Abstract
Ultrasonic extraction followed by Stir Bar Sorptive Extraction (SBSE) and thermal desorption inline coupled with Gas Chromatography and Mass Spectrometry (TD/GC/MS)was used to perform a comprehensive determination of soil-borne polycyclic aromatic hydrocarbons (PAHs) in El Paso, Texas. The method provided good sensitivity and faster processing time for the analysis. The total PAHs in El Paso soil ranged from 0.1 to 2225.5 µg kg−1. Although the majority of PAH concentrations did not exceed the soil screening levels regulated by the United States Environmental Protection Agency, the existence of PAHs in this ecosystem is ubiquitous. Naphthalene were found in 100% of the soil samples; while the heavy PAHs (five- and six-ring) were not often detected and mostly remained in closer proximity to industrial areas and major traffic points. The results ruled out the possibility of petroleum refining as the significant source of local soil-borne PAH contamination, but they suggested that the PAHs found in El Paso soil were closely linked to human activities and possible other industrial processes.
Keywords: Persistent organic pollutants (POPs), Stir Bar Sorptive Extraction (SBSE), Border, Soil, Diagnostic ratio
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds consisting of two or more fused aromatic rings [1]. They belong to persistent organic pollutants (POPs), a group of chemicals which are characterized by their persistence in the environment, toxicity, and bioaccumulation in the food chain. Once introduced, POPs are transported, mainly through the atmospheric pathway, over short and long distance from their sources. This movement occurs either on suspended particles or through a process called global distillation and cold condensation [2]. PAHs can occur naturally (e.g. volcanic activities), and also be released and generated by anthropogenic activities (e.g. coke production, ferrous and nonferrous metallurgic processes, and vehicle emissions). In addition, PAHs are formed during the incomplete burning of oil, gas, electric power generation, coal, wood, garbage, or other organic substances, such as tobacco and charbroiled meat [1]. As a result, PAHs are ubiquitous. They have been found in every part of the world, even in remote areas like the Arctic, where no substantial pollution sources exist [3].
In order to understand the global distribution of POPs, numerous screening studies have been performed around the world. The El Paso Texas area is a unique region for such an environmental study. The City of El Paso with its twin city, Ciudad Juarez, Mexico, is located approximately midway between the Pacific Ocean and the Gulf Coast. The City of El Paso shares airsheds and water resources with the city of Ciudad Juarez. The Rio Grande forms the international boundary between the two cities, and this area is one of the largest semi-arid international border communities in the world. The North America Free Trade Agreement (NAFTA) has increased industrial development on the Mexican side. As a result, there are more than 300 U.S.-owned factories, called maquiladoras, in Ciudad Juarez [4], and the population on both sides of the border has increased significantly in the past two decades. The increasing release of pollutants into the border region has raised concerns about the environment and public-health-related issues.
Soil is the primary environmental reservoir for semi-volatile organic compounds including PAHs [5,6]. PAHs in soil mostly resulted from the stationary and mobile sources, which dispersed the PAHs in the atmosphere. By going through the transportation process in the atmosphere, PAHS can deposited on the soil by dry and/or wet depositions, and finally sink into the soil. Because of their persistence and hydrophobicity, PAHs accumulate in soils, especially in organic matters, where they can be retained for many years [7,8]. Since humans can be exposed to PAHs through the soil–(air)–plant–animal–human pathway [7], soil-borne PAHs can be an assessment of health impact. Therefore, this study focused on soil-borne PAHs to gain knowledge of an understudied environmental issue.
Environmental study relies heavily on chemical analysis. To prepare samples for analysis, the ideal preparation steps should be rapid, easy to operate, cost effective, generating minimal or no hazardous wastes, and providing good recovery to allow sensitive measurement of the analytes. USEPA SW-846 methods suggest the following methods for extracting PAHs from solid samples: Methods 3540C and 3541 (Soxhlet Extraction), Method 3545A (Pressurized Fluid Extraction), Method 3546 (Microwave Extraction), Method 3550C (Ultrasonic Extraction), Methods 3560 and 3561 (Supercritical Fluid Extraction). Traditionally, the extract will be further concentrated or enriched (using K-D condenser or N2 flow) followed by cleanup procedures. After further enrichment, 1–3 µL of the concentrated extract (which usually has a final volume of 100 µL to 1 mL) will be directly injected to GC for analysis. Though Soxhlet extraction is commonly considered as the benchmark technique, the sample preparation processes usually demand high solvent consumption, time, and manpower. Nonetheless, a significant amount of analytes can be lost during the processes, which could result in lack of analytical sensitivity [9]. Alternative methods, such as supercritical fluid extraction (SFE) [10], accelerated solvent extraction (ASE) [11] and subcritical water extraction [10], microwave-assisted extraction (MAE) [12], are considered more environmental friendly procedures that use less solvent, have shorter extraction time, provide higher sample throughput, and improve the sensitivity of the analysis; they also were found to have better or comparable extraction efficiency to Soxhlet extraction [13,14]. We further found that ultrasonic extraction could effectively extract PAHs from solid matrix, e.g. SRM 1649a, when compared to MAE recovery results (unpublished data).
In this study, we used sonication to extract PAHs from the soil followed by a solventless technique for enrichment processes. The technique is called Stir Bar Sorptive Extraction (SBSE), which was developed in 1999 to extract organic compounds from aqueous samples [9]. The Stir Bar (also called Twister™) coated with 50–300 µL of polydimethylsiloxane (PDMS), is put into the solution (or extract solution in this case) and stirred for a pre-determined time; and the solutes partition from the aqueous phase into the extractant, PDMS [15]. The equilibrium is controlled by the partition coefficient, KPMDS/w, between the PDMS phase and the aqueous phase. KPMDS/w values increase with the octanol–water partition coefficient Kow of the analytes [9,15,16]; therefore, compounds, such as PAHs, with high Kow are prone to partition into the PDMS phase and be extracted from aqueous solutions [16]. After stirring, the Stir Bar is removed from the solution and the adsorbed compounds are thermally desorbed from the PDMS phase on the Stir Bar in a thermal desorption unit (TDU) and analyzed by Gas Chromatography/Mass Spectrometry (GC/MS).
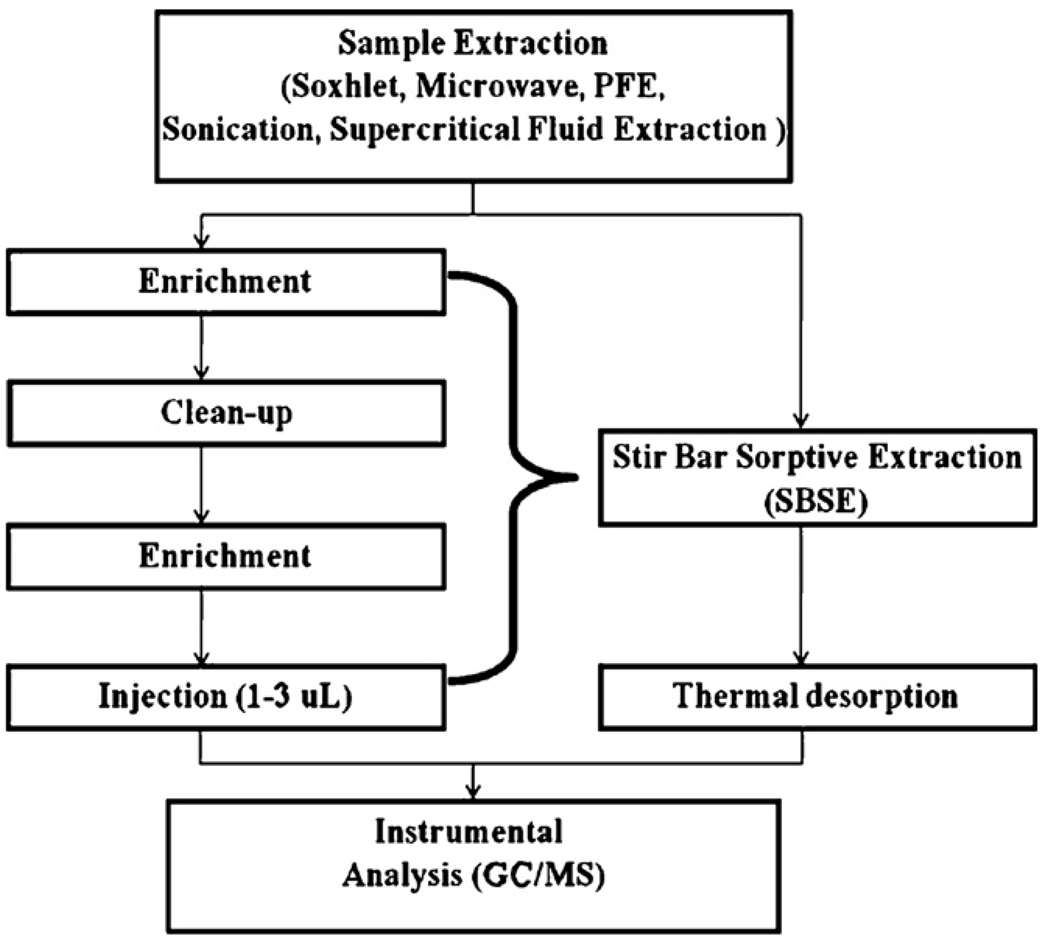
Although SBSE was originally developed for the extraction of aqueous samples, the technique has been successfully applied to the trace analysis of various target analytes in environmental samples such as soil [9,17], vegetables [18], and biological samples [9,19]. Combined with various extraction methods (as illustrated in Fig. 1), SBSE is found to avoid clean-up and concentration procedures, reduce matrix effect, have higher throughput capacity for measuring chemical composition, and improve detection limits [14,17].
Fig. 1.
Schematic illustration of the analytical procedures for semivolatile organic compounds from solid samples. The traditional procedures are demonstrated on the left side of the flow chart; while the technique used in this research is shown on the right side.
Currently, data for the occurrence of PAHs and their impact on public health are lacking in the United States/Mexico border area. Herein, we aimed to study the concentration and occurrence of soil-borne PAHs in the El Paso region. Our data would be the first analysis of PAHs in soil from this geographical location, which could be correlated to other environmental and health issues. The optimization of ultrasonic extraction–SBSE–thermal desorption–GC/MS techniques for soil-borne PAHs was also investigated and is reported in this study.
2. Materials and methods
2.1. Materials
PAH standard mixtures (Ultra Scientific; Kingstown, RI; Cat # US-106N; 2000 µg mL−1), internal standard spiking solutions (dibutyl chlorendate, Ultra Scientific; Kingstown, RI; Cat # US-STS-280N) and GC grade methanol were supplied by Fisher Scientific Inc. PAH stock solutions of 10 mg L−1 were prepared in methanol, and stored in amber vials at 4 °C. Ultrapure quality water was produced by Festa Ultrapure water production system (Festa/Ing, Chihuahua Mexico).
2.2. Description of sampling sites
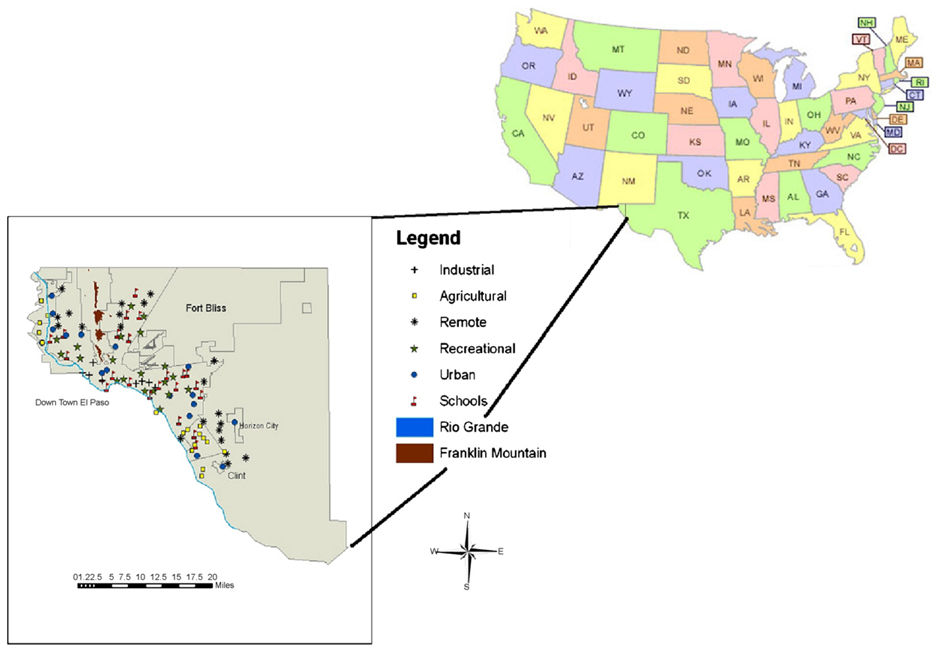
One hundred and seven samples were collected from February 2004 to May 2004 across the El Paso, TX area (Fig. 2). Sampling sites were selected randomly but evenly distributed throughout the El Paso area. The sampling sites were categorized into six types including industrial, agricultural, urban, recreational, school and remote sites. Industrial sites were classified as areas in which land use is mainly for oil refining and other industrial activities, or as an area within 1 mile from these facilities. Agricultural sites are the regions exclusively adopted for croplands. These areas are mainly located along the Rio Grande. Urban sites were composed primarily of residential housing and community development. Samples were collected from empty spaces located among houses and in places surrounded by urban activities (e.g. malls and roads). The recreational sites were designated for city or county parks. Remote areas were classified as locations far from human activities (at least 15 miles away) or sparsely populated. The school sites are well distributed among urban, industrial, and agricultural areas.
Fig. 2.
Location of sampling sites. Sites were randomly selected throughout El Paso County. No sample was collected in the Ford Bliss area due to security concerns.
The vegetation covering in the majority of the samples sites is sparse, typical of a desert environment. Creosote bush (Larrea tridentata) was the most abundant plant in urban areas. The vegetation in the remote areas was composed of scatter but variable vegetation including creosotes bush, saltbush (Atriplex canescens), jimmy weeds (Isocoma pluriflora), summer cypress (Kochia scorpia), and tumbleweed (Salsola kali). Vegetation in school and recreational sites were dominated by grass.
In this research, the working hypothesis was that the industrial areas might be the source of PAH contamination and therefore they were used as the centers of interest. Circles were drawn with approximated 1, 2, 5, 10, 15, and 20 miles as the radii from the centers to study the spatial distribution of PAHs. Each sampling site was at least 1 mile away from the others.
2.3. Sampling methods
At least 1000 g of surface soil (10–15 cm of depth)were collected from each site with a stainless steel shovel. Each sample was a composite of 10 sub-samples from an approximated 50 m × 50 m area at each individual site. The site location coordinates were determined with a handheld Global Position System receiver (Trimble Geoexplorer II) with 2–5m precision. Upon returning to the laboratory, the soil samples were air-dried (if necessary), and each sample was sieved to 1 mm to eliminate organic fragments and aggregates. Subsequently, they were transferred into clean glass-amber containers and stored at ambient temperature prior to analysis. Characterization of the soil samples was performed. Ninety-five percent of the soil samples had moisture contents ranging from 0.04 to 2.98% and organic carbon content ranging from 0.4 to 8.8% (data not shown). The soil texture analysis indicated that 59% of the samples were sandy soil, 29% are loamy sandy and 12% are sandy loamy soil.
2.4. Sample preparation: ultrasonic extraction coupled with SBSE
Ten grams of soil sample and 30 mL of 100% methanol (extractant) were added into a 40-mL vial. The soil was ultrasonically extracted (Fisher Scientific—FS30H sonicator) at room temperature for 30 min to promote the diffusion of PAHs from the soil into the extractant. After sonication, an aliquot of 2.4 mL of the extract was added to a 20-mL headspace vial along with 100 µL of 10 ppm of internal standard spiking solution and 7.5 mL of purified water for the SBSE enrichment process. The analytes were extracted with a Stir Bar (Twister™, 10 mm length and 0.5 mm thickness, Gerstel, Inc., Baltimore, MD, USA) for 4 h at 1000 rpm under ambient temperature. After stirring, the Stir Bar was removed with tweezers, rinsed with ultrapure water, dried with a lint free tissue, and placed in an empty glass thermal desorption tube. The Stir Bar was placed in a TDU system, and PAHs were thermally desorbed from the Stir Bar and analyzed by a GC–MS.
2.5. TD–GC–MS analysis
Thermal desorption–Gas Chromatography–Mass Spectrometry (TD–GC–MS) analysis was performed on a GERSTEL Twister™ Desorption Unit (TDU) with a CIS 4 cryo-injector (Gerstel, Inc., Baltimore, MD, USA) coupled with a GC/MS system (Agilent 6890 GC/5973N MSD, Agilent Technology, Santa Clara, CA, USA). The Stir Bars were thermally desorbed in the TDU under splitless mode. The thermal desorption process was programmed as follows: initial temperature at 40 °C with ramp at 60 °C/min to 300 °C (held for 5 min). The TDU transfer line temperature was set at 320 °C. The desorbed PAHs were cryo-focused in the CIS 4 at −40 °C prior to the injection. The CIS 4 temperature was ramped from −40 °C to 320 °C at 12 °C/s and held for 10 min. The separations were performed using a HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm, Agilent Technology). The oven was programmed as follows: 50 °C (held for 2 min) with 25 °C/min ramp to 150 °C, 3 °C/min to 230 °C, 8 °C/min to 300 °C and held for 10 min. The carrier gas was ultra high purity helium at a constant flow of 1.0 mL min−1. The GC transfer line was set at 280 °C. For mass spectrometer parameters, temperature settings of the source and the quad were 230 and 150 °C, respectively. The MS was programmed using a scan mode with m/z ranging from 40 to 500 amu. The identification of compounds was carried out using the NIST library. Data quantification was performed using the MSD ChemStation Software (Agilent technology). Seven-point calibration curves were conducted ranging from 0.01 to 1000 µg L−1. The linear response of the curves produced correlation coefficients (R2) higher than 0.99 for all 16 PAHs (data not shown).
For quality assurance and control, calibration standards were run every 30–40 sample analyses. Two replicates for each sample extract were performed. Should the relative standard deviation (R.S.D.), which is calculated as standard deviation divided by the average, be greater than 25%, a third replicate analysis was performed. Blank runs (empty vial)were carried out every five sample runs. A laboratory blank sample was analyzed every 20 sample runs.
2.6. Geographical Information System (GIS) mapping
Geographical Information System (GIS), ESRI ArcGIS, Geostatistical Analyst extension within ArcMap 9_ program, was used to produce maps of PAH distribution. The position of the sample locations was recorded using a Global Positioning System (GPS), and the distribution GIS map was prepared based on the concentration found at each site. The input data was converted to a vector characterized by points representing each sample location. These vector data were transformed to a grid via interpolation using the GIS geostatistical analyst. When interpolation was performed for non-sample points, surface values were estimated or predicted based on known values of surrounding points [20].
Kriging interpolation was applied to interpolate the concentration values. Kriging interpolation provides unbiased estimation and minimized prediction of mean-square errors [21,22]. This method assumes that the distance or direction between sample points reveals a spatial correlation (spatially dependent data) that can be used to explain variation on the surface [23]. Kriging uses a semivariogram to quantify the spatial correlation in the data, and defines the weights that determine the contribution of each data point for predicting values at the un-sampled locations [22]. The semivariogram is a function of a distance and direction separating two locations, and is calculated as half of the average of squared difference between the components of data pairs [21,22,24,25]:
| (1) |
where N(h) is the number of data pairs separated by the distance h, Z is the data value, and x is the position of the soil sample.
Ordinary Kriging was used as surface estimator. The general formula is
| (2) |
where Z(si) is the measured value at location i, λi is the weight for the measured value at the location i, s0 is the predicted location, and N is the number of measured values, which are obtained from the variogram modeling [26]. Since the distribution of PAHs shows a skewed distribution with few larges values, the PAH concentration were log-transformed to show a better agreement with a normal distribution. This transformation helps to make the variance more constant and to normalize the data.
3. Results and discussion
3.1. Ultrasonic extraction–SBSE method optimization
Ultrasonic extraction was performed to extract PAHs from soil samples. The effect of solvent during extraction procedures was studied by Sandra et al. [18] and in our group (unpublished data). Solvents such as acetonitrile, acetone, and methanol have been evaluated. The extraction efficiency of organic compounds using acetonitrile and methanol was very similar, but was better than using acetone. Methanol was preferred because it is an environmentally friendly solvent. In addition, methanol can be used in the next SBSE procedure and therefore no solvent exchange was needed. We also found that higher methanol content used in sonication provided better extraction efficiency (unpublished data). As a result, 100% methanol was chosen as the extraction solvent used in ultrasonic extraction.
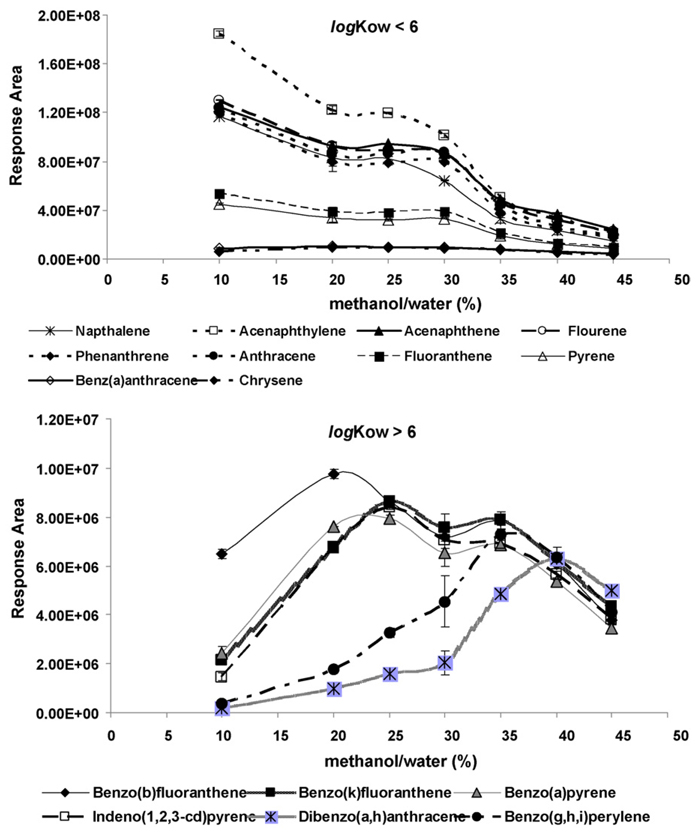
After the ultrasonic extraction, SBSE was used for PAH enrichment from the extract. In SBSE, analytes in the extract solution diffuse into the PDMS phase until they reach equilibrium between both phases. Two factors were investigated for their impact on the equilibrium: (1) organic solvent content and (2) stirring time in the SBSE process. Study showed that organic solvent content in the extract solutions could affect the partitioning of a compound into the PDMS phase on the Stir Bar [27].Methanol is commonly used as the organic solvent in SBSE [16]. To evaluate the effect of the organic solvent content on the recovery of PAHs in SBSE, various levels of methanol in water were examined. The methanol percent levels were prepared at 10%, 20%, 25%, 30%, 35%, 40%, and 45% in water. PAH standard solutions with concentration of 100 µg L−1 were prepared in each of the above methanol/water solution for testing the recovery of PAHs. As seen in Fig. 3, the use of 10% methanol in the SBSE showed better recovery for compounds that have low Kow; while 35% or 40% methanol/water mixtures seemed to have better extraction efficiencies for compounds with higher Kow. Based on the finding, it was decided to use 25% methanol/water mixture as the solvent in the SBSE process since it demonstrated good sensitivity for compounds with broad range of Kow values, and provided sufficient analytical capability to perform quantification for multi-residue extractions.
Fig. 3.
The effect of methanol concentration in the SBSE processes on the PAHs recovery. Error bars were ±standard deviation of the corresponding replicates.
Other studies on the recovery of various organic compounds in aqueous samples using different levels of methanol/water solutions showed that 20% methanol–water gave a high recovery of PAHs with low log Kow (<4.2) and 50% methanol–water was the best solvent for PAHs with high log Kow (>4.2) [27]. Extractions of solutes with the Stir Bars using water as the only solvent have shown poor efficiency and recovery, probably due to the lost of solutes on the walls of glass vials [28]. For compounds with high log Kow (>5.0), solvents can minimize the adsorption of the compounds onto the wall of the glass vial and increase the extraction efficiency and recovery [28]. For compounds with low log Kow (<2.5), organic solvents can decrease the extraction of analytes due to the increases of solubility of the compounds in the solution, which in turn decreases the partition of analytes into the PDMS on the Stir Bar. As a result, the extraction efficiency would decline.
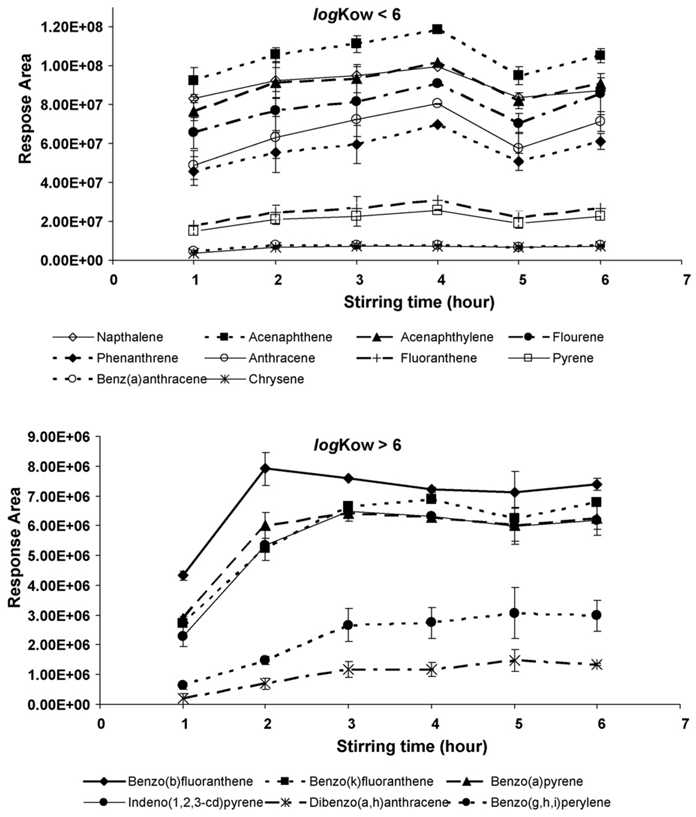
The extraction of organic compounds by SBSE depends not only on the solvents, but also on the time needed to reach the equilibrium between the solvent and PDMS phases. In order to obtain the optimal extraction efficiency, the extraction time needed for the SBSE process was studied. Spiked PAH solution at 100 µg L−1 was prepared in a 25% methanol/water solution. The extraction time varied from 1 to 24 h. As shown in Fig. 4 (24-h data not shown), the equilibrium of the partition of compounds into the PDMS phase varied. A 4-h stirring time seemed to provide sufficient recoveries for the majority of compounds and therefore was selected for SBSE extraction of PAHs.
Fig. 4.
The effect of the extraction time during the SBSE processes on the recovery of various PAHs. Error bars were ±standard deviation of the corresponding replicates.
Spiked soil samples with a known amount of PAHs were used to test the efficiency and recoveries of PAHs from the soil using the optimized analytical procedure as described previously. Spiked soil preparation was made by mixing a PAH standard solution in acetone with 10 g of soil to obtain a final concentration of 50 µg kg−1. The vials were placed in a hood, and the solvent was slowly evaporated (air dried) with constant stirring. Two different kinds of soil were used: sandy soil and Loamy sandy soil which represent 88% of the samples analyzed. The average recovery of PAHs from the soil ranged from 81 to 114%, except for acenaphthylene (Table 1). NIST SRM 1649a was also tested and the recovery of PAHs ranged from 61% to 131%.
Table 1.
Recovery of PAHs from spiked soil
| Compounds | m/z | # of ring | log Kow | Sand | Loamy sand | SRM 1649a | |||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | R.S.D.a(%) | Recovery (%) | R.S.D. (%) | Recovery (%) | R.S.D. (%) | ||||
| Acenaphthene | 152 | 3 | 4.0 | 85.30 | 3.32 | 82.87 | 5.77 | NAb | NA |
| Acenaphthylene | 154 | 3 | 4.7 | 85.34 | 3.15 | 61.66 | 5.38 | NA | NA |
| Anthracene | 178 | 3 | 4.5 | 89.01 | 3.89 | 85.82 | 7.14 | 131.75 | 1.43 |
| Benz[a]anthracene | 228 | 4 | 5.6 | 103.40 | 3.64 | 96.55 | 7.86 | 61.22 | 20.97 |
| Benzo[a]pyrene | 252 | 5 | 6.1 | 103.41 | 2.32 | 88.66 | 8.30 | 74.21 | 1.95 |
| Benzo[b]fluoranthene | 252 | 5 | 6.0 | 114.19 | 3.08 | 101.39 | 11.90 | 120.66 | 3.92 |
| Benzo[k]fluoranthene | 252 | 5 | 6.1 | 100.60 | 3.11 | 90.90 | 9.19 | 96.28 | 1.24 |
| Chrysene | 228 | 4 | 5.2 | 108.70 | 3.63 | 103.46 | 7.59 | 106.73 | 0.61 |
| Fluoranthene | 202 | 4 | 4.9 | 91.78 | 3.90 | 90.30 | 9.81 | 103.67 | 10.88 |
| Fluorene | 166 | 3 | 4.2 | 88.51 | 3.58 | 96.86 | 5.49 | NA | NA |
| Indeno[1,2,3-cd]pyrene | 276 | 6 | 6.6 | 102.61 | 1.89 | 79.83 | 9.34 | NA | NA |
| Naphthalene | 128 | 2 | 3.3 | 80.67 | 1.80 | 82.77 | 1.97 | NA | NA |
| Phenanthrene | 178 | 3 | 4.5 | 88.97 | 3.90 | 83.28 | 7.83 | 87.32 | 0.72 |
| Pyrene | 202 | 4 | 4.2 | 96.43 | 3.93 | 89.60 | 15.37 | 107.85 | 13.80 |
The results were calculated based on seven replicates.
R.S.D. = (standard deviation)/average × 100%.
NA: not applicable; comparison data are not available.
3.2. PAHs in soil samples
The 16 priority polycyclic aromatic hydrocarbons were analyzed in the soil samples collected from the area. Among the 107 samples analyzed, PAHs were detected in all samples from the entire study area (Table 2). The total PAH concentration (the sum of the concentrations of 16 PAHs) in the soil ranged from 0.1 to 2225.5 µg kg−1. The range is similar to those found in Norway [29], China [30], and Japan [31], but is an order of magnitude lower than what were found in Hong Kong [32] and United Kingdom [29].
Table 2.
Concentrations and occurrences of 16 PAHs in 107 soil samples from El Paso, TX
| PAHs concentrations (µg kg−1) from soil in El Paso Texas (n=107) | |||||
|---|---|---|---|---|---|
| Min | Max | Median | Mean | Occurrence % | |
| Acenaphthene | ND | 175.8 | ND | 2.3 | 38.3 |
| Acenaphthylene | ND | 138.1 | ND | 1.7 | 30.8 |
| Anthracene | ND | 131.5 | ND | 4.8 | 47.7 |
| Benzo[a]anthracene | ND | 131.2 | ND | 4.7 | 15.0 |
| Benzo[a]pyrene | ND | 364.6 | ND | 8.4 | 15.0 |
| Benzo[b]fluoranthene | ND | 267.5 | ND | 5.1 | 8.4 |
| Benzo[g,h,i]perylene | ND | 189.2 | ND | 1.9 | 1.9 |
| Benzo[k]fluoranthene | ND | 238.3 | ND | 4.4 | 9.3 |
| Chrysene | ND | 346.6 | ND | 13.3 | 20.6 |
| Dibenz[a,h]anthracene | ND | ND | ND | ND | 0.0 |
| Flouranthene | ND | 538.5 | 4.6 | 26.4 | 82.2 |
| Flourene | ND | 220.4 | ND | 2.9 | 42.1 |
| Indeno[1,2,3-cd]pyrene | ND | 50.5 | ND | 0.6 | 1.9 |
| Naphthalene | ND | 34.2 | 6.4 | 8.3 | 100.0 |
| Phenanthrene | ND | 203.6 | 2.6 | 9.7 | 86.0 |
| Pyrene | ND | 397.1 | 4.5 | 22.0 | 86.9 |
| Total PAHs | 0.1 | 2225.5 | 28.2 | 116.3 | 100.0 |
Occurrence (%) was calculated based on the number of sites found to contain the PAH divided by the total number of soil samples collected. ND: not detected.
The PAH compound with the highest concentration was found to be fluoranthene (538.5 µg kg−1 dry soil), followed by pyrene (397.1 µg kg−1), benzo[a]pyrene (364.6 µg kg−1), and chrysene (346.6 µg kg−1). On the other hand, dibenz[a,h]anthracene was not detected in all samples. The four most frequently detected PAHs among the 16 compounds were fluoranthene, pyrene, phenanthrene, and naphthalene with occurrence of 82.2, 86.9, 86.0, and 100%, respectively. Though naphthalene was found in all samples collected in the area, the concentration found in the samples was not overwhelmingly high, ranging from ND (not detected) to 34.2 µg kg−1 with an average of 8.3 µg kg−1. Overall, the levels of the four frequently detected PAHs were still in the low end with average concentrations of 22.0 µg kg−1 or less. Using the soil screening levels (SSLs) used by the Agency for Toxic Substances and Disease Registry (ATSDR) [33], only 4 out of 107 soil samples were found to exceed the SSLs for benzo[a]pyrene (data not shown). Other PAHs in the samples were below the SSLs. It was noted that the samples with exceeding levels of benzo[a]pyrene were from sites not only close to industrial areas, but also from recreational areas. Though charcoal and other burning was observed, the sources of the high level of benzo[a]pyrene remain unknown.
Breakdown of the concentrations and occurrences of the 16 priority PAHs based on the type of sampling sites is summarized in Table 3 and Table 4, respectively. As expected, the lowest levels of total PAHs were found in the samples from sites registered as agricultural and remotes areas. The highest average concentration of total PAHs was present in the industrial areas (382.3 µg kg−1) followed by recreational and school areas (184.3 and 133.9 µg kg−1, respectively). The highest occurrences of each PAH compound was again found in industrial, recreational, or school areas. This result established that the highest levels of total PAHs were frequently found in the regions where either the industrial facilities are located or high human activities occur. It is worth mentioning that some recreational areas and several schools are near the industrial sites, and most of them are located at the southeast and downtown El Paso. Suffice it to say, industrial processes and human activities (such as burning and traffic) might be the major sources of soil-borne PAHs in our area.
Table 3.
Concentrations of PAHs in the soil by area categories: industrial, agricultural, remote, recreational, urban, and school areas
| Industrial (n = 9) | Agricultural (n=17) | Remotes (n = 22) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Mean | Min | Max | Median | Mean | Min | Max | Median | Mean | |
| Acenaphthene | ND | 1.7 | ND | 0.3 | ND | 1.6 | ND | 0.2 | ND | 2.5 | ND | 0.3 |
| Acenaphthylene | ND | 1.4 | ND | 0.3 | ND | 0.9 | ND | 0.1 | ND | 2.0 | ND | 0.2 |
| Anthracene | ND | 18.3 | 2.5 | 5.5 | ND | 3.2 | ND | 0.2 | ND | 4.0 | ND | 0.2 |
| Benzo[a]anthracene | ND | 130.8 | ND | 18.3 | ND | ND | ND | ND | ND | ND | ND | ND |
| Benzo[a]pyrene | ND | 138.6 | ND | 30.7 | ND | ND | ND | ND | ND | 364.6 | ND | 19.0 |
| Benzo[b]fluoranthene | ND | 267.5 | ND | 33.1 | ND | ND | ND | ND | ND | ND | ND | ND |
| Benzo[g,h,i]perylene | ND | 189.2 | ND | 22.1 | ND | ND | ND | ND | ND | ND | ND | ND |
| Benzo[k]fluoranthene | ND | 238.3 | ND | 33.6 | ND | ND | ND | ND | ND | ND | ND | ND |
| Chrysene | ND | 312.5 | 18.5 | 59.5 | ND | ND | ND | ND | ND | ND | ND | ND |
| Flouranthene | ND | 437.2 | 39.7 | 77.2 | ND | 11.5 | 0.5 | 2.6 | ND | 15.1 | 0.9 | 2.0 |
| Flourene | ND | 1.3 | 0.2 | 0.4 | ND | 0.6 | ND | 0.1 | ND | 1.9 | 0.1 | 0.2 |
| Indeno[1,2,3-cd]pyrene | ND | 50.5 | ND | 5.6 | ND | ND | ND | ND | ND | ND | ND | ND |
| Naphthalene | 2.1 | 31.6 | 8.2 | 12.0 | 1.9 | 12.5 | 4.2 | 4.3 | 0.4 | 16.1 | 2.6 | 4.6 |
| Phenanthrene | ND | 101.6 | 8.6 | 17.3 | ND | 9.7 | 0.2 | 1.5 | ND | 5.2 | 0.7 | 1.5 |
| Pyrene | ND | 343.7 | 34.8 | 66.6 | ND | 10.4 | 0.4 | 2.3 | ND | 29.6 | 0.7 | 3.8 |
| Total PAHs | 6.3 | 2225.5 | 131.3 | 382.3 | 0.1 | 31.3 | 10.6 | 12.6 | 0.5 | 379.6 | 10.4 | 32.0 |
| Recreational (n = 22) | Urban (n = 14) | Schools (n = 23) | ||||||||||
| Min | Max | Median | Mean | Min | Max | Median | Mean | Min | Max | Median | Mean | |
| Acenaphthene | ND | 8.4 | 0.7 | 1.3 | ND | 1.0 | ND | 0.2 | ND | 175.8 | ND | 8.7 |
| Acenaphthylene | ND | 3.2 | 0.1 | 0.7 | ND | 10.9 | ND | 0.8 | ND | 138.1 | ND | 6.5 |
| Anthracene | ND | 131.5 | 0.4 | 8.8 | ND | 69.5 | ND | 7.4 | ND | 101.0 | 1.6 | 6.9 |
| Benzo[a]anthracene | ND | 131.2 | ND | 11.1 | ND | 2.7 | ND | 0.3 | ND | 60.1 | ND | 4.4 |
| Benzo[a]pyrene | ND | 105.2 | ND | 5.5 | ND | 39.5 | ND | 2.8 | ND | 11.4 | ND | 1.9 |
| Benzo[b]fluoranthene | ND | 197.6 | ND | 10.2 | ND | 4.0 | ND | 0.6 | ND | 22.6 | ND | 1.6 |
| Benzo[g,h,i]perylene | ND | ND | ND | 0.0 | ND | ND | ND | ND | ND | ND | ND | ND |
| Benzo[k]fluoranthene | ND | 100.4 | ND | 4.8 | ND | 6.4 | ND | 0.6 | ND | 24.0 | ND | 2.3 |
| Chrysene | ND | 346.6 | ND | 25.3 | ND | 31.2 | ND | 2.7 | ND | 202.4 | ND | 12.6 |
| Flouranthene | ND | 538.5 | 22.9 | 47.6 | ND | 125.9 | 5.0 | 19.2 | ND | 468.3 | 6.7 | 31.5 |
| Flourene | ND | 2.2 | 0.2 | 0.5 | ND | 6.8 | ND | 0.7 | ND | 220.4 | ND | 11.5 |
| Indeno[1,2,3-cd]pyrene | ND | 13.8 | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND | ND |
| Naphthalene | 1.4 | 32.3 | 10.4 | 12.7 | 0.9 | 34.2 | 5.3 | 7.9 | 2.5 | 23.4 | 7.1 | 8.9 |
| Phenanthrene | ND | 111.5 | 10.4 | 16.9 | ND | 76.2 | 4.3 | 11.8 | ND | 203.6 | 2.4 | 12.3 |
| Pyrene | ND | 397.1 | 20.4 | 38.0 | ND | 94.2 | 4.7 | 15.9 | ND | 370.9 | 5.5 | 24.9 |
| Total PAHs | 5.7 | 2092.5 | 94.1 | 184.3 | 1.3 | 320.1 | 38.3 | 57.4 | 11.9 | 1493.7 | 31.6 | 133.9 |
ND: not detected. All levels were reported in µg kg−1 dry weight basis.
Table 4.
Occurrences (%) of PAHs in the soil by the categories of sampling areas: industrial, agricultural, remote, recreational, urban, and school area
| PAHs | Industrial | Recreational | Urban | Agricultural | Remote | School |
|---|---|---|---|---|---|---|
| Acenaphthene | 22.2 | 59.1 | 35.7 | 35.3 | 31.8 | 21.7 |
| Acenaphthylene | 22.2 | 50.0 | 28.6 | 41.2 | 27.3 | 17.4 |
| Anthracene | 55.6 | 50.0 | 42.9 | 5.9 | 13.6 | 91.3 |
| Benzo[a]anthracene | 22.2 | 36.4 | 14.3 | 0.0 | 0.0 | 21.7 |
| Benzo[a]pyrene | 22.2 | 22.7 | 7.1 | 0.0 | 9.1 | 30.4 |
| Benzo[b]fluoranthene | 22.2 | 13.6 | 21.4 | 0.0 | 0.0 | 13.0 |
| Benzo[g,h,i]perylene | 22.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Benzo[k]fluoranthene | 22.2 | 13.6 | 14.3 | 0.0 | 0.0 | 21.7 |
| Chrysene | 55.6 | 40.1 | 21.4 | 0.0 | 0.0 | 26.1 |
| Flouranthene | 88.9 | 100.0 | 85.7 | 64.7 | 63.6 | 91.3 |
| Flourene | 44.4 | 59.1 | 50.0 | 29.4 | 45.5 | 26.1 |
| Indeno[1,2,3-cd]pyrene | 7.1 | 4.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Naphthalene | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Phenanthrene | 88.9 | 90.9 | 92.9 | 58.8 | 77.3 | 91.3 |
| Pyrene | 88.9 | 95.5 | 92.9 | 76.5 | 63.6 | 91.3 |
Occurrence (%) was calculated based on the number of sites found to contain the PAH divided by the number of soil samples collected under the same category.
The toxicity equivalent factor (TEF) approach has been used extensively for the assessment of different classes of toxic chemical mixtures [34–37]. The carcinogenic potency associated with the exposure of a given PAH compound can be estimated by calculating its benzo[a]pyrene (BaP) equivalent concentration (BaPeq). The BaPeq for each individual PAH species is calculated by multiplying the concentration of the compound with its TEF, i.e. BaPeq = conc.× TEF. The TEF of a given species is the toxicity factor relative to BaP carcinogenic potency. The carcinogenic potency of a mixture could then be estimated from the sum of the BaPeq of each individual component, i.e. (total BaPeq) =∑conc.×TEF. Several TEFs of the 16 PAHs had been proposed [38–40], and the values completed by US EPA [39] were adopted in this study. Table 5 shows the BaPeq (µg kg−1) of individual PAH and total PAHs (∑ 16PAHs) in the samples from the 6 sampling zones based on their average concentrations found in soils. Total BaPeq for the seven carcinogenic PAHs (∑7carcPAHs) was also presented. The seven carcinogenic PAHs are benzo[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, chrysene, dibenzo[a,h]anthracene, and indeno[1,2,3-cd]pyrene. Our result showed that the toxicological impact from the local soil is controlled by seven carcinogenic PAHs since the BaPeq values of ∑16PAHs and ∑7carcPAHs are almost identical. Soil of the industrial area showed the highest total BaPeq, while soil from the agricultural has 0 BaPeq value. The total BaPeq levels in recreational, urban and school areas are low compared with industrial areas. Remote areas revealed an unexpectedly high BaPeq level owing to high concentration of benzo[a]pyrene detected at two sites where illegal dumping were suspected but not confirmed. However the occurrence of benzo[a]pyrene in those remote sites was only 9.1% (2 out of 22 samples).
Table 5.
BaPeq profiles in soils collected from various types of sampling sites in El Paso, Texas, expressed in µg kg−1 (dry weight)
| PAH | BaPeq (µg kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| TEFsa | Industrial | Recreational | Urban | Agricultural | Remote | School | |
| Acenaphthene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acenaphthylene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Anthracene | 0.010 | 0.06 | 0.09 | 0.07 | 0.00 | 0.00 | 0.07 |
| Benzo[a]anthracene | 0.0 10 | 0.18 | 0.11 | 0.00 | NDb | ND | 0.04 |
| Benzo[a]pyrene | 1.000 | 30.70 | 5.49 | 2.82 | ND | 19.04 | 1.86 |
| Benzo[b]fluoranthene | 0.100 | 3.31 | 1.02 | 0.06 | ND | ND | 0.16 |
| Benzo[g,h,i]perylene | 0.010 | 0.22 | 0.00 | ND | ND | ND | ND |
| Benzo[k]fluoranthene | 0.100 | 3.36 | 0.48 | 0.06 | ND | ND | 0.23 |
| Chrysene | 0.010 | 0.59 | 0.25 | 0.03 | ND | ND | 0.13 |
| Dibenzo[a,h]anthracene | 1.000 | ND | ND | ND | ND | ND | ND |
| Flouranthene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Flourene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Indeno[1,2,3-cd]pyrene | 0.100 | 0.56 | 0.06 | ND | ND | ND | ND |
| Naphthalene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phenanthrene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pyrene | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ∑16PAHs | 38.98 | 7.51 | 3.05 | 0.00 | 19.05 | 2.49 | |
| ∑7carcPAHs | 38.75 | 7.31 | 2.97 | 0.00 | 19.04 | 2.37 | |
Values adopted from Ref. [39].
ND: not detected.
3.3. Possible sources of PAHs in soil samples
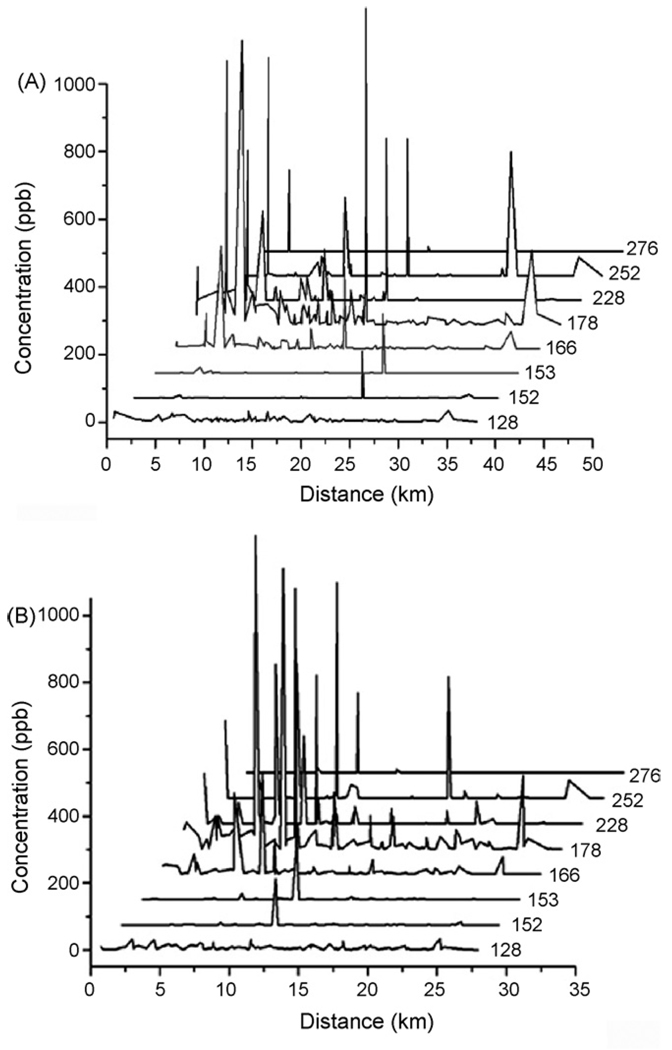
PAHs have different physical and chemical properties that affect their distribution in the atmosphere and hence the concentration in soil [41,42]. Low molecular weight PAHs (e.g. naphthalene, acenaphthylene, and acenaphthene) are likely to be found in gaseous form and their presence would be more related to long-range transport [43]. Heavy PAHs with four to six rings consequently would settle more easily than light PAHs near the point of emission [44], therefore they can be the indicators of the source. In order to see the distribution pattern, a GIS was used to map the distribution of PAHs in the El Paso area. Naphthalene was selected as the model compound for low molecular weight PAHs. Our GIS results showed that light PAHs in the soil were spread throughout the area (Fig. 5). On the contrary, the total PAHs seemed to be more concentrated around the major traffic as well as where the industrial facilities are located (Fig. 6). As shown in Table 4, 10 out of 15 PAHs were found to have the highest average concentrations in soil samples collected from industrial areas among all sites. The finding agrees with the hypothesis that the industrial processes might have contributed PAHs into the area. Thus, a correlation of PAH concentrations and the distances of the sampling sites to the industrial facilities was investigated. PAHs were grouped based on their molecular weight: 276 for Indeno[1,2,3-cd]pyrene and benzo[g,h,i]perylene; 252 for benzo[a]pyrene, benzo[k]fluoranthene, and benzo[b]fluoranthene; 228 for benz[a]anthracene and chrysene; 202 for fluoranthene and pyrene (202); 178 for phenanthrene and anthracene; 166 for fluorene; 154 for acenaphthylene; 152 for acenaphthene; and 128 for naphthalene. The concentration of each PAH group was plotted against the distances between the sampling sites and the closest industrial areas (which are located either in westside El Paso close to downtown, or in the east side of the area). As shown in Fig. 7A and B, light PAHs were more evenly distributed along the distance; while heavy PAHs tended to be more concentrated within the proximity to the industrial areas. However, the distribution pattern of heavy PAHs in Fig. 7A was not as clear as what was observed in Fig. 7B. It is interesting to know that the industrial area in the east side of El Paso hosts an oil refinery plant; whereas there is currently no major factory operating in the west side of the region. Again, the results support the finding that industrial activities were likely to be responsible for the higher levels of PAHs in the region.
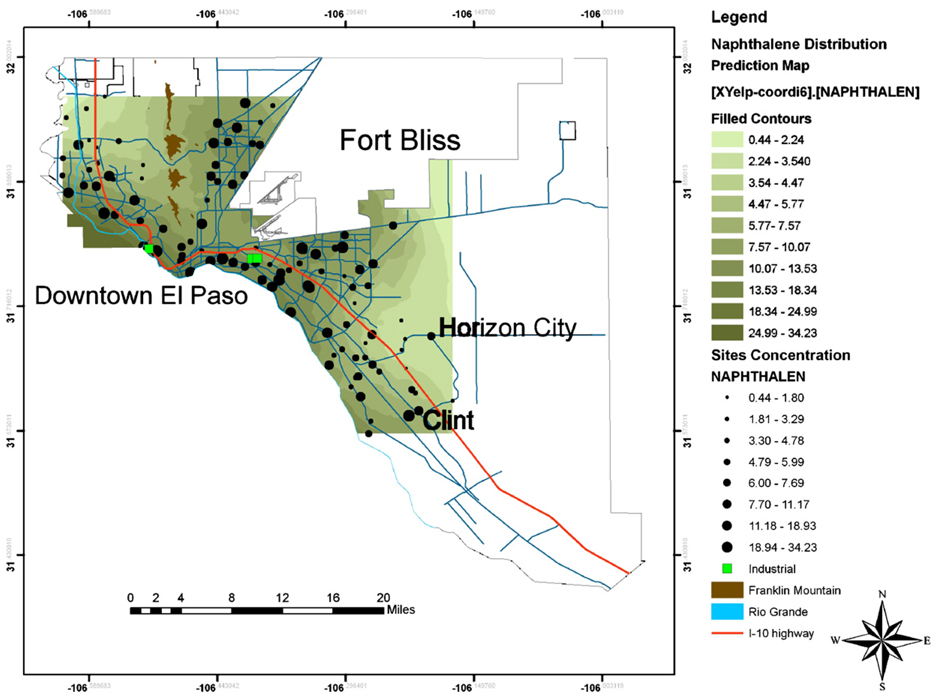
Fig. 5.
A GIS map of the distribution of naphthalene in soil from El Paso, Texas. Concentration in µg Kg−1.Dark blue lines represent major roads.
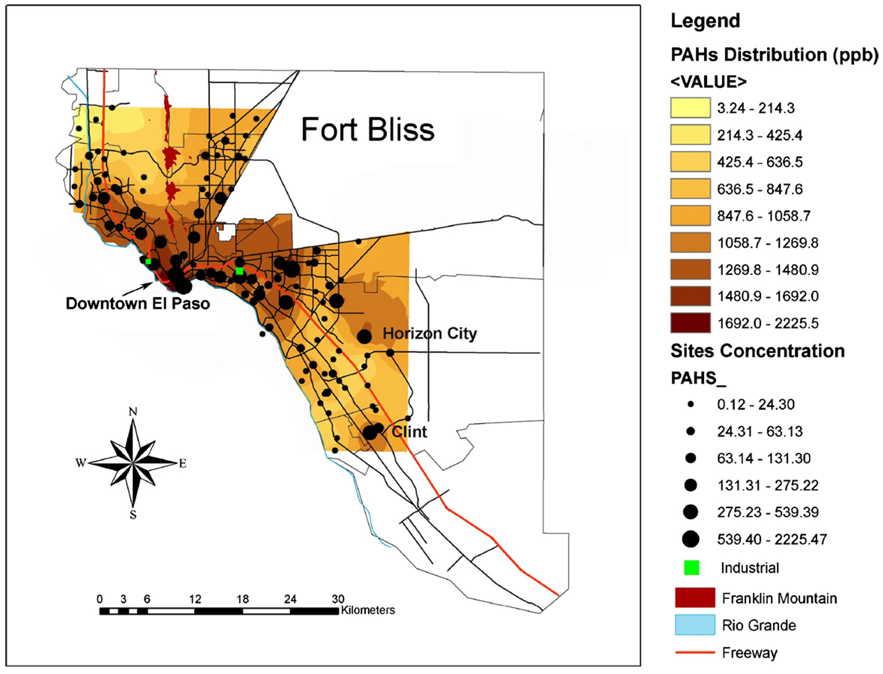
Fig. 6.
A GIS map of the distribution of total PAHs in soil from El Paso, Texas. Black lines represent major roads.
Fig. 7.
PAHs distribution profiles based on molecular weight and the distance between the sampling sites and the closest industrial area. (A) The distance was measured between the sampling sites and the industrial area at westside/downtown El Paso; (B) the distance was measured between the sampling sites and the industrial area in the east side of El Paso. PAHs were grouped based on their molecular weights.
It is noteworthy to point out again that industrial areas are located in a heavy traffic zone and close to Ciudad Juarez/El Paso border. The total PAH distribution map (Fig. 6) also indicated that PAHs seemed to concentrate in downtown as well as along the major interstate highway. A study performed in Southern California showed that the spatial distribution pattern of PAH emission is highly correlated with city centers and major roadways [45]. Based on the GIS maps created in this study, the spatial distribution of PAHs in this study follows similar patterns where the predicted concentration was higher in the city and principal road ways, and decreased with distance. It is reasonable to say that the sources of PAHs in the soil were either from industrial processes or traffic related activities. Therefore, it warrants further analysis for the identification for the major contributors of the local PAH contamination.
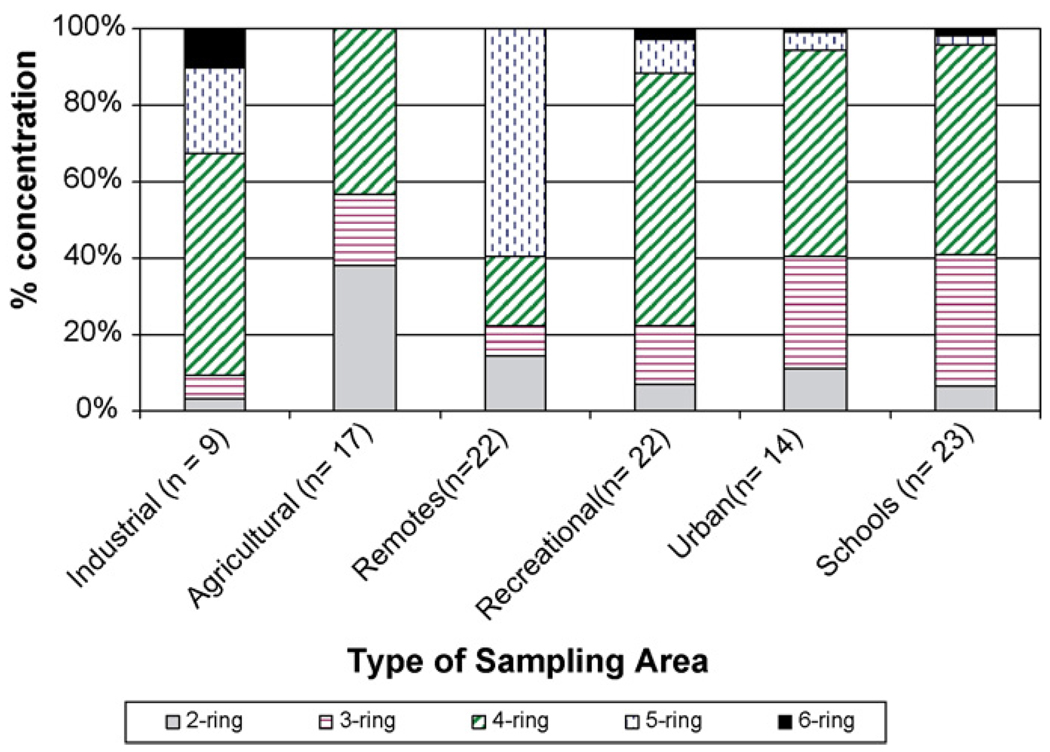
PAHs can also be released to the environment as a result of pyrogenic and petrogenic processes. Pyrogenic processes include combustion of organic materials during industrial activities, residential heating, power generation, incineration, and vehicle emissions; and petrogenic processes take account of emission from petrochemical refining and chemical manufacturing [46]. In general, PAHs from a petrogenic source have lower molecular weight (2- and 3-ring), whereas higher molecular weight PAHs are abundant from pyrogenic sources [47]. Interms of relative concentration, when the value of PAHs with four to six rings is higher than 50% of the total concentration, it indicates a dominance of combustion; when the value of PAHs with two and three rings is higher than 50%, it indicates a dominance of petroleum pollution. As shown in Fig. 8, PAHs with four rings or more are dominant in all areas except for agricultural sites. These results suggest that the PAHs found in El Paso soil were likely to be from pyrogenic activities rather than from petrochemical refining. This observation directed the sources of PAHs in local soil to human activities such as residential burning, vehicle emissions, or power generation. The only exception occurred in agricultural soil samples. The relatively abundant two- and three-ring PAHs in the soil might have been due to long-range transport as observed in Fig. 5.
Fig. 8.
Relative concentration values of PAHs in samples from each category of sampling sites based on the size of PAHs. PAHs were grouped into two- to six-ring compounds. All sites (except for those from agricultural area) contain greater than 50% of the PAHs of four-ring or higher, which indicates a dominance of combustion sources.
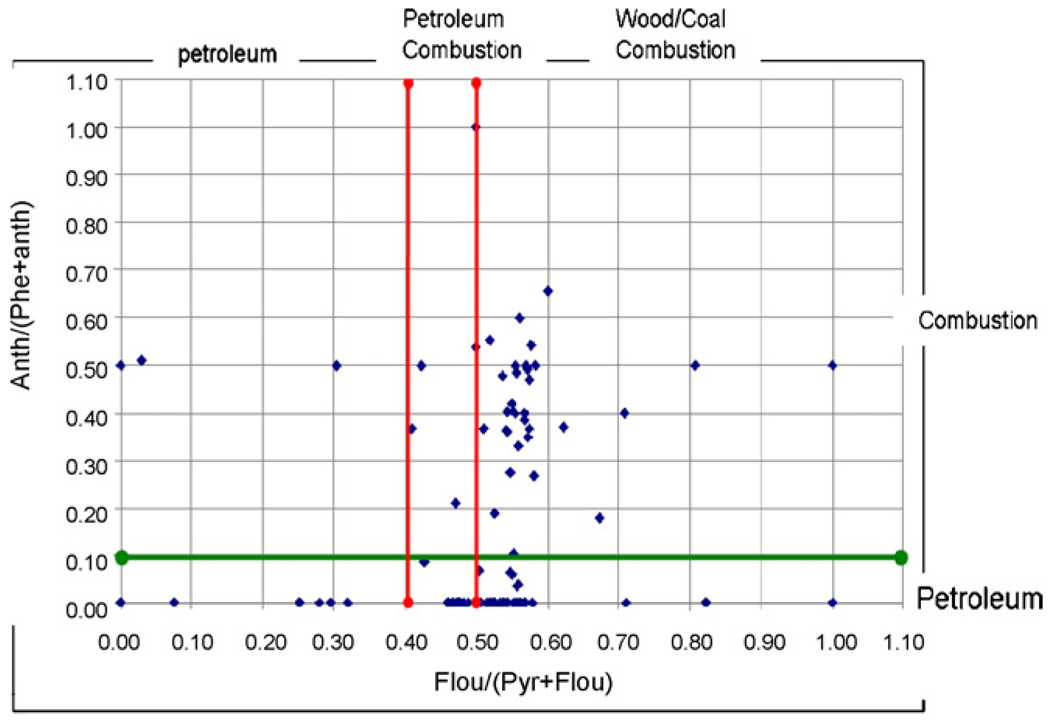
The ratios of PAH isomers was used as an additional tool for source identification. In most cases, the ratios of fluoranthene to fluoranthene plus pyrene (Fluo/(Fluo + Pyr)) being less than 0.4 indicates petroleum input; while the ratios between 0.4 and 0.5 suggest fossil fuel combustion, and the ratios greater than 0.5 imply grass, wood or coal combustion [48]. In addition, the ratios of anthracene to phenanthrene plus anthracene (Anth/(Phe + Anth)) less than 0.1 usually indicates petrogenic input; and a ratio greater than 0.1 points to a dominance of combustion [30]. As shown in Fig. 9, the values of Fluo/(Fluo + Pyr) ratios in the local soil samples by and large fell between 0.5 and 0.6, and Anth/(Anth + Phe) ratios frequently above 0.1. These values suggest that combustion could have been the major origin of PAHs in the region. Another study has identified the Fluo/(Fluo + Pyr) ratios in the particulates from tire, diesel, and wood burning to be 0.54, 0.50 and 0.51, respectively [49], which supports the likelihood of the soil-borne PAHs being from pyrogenic processes. These results ruled out the possibility of petroleum refining as the source of local PAH contamination. Thus, residential heating, vehicle emissions, and power generation at industrial facilities are most likely to be the major contributors of PAH contamination.
Fig. 9.
Diagnostic ratios of Anth/(Anth + Phen) vs. (Flur/Flur + Pye) in the soil samples.
PAH contamination in the El Paso area by pyrogenic processes can be also related to activities occurring in Ciudad Juarez, Mexico. Burning of tires and wood for heating and cooking purposes is common in Ciudad Juarez, especially in the less economically privileged area. Moreover maquiladoras and brick kilns located in Ciudad Juarez might be possible sources of air pollution which can affect El Paso area. Brick makers typically use scrap fuels such as sawdust, tires, and lumber to fire the bricks. Analysis of air samples from a station close to maquiladora area and brick-making district in Ciudad Juarez revealed higher concentration of PAHs in particular matter compared to two stations located in El Paso [50]. Further analysis of soil and air is needed on both site of the border to understand the transportation of PAHs in this area, and the impact of those activities in Ciudad Juarez on the PAH contamination.
There are approximately 2.3 million people living in the El Paso/Juarez area (Census data). The traffic situation at the U.S. Mexico border crossing associated with NAFTA is estimated to have more than 80,000 vehicles each day between El Paso County and Ciudad Juarez, MX [51]. This study had contributed the sources of PAHs in this area likely to be pyrogenic processes. The traffic in this growing metropolitan area and at the border crossing, and the use of wood, tires and other scrap fuels for both residential heating and the production of bricks, may be creating a potential problem for PAH contamination in this region.
4. Conclusions
An analytical method using ultrasonic extraction combined with Stir Bar Sorptive Extraction–thermal desorption–Gas Chromatography/Mass Spectroscopy was optimized for PAH analysis in soil. This method provided time efficient and sensitive results for this study.
A total of 107 soil samples were collected throughout El Paso, TX. Based on the land uses, sampling sites were categorized into industrial, agricultural, remote, recreational, urban, and school areas. Sixteen priority PAHs were analyzed and the levels of PAHs were mostly below the EPA soil screening levels (SSLs). Only 4 out of 107 soil samples were found to contain benzo[a]pyrene exceeding the SSLs. However, the data showed that PAHs existed in all samples collected. Through long-range transport, light PAHs (such as fluoranthene, pyrene, phenanthrene, and naphthalene) were found in 80–100% of the samples analyzed. Heavy PAHs (such as benzo[a]pyrene, benz[a]anthracene and pyrene) were less frequently detected, and mostly found in soil collected from industrial, recreational, or school areas where involving intensive human activities.
GIS maps, relative abundance, and diagnostic ratios were used to identify the sources of PAHs in the local soil. The GIS maps showed that higher concentrations of total PAHs were found in the areas with heavy anthropogenic activities. The diagnostic analysis further specified that the PAHs found in our local soil are likely to come from pyrogenic processes. Thus, residential heating, vehicle emissions, and power generation at industrial facilities are most likely to be the major contributors of PAH contamination. Industrial power generation might have also added PAHs into the environment but petroleum refining did not seem to have significant effect on the PAH contamination in the soil.
This research generated the first comprehensive data of concentration, occurrence, and the possible sources of soil-borne PAHs in the El Paso/Juarez area and is the first study of its kind in the U.S./Mexico border. With the growing population and unique traffic situation at the border crossing, this research has identified a potential environmental problem for PAHs in this region. It provides the foundation for further studies on the impacts of PAHs and other persistent organic pollutants on the environment and public health on both side of the border.
Acknowledgements
The authors acknowledge the financial supports by the National Science Foundation (Grant No. 0245071), HBCU/MI Environment Technology Consortium (project # 633254-H010016), and EPA Southwest Consortium for Environmental Research and Policy (SCERP) project A-06-01. The assistance provided by the Center for Environmental Resource Management at UTEP is gratefully recognized. Special thanks to Ms. Sharon Mitchell for assisting in sampling at the school sites.
References
- 1.Douben PET, editor. PAHs: An Ecotoxicological Perspective. United Kingdom: John Wiley And Sons Ltd; 2003. [Google Scholar]
- 2.Bidleman TF, Falconer RL. Using enantiomers to trace pesticide emissions. Environ. Sci. Technol. 1999;33:206A–209A. doi: 10.1021/es992773x. [DOI] [PubMed] [Google Scholar]
- 3.Hild CM, Beckman KB, Berner JE, Kaatz Chary L, Hamrick KJ, Johnson PC, Krahn PM, Marcy SKM, Matkin CO, Rubin CH, See MG, Smolen MJ, Verbrugge LA Office of Research and Development. National Center for Environmental Assessment. Washington, DC: U.S. Environmental Protection Agency; 2002. Mar, The foundation for global action on persistent organic pollutants: a United States perspective, USEPA EPA/600/P-01/003F. [Google Scholar]
- 4.Blackman A, Batz M, Evans D. Maquiladoras, Air Pollution, and Human Health in Ciudad Juarez and El Paso, Discussion Papers dp-03-18. Resources for the Future. http://www.rff.org/documents/RFF-DP-03-18.pdf.
- 5.Jones KC, de Voogt P. Persistent organic pollutants (POPs): state of the science. Environ. Pollut. 1999;100:209–221. doi: 10.1016/s0269-7491(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 6.Cousins IT, Beck AJ, Jones KC. A review of the processes involved in the exchange of semivolatile organic compounds (SVOC) across the air-soil interface. Sci. Tot. Environ. 1999;228:5–24. [Google Scholar]
- 7.Fu J, Mai B, Sheng G, Zhang G, Wang X, Peng P, Xiao X, Ran R, Cheng F, Peng X, Wang Z, Tang UW. Persistent organic pollutants in environment of the Pearl River Delta, China: an overview. Chemosphere. 2003;52:1411–1422. doi: 10.1016/s0045-6535(03)00477-6. [DOI] [PubMed] [Google Scholar]
- 8.El-Hage A, Moulton DW. Evaluation of selected natural resources in El Paso County, Texas, Resources Protection Division Water Resources Team. Texas Park and Wildlife. 1998 [Google Scholar]
- 9.David F, Sandra P. Review: Stir bar sorptive extraction for trace analysis. J. Chromatogr. A. 2007;1152(1–2):54–69. doi: 10.1016/j.chroma.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Ramos L, Ramos JJ, Brinkman UATh. Miniaturization in sample treatment for environmental analysis. Anal. Bioanal. Chem. 2005;381:119–140. doi: 10.1007/s00216-004-2906-5. [DOI] [PubMed] [Google Scholar]
- 11.Gan J, Papiernik SK, Koskinen WC, Yates SR. Evaluation of accelerated solvent extraction (ASE) for analysis of pesticide residues in soil. Environ. Sci. Technol. 1999;33:3249–3253. [Google Scholar]
- 12.Shu YY, Lao RC, Chiu CH, Turle R. Analysis of polycyclic aromatic hydrocarbons in sediment reference materials by microwave-assisted extraction. Chemosphere. 2000;41:1709–1716. doi: 10.1016/s0045-6535(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 13.Saim N, Dean JR, Abdullah MdP, Zakaria Z. Extraction of polycyclic aromatic hydrocarbons fromcontaminated soil using Soxhlet extraction, pressurised and atmospheric microwave-assisted extraction, supercritical fluid extraction and accelerated solvent extraction. J. Chromatogr. A. 1997;791:361–366. [Google Scholar]
- 14.Alvarez-Avilíes O, Cuadra-Rodríguez L, González-Illán F, Quiñones-González J, Rosario O. Optimization of a novel method for the organic chemical characterization of atmospheric aerosols based on microwave-assisted extraction combined with stir bar sorptive extraction. Anal. Chim. Acta. 2007;597:273–281. doi: 10.1016/j.aca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Baltussen E, Cramers CA, Sandra PJF. Sorptive sample preparation—a review. Anal. Bioanal. Chem. 2002;373:3–22. doi: 10.1007/s00216-002-1266-2. [DOI] [PubMed] [Google Scholar]
- 16.David F, Tienpont B, Sandra P. Stir-bar sorptive extraction of trace organic compounds from aqueous matrices. LC-GC Europe. 2003 July;:1–7. [Google Scholar]
- 17.Rodil R, Popp P. Development of pressurized subcritical water extraction combined with stir bar sorptive extraction for the analysis of organochlorine pesticides and chlorobenzenes in soils. J. Chromatogr. A. 2006;1124:82–90. doi: 10.1016/j.chroma.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Sandra P, Tienpont B, David F. Multi-residue screening of pesticides in vegetables, fruits and baby food by stir bar sorptive extraction-thermal desorption-capillary gas chromatography-mass spectroscopy. J. Chromatogr. A. 2003;1000:299–309. doi: 10.1016/s0021-9673(03)00508-9. [DOI] [PubMed] [Google Scholar]
- 19.Benijts T, Vercammen J, Dams R, Tuan HP, Lambert W, Sandra P. Stir bar sorptive extraction–thermal desorption–capillary gas chromatography–mass spectrometry applied to the analysis of polychlorinated biphenyls in human sperm. J. Chromatogr. B. 2001;755:137–142. doi: 10.1016/s0378-4347(01)00048-2. [DOI] [PubMed] [Google Scholar]
- 20.Childs C. Interpolating surface in ArcGIS9 spatial analyst. ESRI Education Services. 2004 http://www.esri.com/news/arcuser/0704/files/interpolating.pdf.
- 21.Krivoruchko K, Gotway CA. Creating exposure maps using Kriging. Public Health GIS News Inform. 2004;56:11–16. [Google Scholar]
- 22.Liu XM, Zhang MK, Yu XF, Xu JM, Huang JH, Shi JC. Application of geostatistics and GIS technique to characterize spatial variabilities of bioavailable micronutrients in paddy soils. Environ. Geol. 2004;46:189–194. [Google Scholar]
- 23.Krivoruchko K. Introduction to modeling spatial processes using geostatistical Analyst. http://www.esri.com/library/whitepapers/pdfs/intro-modeling.pdf.
- 24.Gribov A, Krivoruchko K, Ver Hoef JM. Modeling the semivariogram: new approach, methods comparison and case study, Book section: Stochastic Modeling II. American Association of Petroleum Geologists (AAPG) 2004 [Google Scholar]
- 25.Cattle JA, McBratney AB, Minasny B. Kriging method evaluation for assessing the spatial distribution of urban soil lead contamination. Environ. Qual. 2002;31:1576–1588. doi: 10.2134/jeq2002.1576. [DOI] [PubMed] [Google Scholar]
- 26.Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of Geographical Information System for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in urban outbreak. Am. J. Trop. Med. Hyg. 2003;69(6):634–640. [PubMed] [Google Scholar]
- 27.Leon VM, Ivarez BA, Cobollo MA, Muñoz S, Valor I. Analysis of 35 priority semivolatile compounds in water by stir bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry. I. Method optimization. J. Chromatogr. A. 2003;999:91–101. doi: 10.1016/s0021-9673(03)00600-9. [DOI] [PubMed] [Google Scholar]
- 28.Kolahgar B, Hoffmann A, Heiden AC. Application of stir bar sorptive extraction to the determination of polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A. 2002;963:225–230. doi: 10.1016/s0021-9673(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 29.Nam JJ, Thomas GO, Jaward FM, Steinnes E, Gustafsson O, Jones KC. PAHs in background soils from Western Europe: influence of atmospheric deposition and soil organic matter. Chemosphere. 2008;70:1596–1602. doi: 10.1016/j.chemosphere.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L, Chen Y, Zhou R. Distribution of polycyclic aromatic hydrocarbons in water, sediment and soil in drinking water resource of Zhejiang Province, China. J. Hazard. Mater. 2008;150:308–316. doi: 10.1016/j.jhazmat.2007.04.102. [DOI] [PubMed] [Google Scholar]
- 31.Honda K, Mizukami M, Ueda Y, Hamada N, Seike N. Residue level of polycyclic aromatic hydrocarbons in Japanese paddy soils from 1959 to 2002. Chemosphere. 2007;68:1763–1771. doi: 10.1016/j.chemosphere.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 32.Chung MK, Hu R, Cheung KC, Wong MH. Pollutants in Hong Kong soils: polycyclic aromatic hydrocarbons. Chemosphere. 2007;67:464–473. doi: 10.1016/j.chemosphere.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 33.Agency for Toxic Substances and Disease Registry (ATSDR) Public Health Assessment. http://www.atsdr.cdc.gov/HAC/PHA/middlesexsampling/msp p2.html.
- 34.Nadal M, Schuhmacher M, Domingo JL. Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environ. Pollut. 2004;132:1–11. doi: 10.1016/j.envpol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Fang GC, Chang KF, Lu C, Bai H. Estimation of PAHs dry deposition and BaP toxic equivalency factors (TEFs) study at Urban, Industry Park and rural sampling sites in central Taiwan, Taichung. Chemosphere. 2004;55:787–796. doi: 10.1016/j.chemosphere.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Crnković D, Ristić M, Jovanović A, Antonović D. Levels of PAHs in the soils of Belgrade and its environs. Environ. Monit. Assess. 2007;125:75–84. doi: 10.1007/s10661-006-9240-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen SC, Liao CM. Health risk assessment on humans exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Tot. Environ. 2006;366:112–123. doi: 10.1016/j.scitotenv.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 38.Nisbet ICT, LaGoy PK. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs) Regul. Toxicol. Pharmacol. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- 39.US. EPA. U.S. Environmental Protection Agency. Washington, DC: Office of Research and Development; 1993. Provisional Guidance for Quantitative Risk Assessment of Polycylic Aromatic Hydrocarbons. [Google Scholar]
- 40.Malcom HM, Dobson S. The Calculation of an Environmental Assessment Level (EAL) for Atmospheric PAHs using Relative Potencies. London, UK: Department of the Environment; 1994. pp. 34–46. [Google Scholar]
- 41.Iturbe R, Flores RM, Torres LG. Subsoil contamination by hydrocarbons in an out-of-service oil distribution and storage station in Zacatecas, Mexico. Environ. Geol. 2003;44:602–620. [Google Scholar]
- 42.Hautula EL, Rekilä R, Tarhaben J, Ruuskanen J. Deposition of motor vehicle emission and winter maintenance along roadside assessed by snow analysis. Environ. Pollut. 1995;87:45–49. doi: 10.1016/s0269-7491(99)80006-2. [DOI] [PubMed] [Google Scholar]
- 43.Meharg AA, Wrigth J, Dyke H. Polycyclic aromatic hydrocarbon (PAH) dispersion and deposition to vegetation and soil following a large scale chemical fire. Environ. Pollut. 1998;99:29–36. doi: 10.1016/s0269-7491(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 44.Motelay-Massei A, Ollivon D, Garban B, Chevreuil M. Polycyclic aromatic hydrocarbons in bulk deposition at a suburban site: assessment by principal component analysis of the influence of meteorological parameters. Atmos. Environ. 2003;37:3135–3146. [Google Scholar]
- 45.Lua R, Wub J, Turcoa RP, Winerb AM, Atkinsonc R, Areyc J, Paulsona SE, Lurmannd FW, Miguele AH, Eiguren-Fernandez A. Naphthalene distributions and human exposure in Southern California. Atmos. Environ. 2005;39:489–507. [Google Scholar]
- 46.Li YT, Li FB, Chen JJ, Yang GY, Wan HF, Johnson PC, Zeng XD, Liu JM. The concentrations, distribution and sources of PAHs in agricultural soils and vegetables from Shunde, Guangdong, China. Environ. Monit. Assess. 2008;139:61–76. doi: 10.1007/s10661-007-9816-x. [DOI] [PubMed] [Google Scholar]
- 47.Ping LF, Luo YM, Zhang HB, Li QB, Wu LH. Distribution of polycyclic aromatic hydrocarbons in thirty typical soil profiles in the Yangtze River Delta region, east China. Environ. Pollut. 2007;147:358–365. doi: 10.1016/j.envpol.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002;33:489–515. [Google Scholar]
- 49.Shi Y, Murr LE, Soto KF, Lee W-Y, Guerrero PA, Ramirez DA. Characterization and comparison of speciated atmospheric carbonaceous particulates and their polycyclic aromatic hydrocarbon contents in the context of the Paso del Norte airshed along the U.S.–Mexico border. Polycycl. Aromat. Compd. 2007;27:361–400. [Google Scholar]
- 50.Arrieta DE, Ontiveros CC, Li WW, Garcia JH, Denison MS, McDonald JD, Burchiel SW, Washburn BS. Aryl hydrocarbon receptor-mediated activity of particulate organic matter from the Paso del Norte Airshed along the U.S.–Mexico border. Environ. Health Persp. 2003;111:1299–1305. doi: 10.1289/ehp.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.TX DOT. District Highway Traffic Map, El Paso District. Prepared by the Texas Department of Transportation with the Transportation Planning and Programming Division. 2005 [Google Scholar]