prevents TNF-dependent acute hepatic inflammation. PNAS. 2005;102:13622–13627. doi: 10.1073/pnas.0501758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand J-B, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 19.Yeon Hee P, Hae Jeong P, Bong-Seog K, Eunyoung H, Kyung Hee J, Seo Hyun Y, Sung Vin Y, Joo-Ho C. BNP as a marker of the heart failure in the treatment of imatinib mesylate. Cancer letters. 2006;243:16–22. doi: 10.1016/j.canlet.2005.11.014. [DOI] [PubMed] [Google Scholar]