Abstract

A bis-pyrrol-2-yl-2,5-diamidopyrrole has been synthesized and shown to have a significantly higher affinity for oxo-anions than previous generation 2,5-diamidopyrroles.
The ubiquity of anions in nature makes the study of their interaction with natural and artificial receptors a topic of considerable current interest.1,2 The synthesis of artificial anion receptor systems is driven also by their potential utility in a wide range of applications as different as separations and waste remediation to biomedical analysis and therapy.3 Within the general context of supramolecular chemistry, the field of synthetic anion receptor chemistry is one of the fastest growing disciplines.4 To date, a wide variety of heterocyclic anion receptors have been reported in the literature. These include systems that run the gamut from simple linear monomeric pyrroles to open chain polypyrroles, as well as cyclic pyrrolic structures.5 Acyclic 2,5-diamidopyrroles, e.g. 2, reported by Gale and co-workers,6 are among the most versatile and easy-to-synthesize of the known pyrrole-based anion receptors. These systems have been found to interact particularly strongly with benzoate and dihydrogen phosphate anions in DMSO or DMSO/water solution.
We were thus curious to explore whether the incorporation of additional pyrrole NH hydrogen bond donor elements would lead to enhancements in affinity or modifications in the inherent anion binding selectivity. Towards this end we have prepared receptor 1, a system whose synthesis is predicated on the availability of a functionalized 2-aminopyrrole precursor.
2-Aminopyrroles are not readily available precursors. In general, such species are hard to make and are notoriously prone to decomposition. An exception is systems bearing electron withdrawing groups in the beta-pyrrolic positions, such as diethyl 2-aminopyrrole-3,4-dicarboxylate, 3.7 This latter pyrrole was prepared readily using the procedure first described by Duffy and Wibberley.7
Once this key precursor was in hand, it was reacted in dry CH2Cl2 with 0.5 equiv of 3,4-diphenylpyrrole-2,5-dicarbonyl dichloride 4,8 in the presence of Et3N. Following purification via column chromatography (silica gel, 3% MeOH in CH2Cl2 eluent), compound 1 was isolated in 47% yield (Figure 2). The moderate yields for this reaction are ascribed to the inherent instability of the diacid chloride, 4, and to the formation of the three-ring dimer species 5.9 The formation of such dimeric species has been noted previously in the context of forming 2 and its analogues.6
Figure 2.
Synthesis of receptor 1
Structural proof for receptor 1 came from a single crystal X-ray diffraction analysis of crystals of 1 grown by slow evaporation of an CHCl3 solution of the receptor (See Supplementary Information). Figures 3 and 4 show the top and side views of 1, and intermolecular hydrogen bonding interactions between adjacent species in the solid state respectively.
Figure 3.
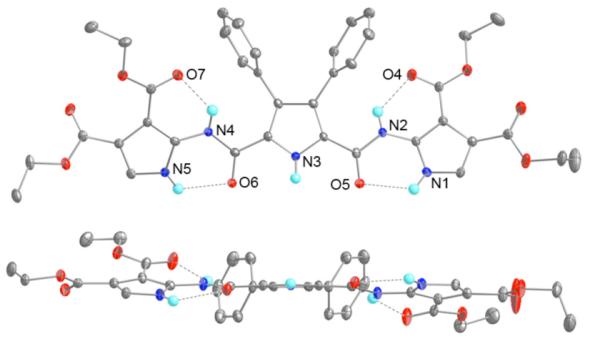
Top and side view of 1. Dashed lines indicate hydrogen bonding interactions. The thermal ellipsoids are scaled to the 50% probability level. Most hydrogen atoms have been removed for clarity.
Figure 4.

View of the crystal structure of 1 showing interactions between adjacent receptors in the solid state
There are a number of different hydrogen bonding interactions observed in the solid state structure of 1. The first, depicted in Figure 3, is of an intramolecular nature and is formed between the pyrrole NH proton and the carbonyl oxygen of the amide moiety (N1H···O5, 2.73 Å, 118°; N5H···O6, 2.72 Å, 116°). A second intramolecular interaction involves the amide NH proton and the carbonyl oxygen of the ester functionality on the β-position of the terminal pyrrole (N2H···O4, 2.75 Å, 126°; N4H···O7, 2.74 Å, 124°). In addition to these two intramolecular interactions, there are six intermolecular hydrogen bond interactions. The intermolecular interactions serve to assemble compound 1 into what can be described as an extended dual sheet structure. This unusual structure reflects the fact that half of a molecule is hydrogen bonded to one complementary molecule, while the other half is hydrogen bound to a second, independent but also complementary molecule. The actual hydrogen bonding interactions involve the pyrrole NH protons and the corresponding amide carbonyl oxygen atoms from one of the molecular partners in the dual sheet. Four of the hydrogen bond interactions are considered to be fairly strong, as evidenced by the bond lengths between 2.81 and 2.92 Å, while the other two, with identical lengths of 3.22 Å, are judged to be weaker.
The fact that extensive intermolecular interactions were observed for 1 in the solid state, led to concerns that aggregation might be observed in solution. Such aggregation behavior, to the extent it was observed, would complicate the anion binding behavior of this putative receptor. Accordingly, its interactions with representative anions (in the form of their tetrabutylammonium salts) were studied in DMSO-d6 solution using 1H-NMR spectroscopic techniques. This solvent, which was also the one used previously for the study of 2, was expected to stabilize the monomeric form of 1. Consistent with this supposition, the binding constants obtained from fits of the 1H-NMR spectroscopic titration curves (cf. Figure 5) were found to be concentration independent over the concentration range of 2.2 - 4.9 mM (0.95 mM - 9.97 mM in the case of benzoate). Standard curve protocols, as used previously by our groups10 (and others), were used to calculate the binding constants, with the host-guest stoichiometries being determined using the Job plot method. The results of these analyses are summarized in Table 1.
Figure 5.

Curve-fitting trace of the data obtained by following the amide NH proton shift in the 1H-NMR spectral titration of 1 with TBAbenzoate. The inset shows part of the 1H-NMR spectrum of 1 recorded in the absence (blue trace) and in the presence (red trace) of benzoate anion (6.14 equiv).
Table 1.
Affinity constantsa for the binding of anions by receptors 1 and 2 as determined from 1H-NMR spectroscopic titrations in DMSO-d6. The anions studied were in the form of their tetrabutylammonium salts
All studies were carried out at an initial receptor concentration of 3.0 mM. The tabulated values are the average of at least two independent determinations; error ≤ 15%
from ref. 6
NB = no binding, as inferred from the lack of observable change in the 1H-NMR spectrum upon the addition of the indicated anion
not determined
1:1 stoichiometry as confirmed by Job plots.
Inspection of Table 1 reveals that in the series of anions studied only dihydrogenphosphate and benzoate anions are bound appreciably by receptor 1 in DMSO. In accord with the design expectations, receptor 1 displays a much higher affinity for these two anions than 2.6 It also appears to be even more selective for these oxyanions than these previously reported 2,5-diamidopyrrole anion receptor systems. In fact, in contrast to 2, receptor 1 displays no detectable affinity for chloride anion and, in analogy to these analogues, likewise shows no appreciable interaction with either bromide or hydrogen sulfate anions. On the other hand, receptor 1 displays a dihydrogenphosphate / benzoate selectivity that is actually reversed compared to that seen in the case of receptor 2.
These results can be rationalized by the presence of the two new pyrrole rings in 1 and hence a beneficial increase in the number of hydrogen bond donor sites as compared to compound 2. The selectivty of 1 towards oxo-anions over halide anions may be explained by the presence of intramolecular hydrogen bond interactions between the amide NH protons and one of the β-pyrrolic ester moieties (Fig. 3: N4H···O7 and N2H···O4). These interactions, observed in the solid state, are maintained in solution as judged by the significant difference seen in the chemical shift of the amide NH proton seen in the case of 1 (11.99 ppm) and 2 (9.37ppm).6 Support for this conclusion also comes from the fact that the amide NH proton of 1 undergoes an upfield shift upon addition of an oxo-anion. Interactions with such anions serve to break up these intramolecular NH···O H-bonds, replacing them with a number of NH···A (A = anion) contacts. Each of these individual NH···A hydrogen bond is likely weaker than the original internal hydrogen bonds. As a result, a net upfield shift in the NH signal is observed. For the same reason, strong binding is seen only the case of substrates, such as oxo-anions (e.g., benzoate, dihydrogen phosphate), but not halides, that are of an appropriate size and shape to stabilize a large number of NH···A hydrogen bonding interactions.
In summary, we have shown that the addition of supplementary NH hydrogen bond donors on to an easy-to-make pyrrole-amide anion recognition platform, such as that defined by the parent system 2, can lead to demonstrable increases in anion affinity. The fact that in 1 these additional NH donor moieties are constrained within a quasi-planar receptor framework via intramolecular hydrogen bond interactions in the absence of an added anion guest, favors interaction with oxo-anions, such as benzoate and dihydrogen phosphate, and imparts selectivity relative to halide-anions.
Current work is focused on probing further the utility of this approach, as well as studying the intermolecular interactions of systems such as 1 and 2 in less competitive solvent environments.
Supplementary Material
Figure 1.
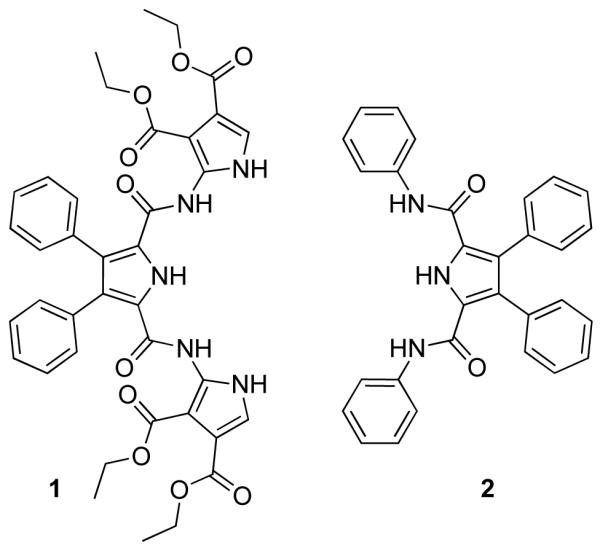
Receptor 1 and the “parent” compound receptor 2
Acknowledgment
This work was supported in part by the NIH (grant no. GM 58907 to J.L.S.) P.A.G. thanks the Royal Society for a University Research Fellowship and Professor Mike Hursthouse and the EPSRC for access to the crystallographic facilities at the University of Southampton.
References
- 1.Sessler JL, Gale PA, Cho WS. In: Anion Receptor Chemistry (Monographs in Supramolecular Chemistry. Stoddart JF, editor. RSC, Cambridge: 2006. [Google Scholar]
- 2.Bianchi A, Bowman-James K, Garcia-España E, editors. Supramolecular Chemistry of Anions. Wiley - VCH; New York: 1997. [Google Scholar]; Schrader T, Hamilton A, editors. Functional synthetic receptors. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- 3.Moyer BA, Singh RP, editors. Fundamentals and Applications of Anion Separations. Kluwer Academic/Plenum Publishers; New York: 2004. [Google Scholar]
- 4.Schmidtchen FP. Top. Curr. Chem. 2005;255:1–30. [Google Scholar]; Stibor I, Zlatušková P. Top. Curr. Chem. 2005;255:31–64. [Google Scholar]; Lhoták P. Top. Curr. Chem. 2005;255:65–96. [Google Scholar]; Davis F, Collyer FD, Higson SPJ. Top. Curr. Chem. 2005;255:97–124. [Google Scholar]; Beer PD, Bayly SR. Top. Curr. Chem. 2005;255:125–162. [Google Scholar]; Suksai C, Tuntulani T. Top. Curr. Chem. 2005;255:163–198. [Google Scholar]; Houk RJT, Tobey SL, Anslyn EV. Top. Curr. Chem. 2005;255:199–220. [Google Scholar]; Gale PA, editor. Coord. Chem. Rev. Vol. 240 2003. [Google Scholar]
- 5.Cho W-S, Sessler JL. In: Functional synthetic receptors. Schrader T, Hamilton A, editors. Wiley-VCH; Weinheim: 2005. pp. 165–256. [Google Scholar]
- 6.Gale PA. Chem. Commun. 2005:3761–3772. doi: 10.1039/b504596g.Gale PA, Light ME, McNally B, Navakhun K, Sliwinski KE, Smith BD. Chem. Commun. 2005:3773–3775. doi: 10.1039/b503906a.Camiolo S, Gale PA, Light ME, Hursthouse MB. Org. Biomol. Chem. 2003;1:741–744. doi: 10.1039/b210848h.Gale PA, Camiolo S, Chapman CP, Light ME, Hursthouse MB. Tetrahedron Lett. 2001;42:5095–5097.Gale PA, Camiolo S, Tizzard GJ, Chapman CP, Light ME, Coles SJ, Hursthouse MB. J. Org. Chem. 2001;66:7849–7853. doi: 10.1021/jo016020g.Denuault G, Gale PA, Hursthouse MB, Light ME, Warriner CN. New J. Chem. 2002:811–813.Camiolo S, Gale PA, Hursthouse MB, Light ME, Shi AJ. Chem. Commun. 2002:758–760. doi: 10.1039/b200980c.Camiolo S, Gale PA, Hursthouse MB, Light ME. Tetrahedron Lett. 2002;43:6995–6996.Gale PA, Navakhun K, Camiolo S, Light ME, Hursthouse MB. J. Am. Chem. Soc. 2002;124:11228–11229. doi: 10.1021/ja027118v.for acyclic diamidodipyrrolylmethanes which show a significantly enhanced affinity for oxo-aniosn as compared to the 2,5-diamidopyrrole see:Vega IED, Gale PA, Hursthouse MB, Light ME. Org. Biomol. Chem. 2004;2:2935–2941. doi: 10.1039/B409115A.Vega IED, Camiolo S, Gale PA, Hursthouse MB, Light ME. Chem. Commun. 2003:1686–1688.
- 7.Duffy TD, Wibberley DG. J. Chem. Soc., Perkin I. 1974:1921–1929. [Google Scholar]
- 8.Friedman M. J. Org. Chem. 1965;30:859–863. [Google Scholar]
- 9.Boatman RJ, Whitlock HW. J. Org. Chem. 1976;41:3050–3051. [Google Scholar]
- 10.Camiolo S, Gale PA. Chem. Commun. 2000:1129–1131. [Google Scholar]; Sessler JL, Pantos GD, Katayev E, Lynch V. Org. Lett. 2003;5:4141–4144. doi: 10.1021/ol0355635. [DOI] [PubMed] [Google Scholar]; Li R, Evans LS, Larsen DS, Gale PA, Brooker S. New J. Chem. 2004;28:1340–1343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



