Abstract
The molecules concerned with costimulation belong either to the immunoglobulin (Ig) or tumor necrosis factor (TNF) superfamilies. The tumor necrosis superfamily comprises molecules capable of providing both costimulation and cell death. In this review we briefly summarize certain TNF superfamily receptor-ligand pairs that are endowed with costimulatory properties and their importance in health and disease.
1. Introduction
Clonal expansion of T cells requires both a ligand which engages its receptor (TcR) and a functionally defined second signal (also called a co-stimulatory signal). The participation of a costimulatory signal in T cell activation is of paramount importance as it results in two potential outcomes, activation or clonal anergy (Jenkins, 1992; Mueller et al., 1989). The two different outcomes of antigen recognition, by T cells, are first explained by the dual signal model of T cell activation by Bretscher and Cohn (1970). The nature or identity of this accessory signal was initially thought to be a soluble factor but later studies have established that it is a cell surface- derived event and occurs during cognate interaction between an antigen presenting cell (APC) and partnering T cell (Jenkins and Johnson, 1993).
Based on their molecular structure, the costimulatory molecules have been divided into two major groups belonging either to the immunoglobulin (Ig) or to the tumor necrosis factor (TNF) superfamily. The members of the TNF superfamily have distinctive cytoplasmic death domains and can induce apoptosis as well as receptors with no apparent homology in the cytoplasmic tail. This latter group of receptors is involved in gene activation and anti-apoptotic signaling.
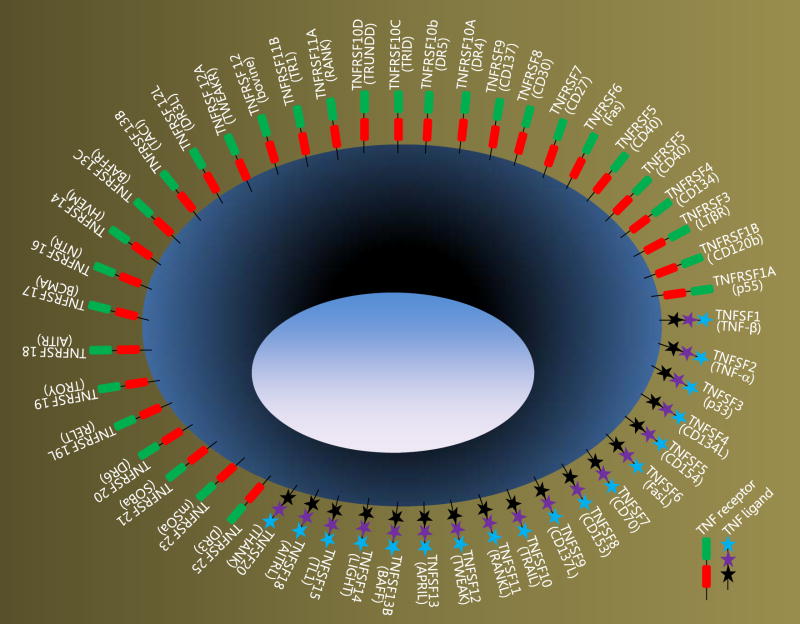
The inventory of the TNF superfamily is increasing rapidly (Fig. 1) and it is impossible to cover all aspects of this superfamily in a short chapter. In this chapter, as well as briefly summarizing key features about this superfamily, we describe how the well-characterized members of this family concerned with positive immune regulation are coordinated (Fig. 2) and their role in clinical applications.
Figure 1. Schematic cartoon depicting members of TNF superfamily.
Each member of the TNF superfamily is indicated by its scientific nomenclature. The description in the parentheses denotes their common name. Since the expression of a particular TNF receptors or its ligand is not exclusive to a particular cell type and sometimes present on the same cell, a generalized depiction illustrated.
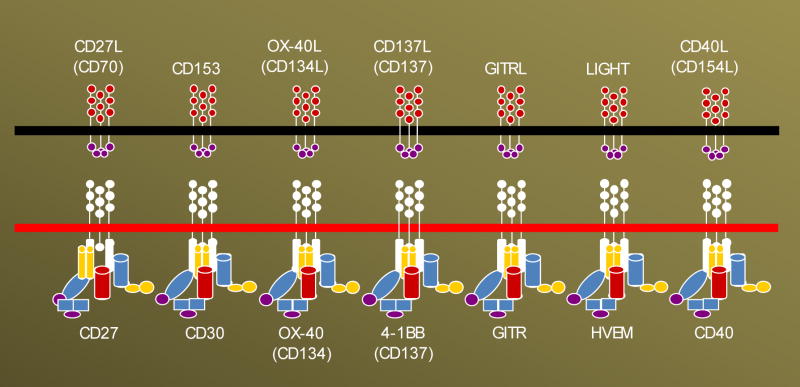
Figure 2. Schematic representation of important members of TNF superfamily.
Although some the members of the family are constitutively expressed (low levels), in most cases their expression is activation dependent. The expression of given receptor or ligand is not restricted to a particular cell and can sometimes present on both T lymphocyte as well as antigen presenting cell enabling a bidirectional signaling cascade.
CD27-CD70
CD27, a type I disulfide-linked glycoprotein, was discovered more than a decade ago on human resting peripheral blood T cells and medullary thymocytes. Both in humans and mice, CD27 is expressed on naive and memory-type T cells, antigen-primed B cells, and subsets of natural killer (NK) cells (Borst et al., 2005). The CD27 ligand CD70 is transiently and stimulation-dependently expressed on T, B, and dendritic cells (Lens et al., 1997) and constitutively on APCs in the murine intestine (Laouar et al., 2005). Interestingly, CD27 is also expressed by many T cells presumably to modulate the effects of CD70 on B cells by acting as decoy receptors (Hendriks et al., 2000; Kobata et al., 1995).
CD27 costimulation of anti-CD3 primed CD4+ T cells promotes cell division, enhances BcL-xL, and promotes IFN-γ induction (van Oosterwijk et al., 2007). CD27-CD70 signals are important in the terminal differentiation of B cells into antibody-secreting plasma cells (Agematsu et al., 1998; Jacquot et al., 1997; Nagumo et al., 1998). Modulation of the in vivo CD27-CD70 pathway by appropriate agonistic antibodies elicits important functions.
Administration of agonistic anti-CD27 mAbs given without a DC maturation signal completely protects tumor-bearing mice and provides a highly potent reagent for boosting antitumor T-cell immunity (French et al., 2007). Interestingly, triggering CD27 by its ligand CD70 impedes neutralizing antibody production and leads to persistence of lymphocyte choriomeningitis virus (LCMV) infection (Matter et al., 2006). Treatment with an anti-CD70 antibody has been reported to induce long-term survival of organ allografts in CD28-deficient mice by inhibiting the activation of effector and memory CD8+ T cells (Yamada et al., 2005). This finding suggests that the CD27/70 pathway might be an important target for inhibiting rejection resistant to the blockade of conventional costimulatory molecules. Patients with Waldenstrom macroglobulinemia (WM), a B-cell malignancy characterized by an IgM monoclonal gammopathy and bone marrow infiltration with lymphoplasmacytic cells, show elevated soluble CD27 which serves as a marker of disease and as a target in its treatment (Ho et al., 2008). Inb addition, treatment with engineered anti-CD70 Ab has shown promise as anti-tumor agent (McDonagh et al., 2008; Grewal, 2008).
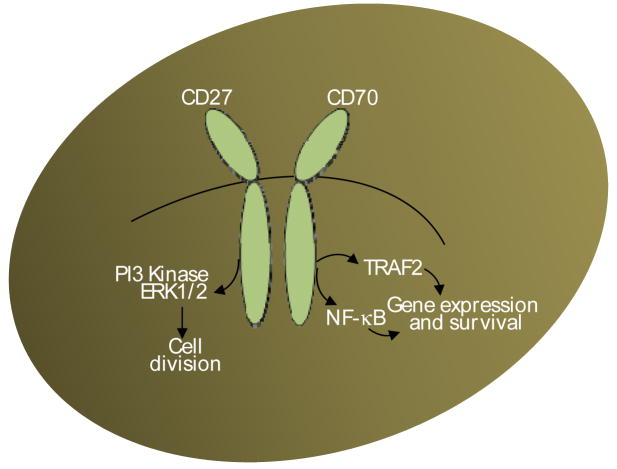
A salient feature of CD27 is its existence in a soluble form. In vivo levels of serum and urine sCD27 correlate with tumor load in patients with leukemia and lymphoma (Lens et al., 1998; van Oers et al., 1993). High soluble levels of CD27 have also been noted in the synovial fluid of rheumatoid arthritis patients and cerebrospinal fluid of multiple scleorosis patients (van Oers et al., 1993). Although CD27 lacks intrinsic kinase activity (Loenen et al., 1998), the C- terminal region associates with TRAF2 and 5 that link to NIK and JNK signaling pathways involving the transcription factors NF-κB and Jun (Loenen et al., 1998). Engagement of CD27 induces a signaling cascade, resulting in activation of NF-κB, promotion of cell survival, and increased T cell effector function (Akiba et al., 1998a; Borst et al., 2005) (Fig. 3). Continuous CD27 signaling, however, leads to T cell depletion (Tesselaar et al., 2003).
Figure 3. CD27-CD70 pathway.
Signaling via CD27-CD70 pathways provides positive immune regulation. When stimulated with appropriate agonist such as anti-CD27 or anti-CD70 or cell lines made to express these molecules relay signals through TRAF2/5 resulting in long-term survival of cells via induction of NIK and NF-κB. Signals also result lead to the expression of PI3 kinase, ERK1/2, PLCγ leading to robust cell division.
Deletion of endogenous CD27 show impaired T cell responses to viral infection (Hendriks et al., 2000), but playing a role in germinal center formation (Xiao et al., 2004). Elimination or blockade of the CD27-CD70 pathway with CD27-deficient mice or blocking with anti-CD70 antibody resulted in improved neutralizing antibody responses and clearance and reduction of viral titers, respectively (Matter et al., 2006).
CD30-CD153
CD30, originally identified in 1982 on tumor cells of Hodgkin’s lymphoma (Schwab et al., 1982), also called Ki-1, is a membrane glycoprotein consisting of two chains with a molecular weight of 120 and 105 kDa. It is expressed by a subset of activated T cells (both CD4+ and CD8+), NK, and B cells and is constitutively expressed in decidual and exocrine pancreatic cells with maximum expression on CD45RO+ memory T cells (Annunziato et al., 2000). CD30 expression has been noted on CD4+CD8+ medullary thymocytes, indicating an important role of CD30/CD153 interactions in the thymus.
CD30 ligand (CD30L; CD153) is 26–40 kDa protein cloned in 1993 and is present on a variety of cells including activated T cells, macrophages, resting B cells, granulocytes, eosinophils, and neutrophils (Smith et al., 1993). Expression of CD153 was also noted on the outer wall of Hassall’s corpuscles and in placenta (Romagnani et al., 1998). As noted in the case of CD27, the soluble form of CD30 (~85/88 kDa) is generated when membrane-bound CD30 protein is cleaved by zinc metalloproteinase (Hansen et al., 1995). Interestingly, soluble CD30 (sCD30) shedding can be found in several neoplastic and reactive diseases (Del Prete et al., 1995; Falini et al., 1995; Pizzolo et al., 1994; Pizzolo et al., 1997; Romagnani et al., 1995) but the significance of sCD30 is not clear. In atopic dermatitis, CD30+ infiltrating T cells in lesions, elevated sCD30 levels, and increased number of circulating CD30+ cells were reported (Dummer et al., 2003). Similarly, elevated sCD30 levels and CD30 on BAL γδ+ T cells were noted in atopic asthma (Heshmat and El-Hadidi et al., 2006). Soluble CD30 was also noted in rhinoconjunctivitis (Bengtsson, 2003). Increased CD30 was also noted in patients with Grave’s disease and Hashimoto’s thyroiditis (Okumura et al., 1997).
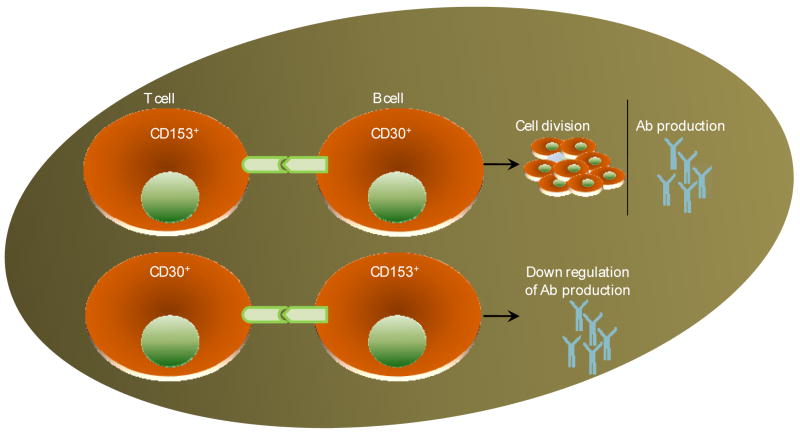
In vitro signaling of CD30 has costimulatory effects on lymphoid cells (Gilffilan et al., 1998). Signaling via CD30 augments proliferation under certain circumstances, but in other cases potentiated apoptosis (Telford et al., 1997) (Fig. 4). In the case of human lymphomas, CD30 signaling reveals reduced proliferation (Lee et al., 1996). Reports available also suggest that CD30 signals regulate Fas-independent apoptosis in CD8+ T cells (Telford et al., 1997). The CD30 cytoplasmic tail interacts with TRAFs 1, 2, 3, and 5, inducing NF-κB (Aizawa et al., 1997; Duckett and Thompson, 1997; Duckett et al., 1997; Gedrich et al., 1996; Lee et al., 1996a).
Figure 4. CD30-CD153 pathway.
Expression of CD30 or CD153 is not exclusive and can be present on both T cells as well as APCs. Such variable expression pattern of CD130-CD153 results in diverse immune responses. Interaction of CD153 bearing T cell with CD30+ B cell enhances survival gene expression leading cell division and increased antibody production. On the other hand, CD30+ T cells interaction with CD153+ B cell results in reduced Antibody production.
An important role for CD30 in the protection against autoimmune disorders has been reported (Kurts et al., 1999). Unmodified anti-CD30 antibodies as well as anti-CD30-based bispecific antibodies, immunotoxins, and radioimmunoconjugates have been examined in pre-clinical trials and clinical studies. Administration of anti-CD30 coupled to ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma demonstrated only a moderate efficacy (Schnell et al., 2002). The increased expression of CD30 on some neoplasms versus its limited expression on normal tissue makes it an excellent target for antibody therapy. CD30-CD30L interaction also is implicated in the induction of Th2 type immunity (Bowen et al., 1996) but a blockade of CD153 could not abrogate the Th2-directed murine Leishmaniasis (Akiba et al., 2000).
CD30 deficient mice show no abnormality in peripheral immune responses but have a defect in the activation-induced death of thymocytes (Amakawa et al., 1996). MHC class-I and class-II disparate skin and heart grafts are rejected much faster in CD30-deficient mice compared with wild-type mice (Beckmann et al., 2001).
CD134-CD134L
CD134 (OX40), one of most important and widely studied TNF superfamily members, was originally described as a cell surface antigen found on activated rat T cells (Paterson et al., 1987). OX40 is transiently expressed following T cell ligation of the TCR and its ligand OX40L (CD252) is expressed on APCs and endothelium. Although CD134 is present on a variety of cells, its role in T cell activation has been thoroughly investigated (So et al., 2008; Sugumura et al., 2004; Weinberg et al., 2002). The ligand for CD134 (CD134L) was originally termed glycoprotein 34 (gp34) and was identified on human T-leukemia virus type 1 transformed cells (Akiba et al., 1998).
Signals via OX40 are co-stimulatory in nature (Watts, 2005) and support late immune responses, enabling effective long-lasting T cell response (Croft, 2003; Salek-Ardakani and Croft, 2006; Weinberg et al., 2004) leading to T cell division, survival, and cytokine induction (Gramaglia et al., 2000; Maxwell et al., 2000; Rogers et al., 2001; Weinberg et al., 1998, 1999) (Fig. 5). The validity of these findings was further substantiated by the determination that in OX40-deficient mice the T helper responses were greatly diminished, while the B cell and CTL responses remained unaffected (Kopf et al., 1999). Also, studies with OX40-Ig fusion protein demonstrate decreased T cell responses under the conditions tested (Weinberg et al., 1999). On the other hand, OX40 signals inhibit Treg cell development and function (So and Croft, 2007; Kroemer et al., 2007; Watts et al., 2005). Signals via OX40 are relayed throughTRAF2, TRAF3, and TRAF5, resulting in NF-κB activation (Arch and Thompson, 1998; Kawamata et al., 1998).
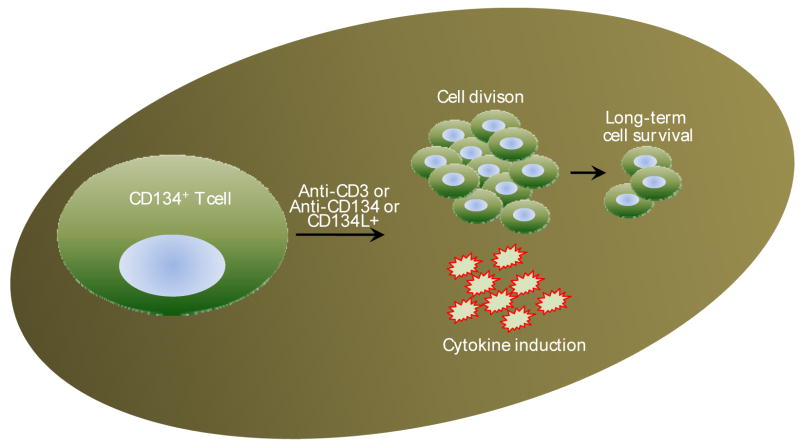
Figure 5. CD134-CD134L pathway.
CD134 is transiently expressed on T cells and is known to support late immune responses. Once expressed and when stimulated via TCR or agonistic anti-CD134 mAbs or cells made to express CD134L supports cell division and IL-2 production resulting in long-term survival of memory T cells.
The importance of the OX40-OX40L pathway in health and disease has been extensively explored (Hori, 2006; Kaleeba et al., 1999; Weinberg et al., 1996, 2005). The first description of enhanced responses through exploitation of the OX40-OX40L pathway was made using OX40L fusion proteins and anti-OX40 mAbs in a tumor model (Pan et al., 2002; Weinberg et al., 2000). OX40 dependent costimulation enhances EAE and is involved in promoting atherosclerotic disease (Gotsman et al., 2008). The role of OX40 signaling is important for allograft response (Demirci and Li, 2008), anti-viral responses (Bertram et al., 2004), and autoimmune processes and cancer (Redmond and Weinberg, 2007). The significance of the OX40 pathway is also explored in allergic reactions (Kroczek and Hamelmann, 2005). Allergen-sensitized and challenged OX40L-deficient mice showed decreased airway hyperactivity, Th2 cytokine production, and serum IgE levels (Aresides et al., 2002).
Besides its role in costimulation of CD4 cells, OX40/OX40L interactions are closely involved in effector functions as well. For example, OX40L crosslinking supported B cell stimulation and antibody production (Stuber et al., 1995), and elevated dendritic cell effector functions (Ohshima et al., 1997). Interfering with this association can inhibit both primary and secondary IgG responses (Stuber and Strober, 1999).
CD137-CD137L
Another important and extensively studied member of the TNF superfamily is the CD137 (4-1BB)-CD137L (4-1BBL) (Vinay and Kwon, 2006a). CD137 was initially discovered in screens for receptors on activated mouse lymphocytes (Kwon and Weissman, 1989). The CD137 is not detected (<3%) on resting T cells and T cell lines. However, when the T cells, in the presence of APCs, are stimulated with a variety of agonists (plate-bound anti-CD3, concanavalin A, phytohemagglutinin, IL-2, IL-4, anti-CD28, PMA, ionomycin alone or in combinations) CD137 upregulates and maintains its expression (Pollok et al., 1993). Interestingly, expression of CD137 is detectable on CD11c+ dendritic cells and CD4+CD25+ Tregs of naïve mice (McHugh et al., 2002; Wilcox et al., 2002). In vitro the CD137 signal provides costimulatory signals to T cells and shows preference for CD8 over CD4 T cells, leading to cellular proliferation, IL-2 production, and increased expression of survival genes (Vinay et al., 2006b). Signals by CD137 are relayed through TRAF1, 2, and 3, which interact with the cytoplasmic domain of CD137; mutation analysis showed the involvement of the runs of acidic residues in the cytoplasmic domain of CD137 (Jang et al., 1998). Jang et al (1998) and Arch and Thompson (1998) reported that CD137 cross-linking induces activation of NF-κB and is inhibited by dominant negative TRAF2 and NF-κB-inducing kinase (NIK). CD137 is secreted in soluble form in sera and lymphocyte secretions in patients with rheumatoid arthritis (Michel et al., 1998). CD137 shares this feature with certain other receptor forms, such as TNFR, NGFR, CD27, CD30, and CD95.
Interestingly, in vivo administration of agonistic antibodies supports robust CD8+ T cell expansion and shrinking of CD4 and B cell numbers and humoral immunity (Vinay et al., 2006b). In depth analysis revealed increased in vivo production of IFN-γ, TNF-α, and TGF-β in anti-CD137 treated animals to be perpetuators of dampened CD4 and humoral responses (Niu et al., 2007; Menoret et al., 2006; Myers et al., 2005; Sun et al., 2002; Vinay et al., 2006c). Others have advocated that in vivo anti-CD137 Abs increase IFN-γ in CD8+ T cells, which in turn upregulate indoleamine 2,3-dioxygenase (IDO) in competent APS which when interacting with CD4+ T cells, bring about their destruction (Choi et al., 2006; Seo et al., 2004) (Fig. 6). Seo et al. (2004) have demonstrated that anti-CD137 mAbs expand a novel CD11c+CD8+ population expressing high levels of IFN-γ and adoptive transfer of these CD11c+CD8+ T cells into susceptible mice ameliorate arthritis. Agonistic anti-CD137 Abs has potent antitumor properties and increases transplant survival and antiviral properties (Croft, 2003; Vinay et al., 2006c).
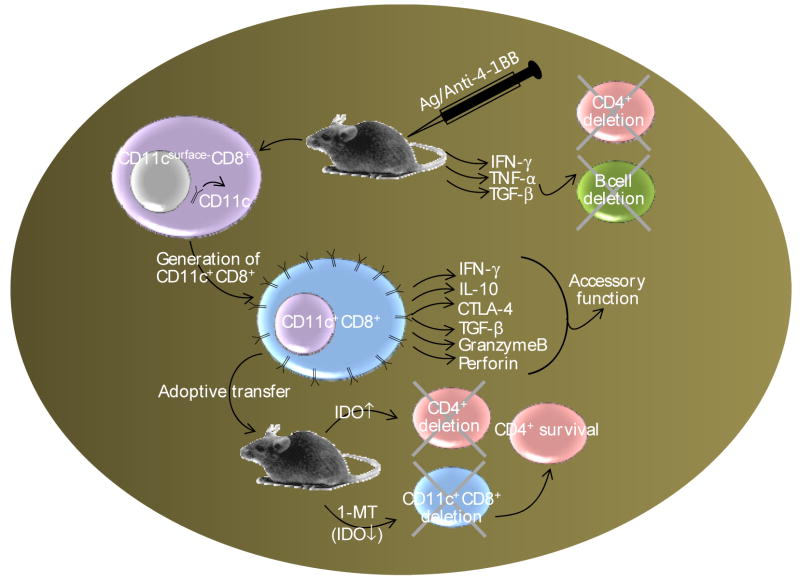
Figure 6. CD137-CD137L pathway.
With a few exceptions expression of CD137 is activation dependent. There is a variability in anti-CD137-mediated signaling. While in vitro anti-CD137 stimulation supports activation of both CD4+ and CD8+ T cells, in vivo effects mediated by anti-CD137 is complex. Administration of agonistic anti-CD137 mAbs supports robust CD8+ T cell expansion and constricts CD4+ T and B cell numbers and function. This latter in vivo effect of anti-CD137 is believed to result from over expression of IFN-γ, IL-10, TGF-β, granzymeB, perforin, and CTLA-4 and expansion of a novel immunoregulatory CD11c+CD8+ T cell subset. The increased IFN-g due to anti-mediated CD11c+CD8+ T cells upregulates indoleamine 2,3-dioxygenase (IDO) in competent cells which when interact with partnering CD4+ T cells causes their deletion. This can be reversed by neutralizing IDO activity by 1-mehtyltryptophan.
CD40-CD154
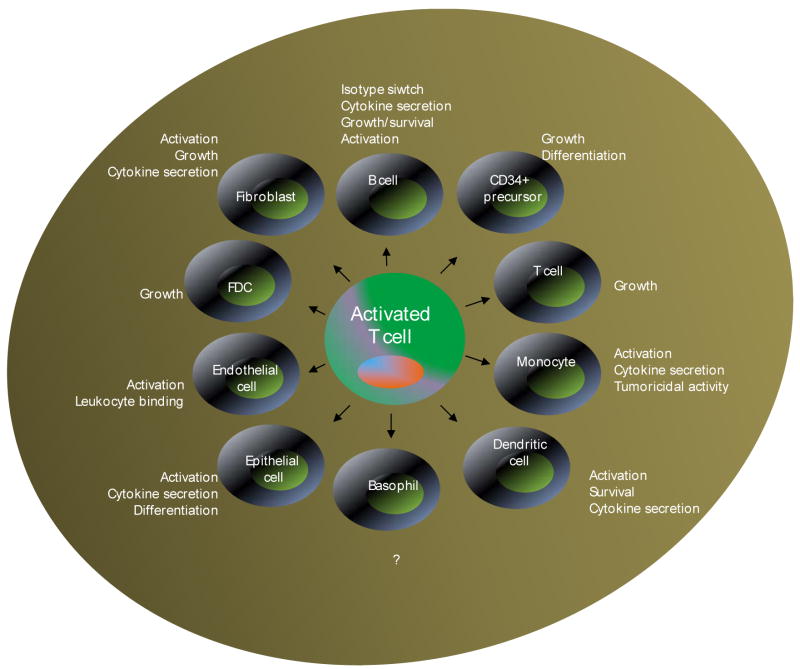
The CD40 pathway remains the most extensively studied TNF superfamily members. The amount of research data available and its success as a therapeutic agent are too vast to cover in this review. CD40 was first identified in 1985 on B cells (Paulie et al., 1985). CD40 received its definition at the 3rd International CD Workshop (Stamenkovic et al., 1989). CD154 (CD40L, gp39, T-Bam or TRAP) is an activation-induced molecule present on CD4+ T cells, monocytes, DCs, and a small proportion of CD8+ cells (Schonbeck and Libby, 2001). The role of CD40 in the regulation of B cell biology is well documented (D’Orlando et al., 2007; Quezada et al., 2004). Besides B cells, CD40 is also present on a variety of antigen-presenting cells; non-antigen-presenting cells including dendritic cells; follicular dendritic cells; monocytes; macrophages; mast cells; fibroblasts; epithelial cells; vascular smooth muscle cells and endothelial cells; and as a functional molecule on CD4+ T cells (Grewal and Flavell, 1998; Munroe et al., 2007) (Fig. 7).
Figure 7. CD40-CD154 pathway.
Expression of CD40 is widespread on a variety cells including CD4+ T cells. CD40 binds an activation induced CD154 molecule. Interaction of CD154+ CD4+ T cells with CD40-bearing cells results in the activation of partnering cells resulting in the expression of cell survival genes, cytokine induction, Ig isotype switching etc.
CD4-CD154 interactions mediate one of the most effective APC-activating signals. Signaling via the dendritic cell CD40 molecule upregulates expression of CD80 and CD86, and induces IL-12 secretion (Cella et al., 1996; Ridge et al., 1998; Schuurhius et al., 2000). Signaling via CD40 activates NF-κB (Lalmanach-Girard et al., 1993; Berberich et al., 1994) and rescues BCR-induced cell death (Schauer et al., 1996). Moreover, the CD40-CD154 pathway is central to germinal center formation and Ig isotype switch as validated by studies using CD40−/− mice (Kawabe et al., 1994).
In vivo, CD40 ligation supports CD4+ and CD8+ T cell growth, resulting in increases in tumor protection and alteration of steady-state tolerance into immunity (Bonifaz et al., 2002; Clarke, 2000; Diehl et al., 1999; French et al., 1999; Lefrancois et al., 2000; Mackey et al., 1998; Toes et al., 1998). On the other hand, the CD40-signaling blockade, mainly through anti-CD40L Ab, inhibits T cell activation and results in tolerance, e.g., to transplants, and control of some autoimmune diseases (Diehl et al, 2000; Iwakoshi et al., 2000). Blockade of the CD40-CD154 pathway has been proven beneficial in transplantation (Bishop, 2002; Mungara et al., 2008; Nathan et al., 2002) and autoimmune diseases (Toubi and Shoenfeld, 2004).
GITR-GITRL
The glucocorticoid-induced tumor necrosis factor receptor (GITR) family-related gene was cloned first from dexamethasone-treated murine T cell hybridoma (3DO) cells using a differential display technique (Nocentini et al., 1997, 2000a). Two groups identified that a novel 25 kDa protein named activation-inducible protein of the TNF receptor (AITR) is the human homolog of the murine GITR (Gurney et al., 1999; Kwon et al., 1999). The AITR, which has 55% identity with murine GITR at the amino acid level, is activated by transducing signals through a TRAF2-mediated mechanism. The expression of AITR is inducible by PMA and ionomycin, anti-CD3 plus anti-CD28 monoclonal antibodies. It is detected as a 1.25 kb mRNA in lymph nodes, PBLs and weakly in the spleen and colorectal adenocarcinoma cell line (SW 480) (Kwon et al., 1999). GITR is a 228 amino acid type I transmembrane protein characterized by three cysteine pseudorepeats in the extracellular domain. It is similar to CD137 in the intracellular domain. The full-length GITR cDNA revealed a 1005 bp long sequence. Northern blot analysis suggested that GITR mRNA is about 1.1 kb long. Subsequent studies showed at least three spliced variants of GITR (Nocentini et al., 2000b).
GITR is not detectable in freshly derived lymphoid tissues (including thymocytes, spleen, and lymph node T cells), liver, kidney, and brain and T cell hybridoma 3D0. However, low levels of GITR mRNA were detected by competitive RT-PCR in T cell hybridoma, thymocytes, spleen, and lymph node T cells. GITR expression in T cells was found to increase 4- to 8-fold upon treatment with immobilized anti-CD3 and Con A and PMA. However, the induction of kinetics were slow with no increase before 6 h (Nocentini et al., 1997). The murine GITR ligand was cloned and characterized in 2003 (Kim et al., 2003). These authors demonstrated that GITRL is detected on immature and mature dendritic cells. In addition, GITRL binding GITR on HEL 293 cells triggers NF-κB activation and the addition of soluble GITRL prevents CD25+CD4+ Treg-mediated suppressive activities (Kim et al., 2003).
Signaling via GITR is costimulatory in nature (Nocentini and Riccardi, 2005). McHugh et al. (2002) were the first demonstrate that GITR is constitutively expressed on CD25+CD4+ Tregs. Simultaneously, Shimizu et al. (2002 determined that GITR plays a key role in dominant immunological self-tolerance maintained by CD25+CD4+ regulatory T cells and could be a suitable molecular target for preventing or treating autoimmune disease (Fig. 8). Interestingly, macrophages were shown to express constitutively both GITR and GITRL and stimulation of these cells with recombinant soluble GITRL results in increased nitric oxide synthase, cyclooxygenase-2 protein, generated significant amounts of prostaglandin E2, and matrix metalloproteinase 9 (Lee et al., 2003; Shin et al., 2000,2002,2003).
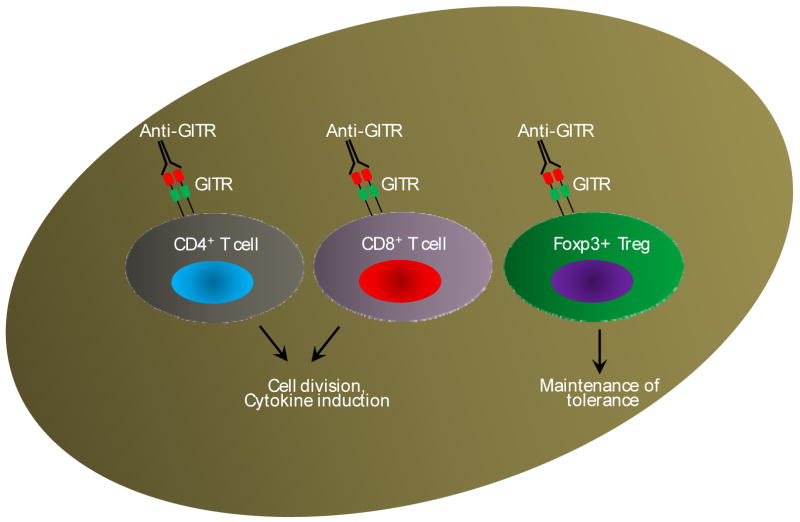
Figure 8. GITR-GITRL pathway.
Expression of GITR is activation dependent with the exception of Foxp3+ Tregs which express this antigen in a constitutive manner. Signals through GITR are co-stimulatory in nature to CD4+ and Cd8+ T cells resulting cell division and cytokine induction. Importantly, GITR provides key signals to FoxP3+ Tregs to maintain immune tolerance and plays a critical role in the control of autoimmune diseases.
Signaling through GITR induces NF-κB activation mediated by TRAF4 and is inhibited by the cytoplasmic protein A20 (Esparza and Arch, 2004). Anti-GITR Ab therapy significantly increased disease severity in an EAE model (Kohm et al., 2004).
The importance of the GITR pathway has begun to be appreciated (Nocentini and Riccardi, 2005). GITR knockout mice develop normally but show increased cell proliferation, IL-2 receptor expression, and IL-2 production compared with control wild-type mice in cultures stimulated with anti-CD3 (Ronchetti et al., 2002). Patients with non-infectious uveitis show more GITR+CD4+ T cells than normal individuals (Li et al., 2003). Treatment of SJL mice with anti-GITR antibody in conjunction with proteolipid protein (PLP 131–151) significantly exacerbated clinical disease severity and CNS inflammation. On the other hand, prior depletion of CD25+CD4+ Tregs failed to result in EAE, suggesting alternative targets for the anti-GITR Ab treatment (Kohm et al., 2004). Administration of anti-GITR Ab in 3-month-old mice results in autoimmune gastritis associated with anti-parietal cell auto-antibodies (Shimizu et al., 2002). In addition, the importance of the GITR-GITRL pathway is underscored in several models including colitis (Uraushihara et al., 2003), autoimmune diabetes (Suri et al., 2003), GVHD (Muriglan et al., 2004), shock due to splanchnic artery occlusion (Cuzzucrea et al., 2004), viral infections (Dittmer et al., 2004), and cancer (Calmels et al., 2005).
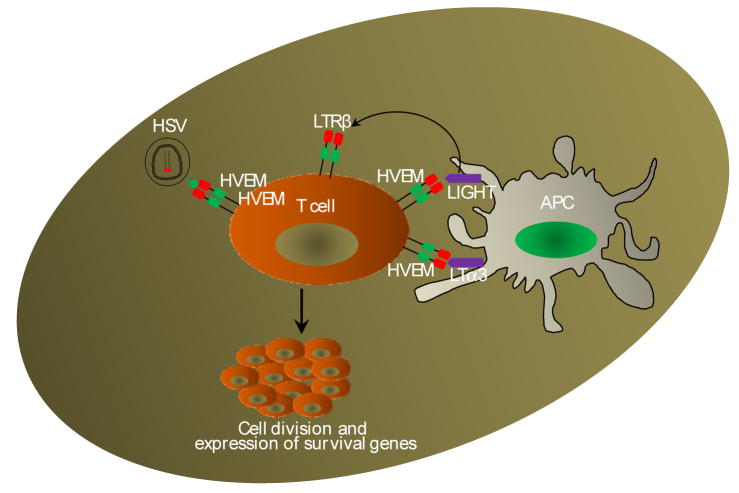
HVEM-LIGHT
Herpes virus entry mediator (HVEM) was identified and cloned in 1996 as one of many entry receptors for α-herpesviruses (Montgomery et al., 1996). HVEM has a wide tissue distribution (Kwon et al., 1997) and is present on a variety of cell types including T and B cells, monocytes, and DCs (Harrop et al., 1998a; Morel et al., 2001).
LIGHT, a 29 kDa type II transmembrane protein, was identified as a ligand for HVEM (Mauri et al., 1998). Although HVEM binds LIGHT, it also binds LTα3 and LIGHT besides binding HVEM, also binds LTRβ, thus complicating the interpretations (Croft, 2003) (Fig. 9). LIGHT is expressed by several cells types, including T cells and DCs (Morel et al., 2000; Tamada et al., 2000a). LIGHT signaling leads to T cell growth and differentiation and has CD28-independent costimulatory activity (Tamada et al., 2000b). LIGHT signaling is important for CD8+ T cell-mediated allo-responses (Liu et al., 2003; Scheu et al., 2002). Transgenic expression of LIGHT in T cells results in acute intestinal inflammation, increased production serum IgA, kidney IgA deposition, and exacerbation of nephritis (Wang et al., 2004).
Figure 9. HVEM-LIGHT pathway.
HVEM was originally identified as entry mediator of herpes virus. HVEM signaling is complex as it binds KIGHt as well as LTα3 and is further complicated as LIGHT besides binding HVEM also binds LTRβ. This complex receptor-ligand interactions as well as HSV-HVEM interplay culminate in the expression of of array of signaling molecules, type I IFNs etc.
HVEM stimulation by LIGHT leads to costimulation T cells and DC activation (Morel et al., 2000; Tamada et al., 2000a). The importance of HVEM in immune regulation was demonstrated in tumor rejection (Tamada et al., 2000b), GVHD (Tamada 2000b, 2002), autoimmune diseases (Shaikh et al., 2000; Wang et al., 2001), and atherosclerosis (Lee et al., 2001). Blockade of LIGHT was shown to hamper early T cell proliferation and cytokine secretion in MLR reaction (Kwon et al., 1997; Harrop et al., 1998b; Tamada et al., 2000a). LIGHT induces apoptosis in tumor cells expressing both LTβR and HVEM, especially when combined with IFN-γ (Mauri et al., 1998; Harrop et al., 1998a; Rooney et al., 2000). The human immunodeficiency virus-1 (HIV-1 Nef) increases expression of LIGHT, resulting in heightened cytokine activity leading to disease progression in infected individuals (Lama and Ware, 2000). Overexpression of LIGHT in MDA-MB-231 breast cancer cells suppressed tumor growth (Zhai et al., 1998).
In summary, the last few years have seen rapid growth in the numbers of members of TNF superfamily. Exploitation of the various unique biological functions of the TNF superfamily members for therapeutic use has shown promise. Further research in this area will undoubtedly unravel keys to effective therapeutic intervention in cancer, transplant survival, antiviral effectiveness, and autoimmunity.
Acknowledgments
This work was supported by grants from the National Cancer Center, Korea (NCC-0890830-2 and NCC-0810720-2); Korean Research Foundation (KRF-2005-084-E00001); Korean Science and Engineering Foundation (Stem Cell-M10641000040 and Discovery of Global New Drug-M10870060009); Korea Health 21 R&D (A050260); NIH RO1-EY013325, LSU Eye Center Core Grant for Vision Research NIH EY02377. The LSUHSC Department of Ophthalmology has an unrestricted grant from Research to Prevent Blindness, New York, New York and receives funding from the Louisiana Lions Eye Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agematsu K, Nagumo H, Oguchi Y, Nakazawa T, Fukushima K, Yasui K, Ito S, Kobata T, Morimoto C, Komiyama A. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 1998;91:173–180. [PubMed] [Google Scholar]
- Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J Biol Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D, Yagita H, Okumura K. CD27, a member of the necrosis factor receptor superfamily, activates NF-κB and stress-activated protein-kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-κB inducing kinase. J Biol Chem. 1998a;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- Akiba H, Atsuta M, Yagita H, Okumura KO. Identification of rat OX40 ligand by molecular cloning. Biochem Biophys Res Commun. 1998b;251:131–136. doi: 10.1006/bbrc.1998.9376. [DOI] [PubMed] [Google Scholar]
- Akiba H, Miyahira Y, Atsuta M, Takeda K, Nohara C, Futagawa H, Aoki T, Yagita H, Okumura KO. Critical contribution of OX ligand to T helper cell type differentiation in experimental Leishmaniasis. J Exp Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amakawa R, Hakem A, Kundig TM, Matsuyama T, Simard JJ, Timms E, Wakeman A, Mittrueker HW, Grieser H, Takimoto H, Schmits R, Shahnian A, Ohashi P, Penninger JM, Mak TW. Impaired negative selection of T cells in Hodgkin’s disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Romagnani P, Mavilia C, Pizzolo G, Stein H, Romagnani S. CD30. In: Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine Ref. Academic Press; UK: 2000. pp. 1669–1684. [Google Scholar]
- Arch RH, Thompson CB. 4-1BB and OX-40 are members of tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor κB. Mol Cell Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arestides RS, He H, Westlake RM, Chen AI, Sharpe AH, Perkin DL, Finn PW. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, Clark EA, Smith CA, Grabstein KH, Cosman D, Spriggs MK. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992:357-80-82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Beckmann J, Kurts C, Klebba I, Bayer B, Klempnauer J, Hoffmann MW. The role of CD30 in skin and heart allograft rejection in the mouse. Transplant Proc. 2001;33:140–41. doi: 10.1016/s0041-1345(00)01943-6. [DOI] [PubMed] [Google Scholar]
- Bengtsson A. The role of CD30 in atopic diseases. Allergy. 2003;56:593–603. doi: 10.1034/j.1398-9995.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J Immunol. 1994;153:4357–4366. [PubMed] [Google Scholar]
- Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol. 2004;16:185–196. doi: 10.1016/j.smim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Bishop DK. The immunobiology of inductive anti-CD40L therapy in transplantation: allograft acceptance is not dependent upon the deletion of graft-reactive T cells. Am J Transplant. 2002;2:323–332. doi: 10.1034/j.1600-6143.2002.20406.x. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on majorhistocompatibility complex class I products and peripheral CD8_ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996:156-442-449. [PubMed] [Google Scholar]
- Bretscher P, Cohn M. Paralysis and induction involve the recognition of one and two determinants of an antigen, respectively. Science. 1970;169:1042–1049. [Google Scholar]
- Calmels B, Paul S, Futin N, Ledoux C, Stoeckel F, Acres B. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12:198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase dependent mechanisms. Cytokine. 2006;34:233–242. doi: 10.1016/j.cyto.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leuko Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity. Nat Rev Immunol. 2003;3:600–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Nocentini G, Di Paola R, Mazzon E, Ronchetti S, Genovese T, Muia C, Caputi AP, Riccardi C. Glucocorticoidinduced TNF receptor family gene (GITR) knockout mice exhibit a resistance to splanchnic artery occlusion (SAO) shock. J Leukoc Biol. 2004;76:933–940. doi: 10.1189/jlb.0204110. [DOI] [PubMed] [Google Scholar]
- D’Orlando O, Gri G, Cattaruzi G, Merluzzi betto E, Gattei V, Pucillo C. Outside inside signaling in CD40-mediated B cell activation. J Biol Regul Homeost Agents. 2007;21:49–62. [PubMed] [Google Scholar]
- Del Prete G, Izzolo ME, Romagnani S. CD30, Th2 cytokines and HIV infection; a complex and fascinating link. Immunol Today. 1995;16:76–80. doi: 10.1016/0167-5699(95)80092-1. [DOI] [PubMed] [Google Scholar]
- Demirci G, Li XC. Novel roles of OX40 in the allograft response. Curr Opin Organ Transplant. 2008;13:26–30. doi: 10.1097/MOT.0b013e3282f3def3. [DOI] [PubMed] [Google Scholar]
- Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- Diehl L, den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- Duckett CS, Thompson CB. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signalling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer W, Brocker EB, Bastian BC. Elevated serum levels of soluble CD30 are associated with atopic dermatitis, but not with respiratory disorders and allergic contact dermatitis. Br J Dermatol. 2003;137:185–187. doi: 10.1046/j.1365-2133.1997.18031887.x. [DOI] [PubMed] [Google Scholar]
- Esparza EM, Arch RH. TRAF4 functions as an intermediate of GITR-induced NF-kappaB activation. Cell Mol Life Sci. 2004;61:3087–3092. doi: 10.1007/s00018-004-4417-0. [DOI] [PubMed] [Google Scholar]
- Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, Martelli MF, Stein H. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- Gedrich RW, Gilfillan MC, Duckett CS, Van Dongen JL, Thompson CB. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J Biol Chem. 1996;271:12852–12858. doi: 10.1074/jbc.271.22.12852. [DOI] [PubMed] [Google Scholar]
- Gilfillan MC, Noel PJ, Podack ER, Reiner SL, Thompson CB. Expression of the costimulatory receptor CD30 is regulated by both CD28 and cytokines. J Immunol. 1998;160:2180–2187. [PubMed] [Google Scholar]
- Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Cir Res. 2008;103:1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- Grewal I, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Grewal IS. CD70 as a therapeutic target in human malignancies. Expert Opin Ther Targets. 2008;12:341–351. doi: 10.1517/14728222.12.3.341. [DOI] [PubMed] [Google Scholar]
- Gurney AL, Marsters SA, Huang RM, Pitti RM, Mark DT, Baldwin DT, Gray AM, Dowd AD, Brush AD, Heldens AD, Schow AD, Goddard AD, Wood WI, Baker KP, Godowski PJ, Ashkenazi A. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9:215–218. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- Hansen HP, Kisseleva T, Kobarg J, Horn-Lohrens O, Havsteen B, Lemke H. A zinc metalloproteinase is responsible for the release of CD30 on human tumor cell lines. Int J Cancer. 1995 Nov 27;63(5):750–756. doi: 10.1002/ijc.2910630524. [DOI] [PubMed] [Google Scholar]
- Harrop JA, McDonnell PC, Brigham-Burke M, Lyn SD, Minton J, Tan KB, Dede K. Herpes virus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998a;273:27548–27556. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- Harrop JA, Reddy M, Dede K, Brigham-Burke M, Ly S, Tan KB, Silverman C, Eichman C, DiPrinzio R, Spampanato J, Porter T, Holmes S, Young PR, Truneh A. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161:1766–1794. [PubMed] [Google Scholar]
- Hendriks J, Loes A, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- Heshmat NM, El-Hadidi ES. Soluble CD30 serum levels in atopic dermatitis and bronchial asthma and its relationship with disease severity in pediatric age. Pediatric Allergy Immunol. 2006;17:297–303. doi: 10.1111/j.1399-3038.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Ho AW, Hatjiharissi E, Ciccarelli BT, Branagan AR, Hunter ZR, Leleu X, Tournilhac O, Xu L, O’Connor K, Manning RJ, Santos DD, Chemaly M, Patterson CJ, Soumerai JD, Munshi NC, McEarchern JA, Law CL, Grewal IS, Treon SP. CD27-CD70 interactions in the pathogenesis of Waldenstrom macroglobulinemia. Blood. 2008;112:4683–4609. doi: 10.1182/blood-2007-04-084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T. Role of OX40 in the pathogensis and control of diseases. Int J Hematol. 2006;83:17–22. doi: 10.1532/IJH97.05151. [DOI] [PubMed] [Google Scholar]; Weinberg AD, Montler R. Modulation of TNF receptor family members to inhibit autoimmune disease. Curr Drug Targets Inflamm Allergy. 2005;4:195–203. doi: 10.2174/1568010053586345. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–2657. [PubMed] [Google Scholar]
- Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB signals are mediated by TRAF2 and activate nuclear factor-κB (NF-κB) Biophys Biochem Res Commun. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5:361–367. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- Jenkins MK. The role of cell division in the induction of clonal anergy. Immunol Today. 1992;13:69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- Kaleeba JA, Offner H, Vandenbark AA, Lublinski A, Weinberg AD. Ox-40 receptor provides a potent cost-imulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1999;10:453–461. doi: 10.1093/intimm/10.4.453. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of Ox-40 signal transduction pathways lead to tumor necrosis factor receptor-associated factor (TRAF)-2 and -5 mediated NF-kappaB activation. J Biol Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- Kim JD, Choi BK, Bae JS, Lee UH, Han IS, Lee HW, Youn BS, Vinay DS, Kwon BS. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–569. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27-CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. Ox-40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- Kroczek R, Hamelmann E. T-cell costimulatory molecules: optimal targets for the treatment of allergic airway disease with monoclonal antibodies. J Allergy Clin Immunol. 2005;116:906–909. doi: 10.1016/j.jaci.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, Chen M, Maki T, Li XC. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179:5584–5591. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- Kurts C, Carbon FR, Krummel MF, Koch KM, Miller JFAP, Heath WR. Signalling through CD30 protects against autoimmune diabetes mediated by CD8T cells. Nature. 1999;398:341–344. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- Kwon B, Yu KY, Ni J, Yu GL, Jang IK, Kim YJ, Xing L, Liu D, Wang SX, Kwon BS. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–61. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- Kwon BS, Tan KB, Ni J, Oh KO, Le ZH, Kim KK, Kim MH, Gentz R, Laing G, Harrop JA, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the TNF superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- Kwon BS, Weismann SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalmanach-Girard AC, Chiles TC, Parker DC, Rothstein TL. T cell-dependent induction of NF-kappa B in B cells. J Exp Med. 1993;177:1215–1219. doi: 10.1084/jem.177.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama J, Ware CF. Human immunodeficiency virus type 1Nef mediates sustained membrane expression of tumor necrosis factor and the related cytokine LIGHT on activated T cells. J Virol. 2000;74:9396–9402. doi: 10.1128/jvi.74.20.9396-9402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RA, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat Immunol. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Shin HH, Kwon BS, Choi HS. Soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) increased MMP-9 activity in murine macrophage. J Cell Biochem. 2003;88:1048–1056. doi: 10.1002/jcb.10456. [DOI] [PubMed] [Google Scholar]
- Lee SY, Park CG, Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J Exp Med. 1996;183:669–674. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Kim SH, Lee BB, Kwon B, Song H, Kwon BS, Park JE. TNFRSF14 is involved in atherogenesis by inducing pro-inflammatory cytokines and matrix matalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee SY, Kandala G, Liou ML, Liou HC, Choi Y. CD30/TNF receptor-associated factor interaction: NF-κB activation and binding specificity. Proc Natl Acad Sci USA. 1996a;93:9699–9703. doi: 10.1073/pnas.93.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- Lens SM, Baars PA, Hooibrink B, Van Oers MH, Van Lier RA. Antigen-presenting cell-derived signals determine expression levels of CD70 on primed T cells. Immunology. 1997;90:38–45. doi: 10.1046/j.1365-2567.1997.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Tesselaar K, Vvan Oers MH, Van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- Li Z, Mahesh SP, Kim BJ, Buggage RR, Nussenblatt RB. Expression of glucocorticoid induced TNF receptor family related protein (GITR) on peripheral T cells from normal human donors and patients with non-infectious uveitis. J Autoimmun. 2003;21:83–92. doi: 10.1016/s0896-8411(03)00085-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Schmidt CS, Zhao F, Okragly AJ, Glasebrook A, Fox N, Galbreath E, Zhang Q, Song HY, Na S, Yang DD. LIGHT-deficiency impairs CD8+ T cell expansion, but not effector function. Int Immunol. 2003;15:861–870. doi: 10.1093/intimm/dxg082. [DOI] [PubMed] [Google Scholar]
- Loenen WA. CD27 and (TNFR) relatives in the immune system: their role in health and disease. Semin Immunol. 1998;10:417–422. doi: 10.1006/smim.1998.0159. [DOI] [PubMed] [Google Scholar]
- Mackey MF, Barth RJ, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukocyte Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–2155. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RA, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and Lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergizes to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- McDonagh CF, Kim KM, Turcott E, Brown LL, Westendorf L, Feist T, Sussman D, Stone I, Anderson M, Miyamoto J, Lyon R, Alley SC, Gerber HP, Carter PJ. Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol Cancer Ther. 2008;7:2913–2923. doi: 10.1158/1535-7163.MCT-08-0295. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccrillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Menoret A, Myers LM, Lee SJ, Mittler RS, Rossi RJ, Vella AT. TGF beta protein processing and activity through TCR triggering of primary Cd8+ T regulatory cells. J Immunol. 2006;177:6091–6097. doi: 10.4049/jimmunol.177.9.6091. [DOI] [PubMed] [Google Scholar]
- Michel J, Langstein J, Hofstadter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998;28:290–295. doi: 10.1002/(SICI)1521-4141(199801)28:01<290::AID-IMMU290>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Morel Y, Schiano de Colella JM, Harrop J, Deen KC, Holmes SD, Wattam TA, Khandekar SS, Truneh A, Sweet RW, Gastaut JA, Olive D, Costello RT. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–4404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus clonal inactivation: a costimulatory signaling pathway determines the outcome of T antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Mungara AK, Brown DL, Bishop DK, Wood SY, Cederna PS. Anti-CD40L monoclonal antibody treatment induces long-term, tissue-specific, immunologic hyporesponsiveness to peripheral nerve allografts. J Reconstr Microsurg. 2008;24:189–195. doi: 10.1055/s-2008-1076085. [DOI] [PubMed] [Google Scholar]
- Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;171:671–682. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, Kochman AA, Tjoe KH, Riccardi C, Pandolfi PP, Sakaguchi S, Houghton AN, Van Den Brink MR. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J Exp Med. 2004;200:149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 regulatory cells use IFN-g to elaborate TGF-β based suppression. J Immunol. 2005;174:7625–7632. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- Nathan MJ, Yin D, Eichwald EJ, Bishop DK. The immunobiology of inductive anti_CD40L therapy in transplantation: allograft acceptance is not dependent upon the deletion of graft-reactive T cells. Am J Transplant. 2002;2:323–332. doi: 10.1034/j.1600-6143.2002.20406.x. [DOI] [PubMed] [Google Scholar]
- Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay Kwon BS, Chen L, Vella AT, Mittler RS. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini G, Bartoli A, Ronchetti S, Giunchi L, Cupelli A, Delfino D, Migliorati G, Riccardi C. Gene structure and chromosomal assignment of mouse GITR, a member of the tumor necrosis factor/nerve growth factor receptor family. DNA Cell Biol. 2000a;19:205–217. doi: 10.1089/104454900314474. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Ronchetti S, Bartoli A, Spinicelli S, Delfino D, Brunetti L, Migliorati G, Riccardi C. Identification of three novel mRNA splice variants of GITR. Cell Death Differ. 2000b;7:408–410. doi: 10.1038/sj.cdd.4400670. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of Ox-40 ligand on human dendritic cells. J Immunol. 1997;189:3838–3848. [PubMed] [Google Scholar]
- Okumura M, Hidaka Y, Kuroda S, Takeoka K, Tada H, Amino N. Increased serum concentration of soluble CD30 in patients with Grave’s disease and Hashimotos’s thyroiditis. J Clin Endocrinol Metabol. 1997;82:1757–1760. doi: 10.1210/jcem.82.6.4000. [DOI] [PubMed] [Google Scholar]
- Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. Ox40 ligation enhances primary and cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Jefferies DJ, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of ~50,000 Mr detected only on CD4+ T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Paulie S, Ehlin-Henriksson B, Mellstedt H, Koho H, N+Ben-Aissa H, Perlmann P. A p50 surface antigen restricted to human urinary bladder carcinomas and B lymphocytes. Cancer Immunol Immunother. 1985;20:23–28. doi: 10.1007/BF00199769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolo F, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, Raiter R, Sinicco A. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997;108:251–253. doi: 10.1046/j.1365-2249.1997.d01-1005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, Sinicco A, Raiteri R, Semenzato G, Stein H, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–5. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Pollok KE, Kim YJ, Zhou Z, Hurtado JC, Kim KK, Pickard RT, Kwon BS. The inducible T cell antigen 4-1BB: analysis of expression and function. J immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interaction at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27:415–436. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4_ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and essential for long-term survival of CD4+ T cells. Immunity. 2001;164:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Manetti R, Mavilia C, Lasagni L, Manuelli C, Vannelli GB, Vanini V, Maggi E, Pupilli C, Romagnani S. High CD30 ligand expression by epithelial cells and Hassal’s corpuscles in the medulla of human thymus. Blood. 1998 May 1;91(9):3323–32. [PubMed] [Google Scholar]
- Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leuk Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–352. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- Rooney IA, Butrovich KD, Glass AA, Borborodu S, Bendict CA, Whitbeck JC. The lymphotoxin β receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000;275:14307–14315. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- Salek-Ardakani S, Croft M. Regulation of CD4 T cell memory by OX40 (CD134) Vaccine. 2006;13:872–883. doi: 10.1016/j.vaccine.2005.07.108. [DOI] [PubMed] [Google Scholar]
- Schauer SL, Wang Z, Sonenshein GE, Rothstein TL. Maintenance of nuclear factor-kappa B/Rel and c-myc expression during CD40 ligand rescue of WEHI 231 early B cells from receptor-mediated apoptosis through modulation of I kappa B proteins. J Immunol. 1996;157:81–86. [PubMed] [Google Scholar]
- Scheu S, Alferink J, Pötzel T, Barchet W, Kalinke U, Pfeffer K, Scheu S, Alferink J, Pötzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell, et al. A phase I study with an anti-CD30 ricin A-chain immunotoxin (ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res. 2002;8:1779–1786. [PubMed] [Google Scholar]
- Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ, van der Voort EI, Rea D, Offringa R, Geuze HJ, Melief CJ, Ossendorp F. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or-dependent stimuli. J Exp Med. 2000;192:145–150. doi: 10.1084/jem.192.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg–Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature. 1982;299:65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- Shaikh RB, Santee S, Granger SW, Buitrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2000;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Shin HH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumour necrosis factor receptor (rGITR) induced COX-2 activity in murine macrophage Raw 264.7 cells. Cytokine. 2002;19:187–192. doi: 10.1006/cyto.2002.1962. [DOI] [PubMed] [Google Scholar]
- Shin HH, Lee HW, Choi HS. Induction of nitric oxide synthase (NOS) by soluble glucocorticoid induced tumor necrosis factor receptor (sGITR) is modulated by IFN-gamma in murine macrophage. Exp Mol Med. 2003;35:175–180. doi: 10.1038/emm.2003.24. [DOI] [PubMed] [Google Scholar]
- Shin HH, Lee MH, Kim SG, Lee YH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumor necrosis factor receptor (rGITR) induces NOS in murine macrophage. FEBS Lett. 2000;514:275–80. doi: 10.1016/s0014-5793(02)02379-7. [DOI] [PubMed] [Google Scholar]
- Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, Brannan CI, Copeland NG, Jenkins NA, Grabstein KH, Gliniak B, McAlister IB, Fanslow W, Alderson M, Falk B, Gimpel S, Gillis S, Din WS, Goodwin RG, Armitage RJ. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I, Clark EA, Seed B. A B lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989;8:1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of Ox-40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Stuber E, Strober W. The T cell-B cell interactions via OX40/OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumura K, Ishii N, Wienberg AD. The therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nature Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fy YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002;8:1405–1413. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific Tcells – a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34:447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000a;164:4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, Ni J, Chen L. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000b;6:283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- Tamada K, Tamura H, Flies D, Fu YX, Celis E, Pease LR, Blazar BR, Chen L. Blockade of LIGHT/LTβ and CD40 signaling induces allospecific T cell anergy, preventing graft-versus-host disease. J Clin Invest. 2002;109:549–557. doi: 10.1172/JCI13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford WG, Nam SY, Podack ER, Miller RA. CD30-regulated apoptosis in murine CD8 T cells after cessation of TCR signals. Cell Immunol. 1997;182:125–136. doi: 10.1006/cimm.1997.1228. [DOI] [PubMed] [Google Scholar]
- Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- Toes RE, Schoenberger SP, van der Voort EI, Offringa R, Melief CJ. CD40-CD40Ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin Immunol. 1998;10:443–448. doi: 10.1006/smim.1998.0147. [DOI] [PubMed] [Google Scholar]
- Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–464. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- Uraushihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, Nakamura T, Watanabe M. Regulation of murine inflammatory bowel disease by CD25+ and CD25− CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol. 2003;171:708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- van Oers MH, Pals ST, Evers LM, van der Schoot CE, Koopman G, Bonfrer JM, Hintzen RQ, von dem Borne AE, van Lier RA. Expression and release of CD27 in human B-cell malignancies. Blood. 1993;82:3430–3436. [PubMed] [Google Scholar]
- van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RA. CD27–CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. Genes, transcripts, and proteins of CD137 receptor and ligand. In: Chen L, editor. CD137 pathway: immunology and diseases. Springer; New York: 2006a. pp. 1–14. [Google Scholar]
- Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006b;84:726–736. doi: 10.1007/s00109-006-0072-2. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Kim JD, Kwon BS. Amelioration of mercury-induced autoimmunity by 4-1BB. J Immunol. 2006c;177:5708–5717. doi: 10.4049/jimmunol.177.8.5708. [DOI] [PubMed] [Google Scholar]
- Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y, Tamada K, Chen L, Fu YX. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108:1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang Y, Wang J, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2004;113:826–835. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Bourdette DN, Sullivan TJ, Lemon M, Wallin JJ, Maziarz R, Davey M, Palida F, Godfrey W, Engleman E, Fulton RJ, Offner H, Vandenbark AA. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nature Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Evans DE, Thahofer C, Shi T, Prell RA. The generation of T cell memory: a review describing the molecular and cellular events following OX40 (CD134) engagement. J Leukoc Biol. 2004;75:962–972. doi: 10.1189/jlb.1103586. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Montler R. Modulation of TNF receptor family members to inhibit autoimmune disease. Curr Drug Targets Inflamm Allergy. 2005;4:195–203. doi: 10.2174/1568010053586345. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Wegmann KW, Funutake C, Whitham RH. Blocking Ox-40/Ox-40 ligand interaction in vitro and in vivo leads to decreased T-cell function and amelioration of EAE. J Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- Weinberg AD. Antibodies to Ox-40 (CD134) can identify and eliminate autoreactive T cells: implications for human auto-immune disease. Mol Med Today. 1998;4:76–83. doi: 10.1016/S1357-4310(97)01181-7. [DOI] [PubMed] [Google Scholar]
- Weinberg AD. OX40: targeted immunotherapy implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, Tamada K, Mittler RS, Tsuchiya H, Pardoll DM, Chen L. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Hendriks J, Langerak P, Jacobs H, Borst J. CD27 Is Acquired by Primed B Cells at the Centroblast Stage and Promotes Germinal Center Formation. J Immunol. 2004;172:7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- Yamada A, Salama AD, Sho M, Najafian N, Ito T, Forman JP, Kewalramani R, Sandner S, Harada H, Clarkson MR, Mandelbrot DA, Sharpe AH, Oshima H, Yagita H, Chalasani G, Lakkis FG, Auchincloss H, Jr, Sayegh MH. CD70 signaling is critical for CD28-independent CD8þ T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, Jiang GW, Lu J, Tan J, Ugustus M, Carter K, Rojas L, Zhu F, Lincoln C, Endress G, Xing L, Wang S, Oh KO, Gentz R, Ruben S, Lippman ME, Hsieh SL, Yang D. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142–1151. doi: 10.1172/JCI3492. [DOI] [PMC free article] [PubMed] [Google Scholar]