Summary
Under harsh environmental conditions Caenorhabditis elegans larvae undergo arrest and form dauer larvae that can attach to other animals to facilitate dispersal[1]. It has been argued that this phenomenon, called phoresy, represents an intermediate step towards parasitism[2, 3]. Indeed, parasitic nematodes invade their hosts as infective larvae, a stage that shows striking morphological similarities to dauer larvae[1]. While the molecular regulation of dauer entry in C. elegans involves insulin and TGF-ß signaling[4-8], studies of TGF-ß orthologues in parasitic nematodes did not provide evidence for a common origin of dauer and infective larvae[9-14]. To identify conserved candidate regulators between Caenorhabditis and parasitic nematodes we used an evolutionary approach involving Pristionchus pacificus as intermediate. We show by mutational and pharmacological analysis that Pristionchus and Caenorhabditis share the dafachronic acid-DAF-12 system as core endocrine module for dauer formation. One of the dafachronic acids, Δ7-DA, has a conserved role in the mammalian parasite Strongyloides papillosus where it controls entry into the infective stage. Application of Δ7-DA blocks formation of infective larvae and results in the generation of free-living animals. The conservation of this small molecule ligand represents a fundamental link between dauer and infective larvae and might provide a general strategy for nematode parasitism.
Results and Discussion
In C. elegans, pheromonal cues that indicate overcrowding, high temperature or starvation are processed through several signaling pathways including insulin/IGF, TGFβ-like, and guanylyl cyclase pathways (Fig. 1A) [4-8, 15-20]. This results in the decrease of a class of steroidal hormones, Δ4-DAfachronic acid and Δ7-DAfachronic acid (DAs) and shifts the nuclear hormone receptor DAF-12 from its ligand-bound form to a ligand-free form, which specifies the dauer fate [21-25]. DAF-12 is strictly required for C. elegans dauer development [26] and its systemic expression suggests a role in the specification of dauer fate at the level of individual tissues [24]. Despite the wealth of knowledge of dauer formation in C. elegans, the extent to which this paradigm applies to other nematodes is not comprehensively investigated. Pharmacological studies in Ancylostoma species, parasitic nematodes related to C. elegans (Fig. 1C), identified potential parallels in the recovery of infective and dauer larvae [27-29]. However, little is known about mechanisms involved in the specification of cell fates during the formation of the infective larvae. Furthermore, studies on the ortholog of daf-7 in Ancylostoma and other parasitic nematodes indicated substantial functional divergence during infective larvae formation [8-14]. Also, dauer pheromones of several rhabditid nematodes were found to be ineffective for C. elegans [15]. Only in the close relative C. briggsae several genes were found to play a similar role as in C. elegans [30].
Fig. 1.
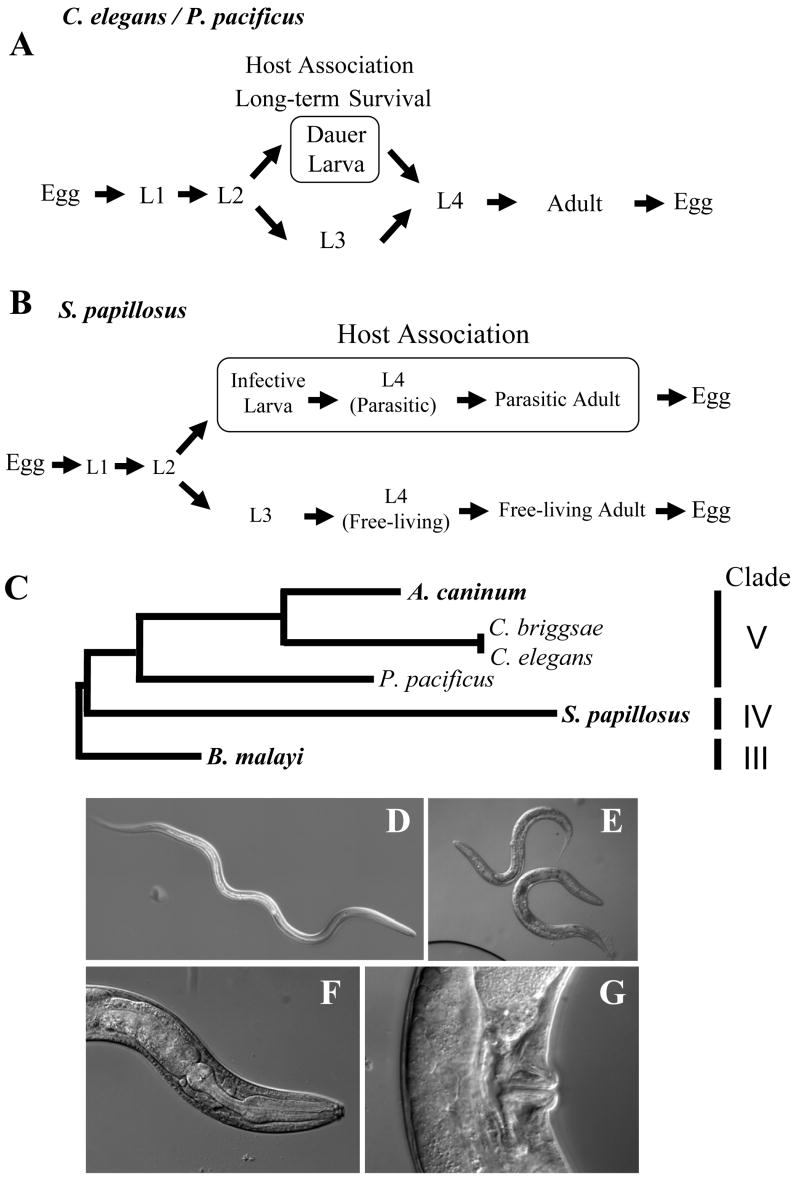
(A) Life cycles of the free-living nematodes C. elegans and P. pacificus. Under favorable conditions, animals go through direct development (L1-L4). In the laboratory, one cycle takes as little as 3-4 days (20° C). Under unfavorable conditions, such as high temperature, high population density or starvation, animals can go into the arrested dauer stage. Note that the importance of the dauer larvae for host association is best known for P. pacificus and its beetle association. (B) A simplified scheme for the life cycle of the vertebrate parasite Strongyloides papillosus. Note that not only the third larval stage, but also the fourth larval stage and the adult stage are specialized for each life style. For a more precise representation of S. papillosus life cycle, see Figure S2. (C) Phylogenetic relationship of the three species used in this study (P. pacificus, C. elegans and S. papillosus), with three other taxa C. briggsae, the hookworm A. caninum, and B. malayi. Parasitic species are indicated in bold. (D) Overall morphology of a S. papillosus infective larva. E-G Phenotype of Δ7-DA-treated progeny S. papillosus that show morphological characteristics of free-living adults. (E) Overall morphology of free-living adults. (F) Rhabdiform esophagus with a grinder in the pharynx, which is only known from free-living adults but not parasitic females. (G) Vulva opening in the mid-body region, a feature that is also only known from free-living females.
Given the complexity of the dauer regulatory network and the limited functional conservation of some dauer control genes, we used an evolutionary approach to identify candidate genes that might be shared with parasitic nematodes. Specifically, we involved a distant relative of C. elegans, P. pacificus as an intermediate (Fig. 1C). P. pacificus is a genetically tractable system that shares many features with C. elegans, such as a short generation time, easy laboratory culture, and a complete genome sequence [31]. Forward and reverse genetic analysis in P. pacificus and the deep position of the last common ancestor of Pristionchus and Caenorhabditis makes Pristionchus an ideal model for exploring the evolution of developmental mechanisms [32, 33]. In the wild, Pristionchus species associate with beetles as dauer larvae, which resume development only after the death of the beetle when they begin feeding on microbes on the carcass [34, 35]. Thus, the dauer stage is important for the adaptation of P. pacificus to its ecological niche.
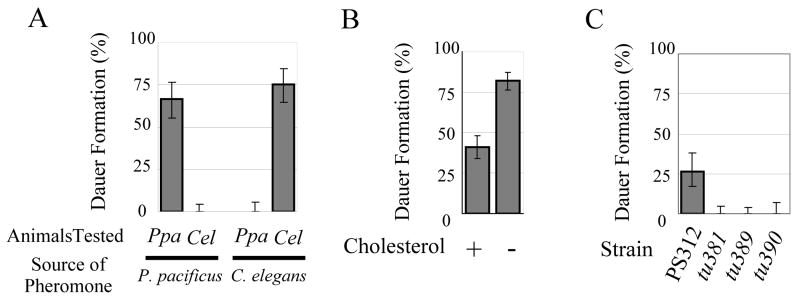
To explore evolutionarily conserved mechanisms of dauer formation, we first asked if a pheromone(s) controls dauer formation in P. pacificus. We found that supernatant prepared from liquid P. pacificus cultures induced eggs and J2 larvae to enter the dauer stage (data not shown). Next, we tested if P. pacificus and C. elegans employ different pheromones by preparing concentrated pheromone extracts from supernatants (Fig. 2A) [36]. In plates containing P. pacificus pheromone extracts, P. pacificus animals were induced to form dauer, whereas only few C. elegans N2 animals went into dauer (p<0.01). Similarly, C. elegans pheromone induced dauer formation in C. elegans, but not in P. pacificus (p<0.01). Thus, P. pacificus and C. elegans use distinct dauer pheromones, a result similar to previous studies with nematodes more closely related to C. elegans [15].
Fig. 2.
Dauer formation assays in P. pacificus wild type and mutant animals. (A) Dauer formation of P. pacificus PS312 (Ppa) and C. elegans N2 (Cel) on agar plates containing either P. pacificus or C. elegans pheromone. (B) Cholesterol restriction enhances dauer formation in P. pacificus PS312. Dauer formation was tested on pheromone plates (as in B) in the presence (5μg/ml) or absence of added cholesterol. (C) Daf-d phenotypes of the three Ppa-daf-12 alleles, tu381, tu389, and tu390. Dauer formation of wild type (PS312) and mutant animals was tested on agar plates containing the same amount of pheromone extract. Error bars denote 95% confidence intervals. Note that the experiments described in Figures 2 A, B and C, were performed with different batches and different amounts of pheromone. See Table S1 for number of animals tested.
In C. elegans, depletion of cholesterol from culture medium induces dauer formation [37]. This is because C. elegans relies on an environmental source for the precursor of the DA hormone, which through binding to DAF-12 inhibits dauer formation. Under laboratory conditions, cholesterol in the medium serves as precursor of DA. To test if cholesterol plays a similar role in P. pacificus dauer formation, we examined the effect of cholesterol restriction in the dauer entry assay. When grown on an agar plate containing pheromone without added cholesterol, a significantly higher number of dauer larvae were formed than on plates with added cholesterol (p<0.01), suggesting that as in C. elegans sterol hormones might regulate dauer formation in P. pacificus (Fig. 2B).
To test if a DA-DAF-12 system controls dauer formation, we set out to isolate dauer formation defective (Daf-d) mutants in forward genetic screens in P. pacificus. To identify Ppa-daf-12 alleles that would be epistatic to cholesterol depletion, we screened for Daf-d mutants using liquid medium without cholesterol. Three Daf-d mutants mapped to the Ppa-daf-12 region of the genome and molecular analysis indeed showed that they carry a molecular lesion in Ppa-daf-12 (Fig. S4). Ppa-daf-12 encodes a nuclear hormone receptor, which shows >92% and 54% amino acid sequence identity to Cel-DAF-12, in the DNA binding domain (DBD) and ligand binding domain (LBD), respectively (Fig. S4B, C). Three segments of the DBD are important for DNA recognition, the proximal (P), distal (D), and direct repeat (DR) boxes, respectively [38, 39]. All three segments are completely conserved, suggesting conservation of the DNA recognition specificity. The three mutations in Ppa-daf-12 are nonsense and splice acceptor mutations that truncate the LBD and parts of the hinge region (Fig. S4A). None of the Ppa-daf-12 mutants formed dauer larvae on an agar plate containing dauer pheromone indicating that Ppa-DAF-12 is essential for dauer entry (Fig. 2C).
Next we wanted to know if DAs, the steroid compounds that serve as small molecule ligands for the DAF-12 receptor, are also conserved between P. pacificus and C. elegans. In C. elegans, DAs can rescue dauer formation constitutive (Daf-c) phenotypes resulting from mutations in multiple signaling pathways acting upstream of DAF-12 (ref. 5). In forward genetic screens for P. pacificus Daf-c mutants, we isolated two incompletely penetrant and two fully penetrant mutations. These mutations map to four separate chromosomal regions (Fig. S1). We tested if Δ4- DA and Δ7-DA could rescue these Ppa-Daf-c mutants. Administration of Δ7-DA strongly suppressed dauer formation in all four P. pacificus Daf-c strains p<0.01; Fig. 3A). Δ4-DA rescued two incompletely penetrant mutations (p<0.01 for both strains), but did not significantly affect the two fully penetrant mutations (p=0.11, and p= 0.56 for Ppa-dfc-3 and Ppa-dfc-4, respectively). These results suggest that DA/DAF-12 is a conserved endocrine module and represents a candidate for conservation in parasitic species.
Fig. 3.
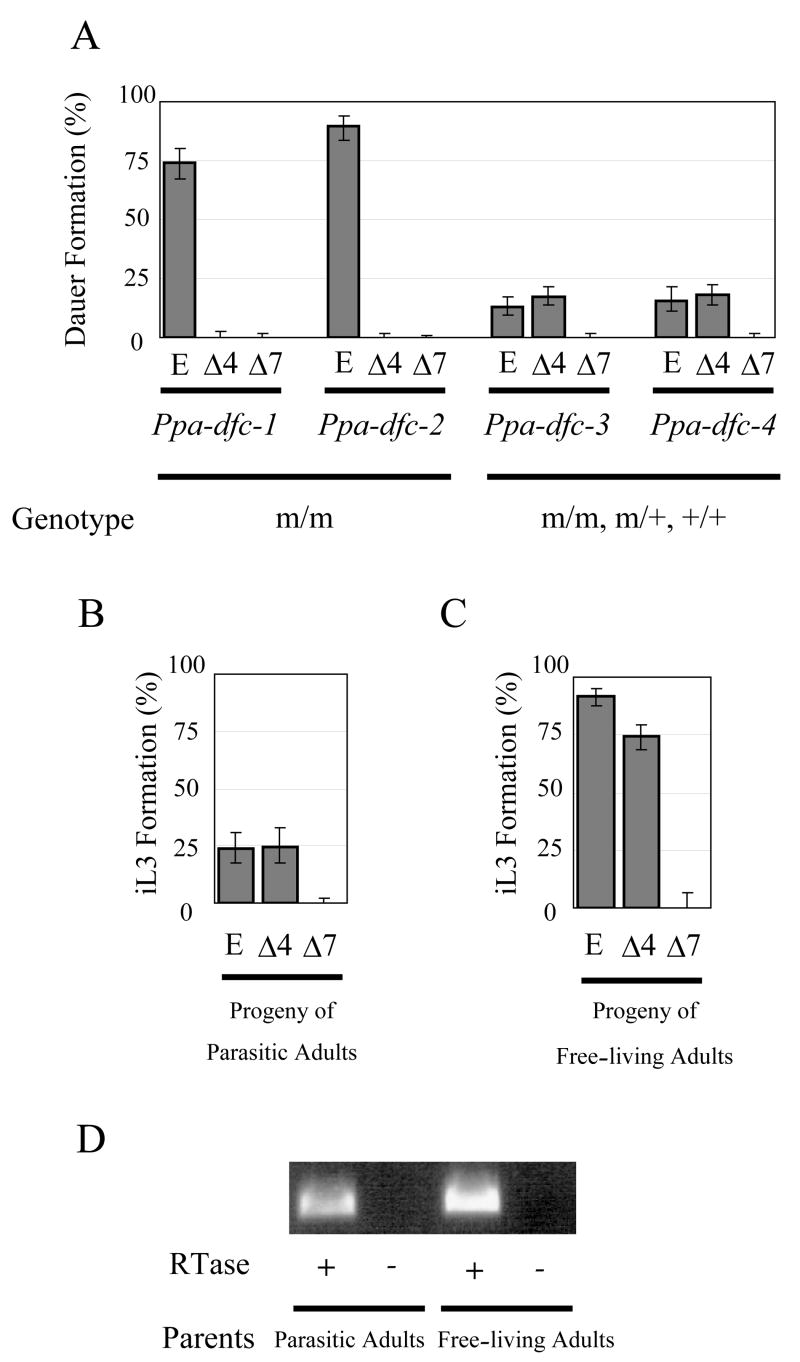
Dauer formation assays in P. pacificus (A) and infective larvae formation assay in S. papillosus (B, C). (A) Effect of DAs on dauer formation on four P. pacificus Daf-c strains. Mutant animals were grown in the presence of either ethanol (E; control), Δ4-DA (Δ4; 250nM), or Δ7-DA (Δ7; 250nM). For two fully penetrant mutants Ppa-dfc-3(tu393) and Ppa-dfc-4(tu394), mixed populations of homozygous, heterozygous and wild type eggs, which are F1 and F2 progeny of single heterozygous hermaphrodites, were tested. Therefore less than 25% of animals are expected to be mutant. Ppa-dfc-1(tu391) and Ppa-dfc-2(tu392) were tested as homozygous animals. (B) Infective third stage larvae (iL3) formation of the progeny of S. papillosus parasitic females in the presence of ethanol (E; control), Δ4-DA (Δ4; 250nM), or Δ7-DA (Δ7; 250nM). (C) iL3 formation of the progeny of S. papillosus free-living adults in the presence of ethanol (E; control), Δ4-DA (Δ4; 250nM), or Δ7-DA (Δ7; 250nM). Note that all the non-iL3s in the plates with ethanol and Δ4-DA were small larvae that were presumably younger than the third stage arrested larvae. The majority of non-iL3s in the plate with Δ7-DA were females as shown in Fig. 1E. Error bars denote 95% confidence intervals. See Table S1 for number of animals tested. (D) RT-PCR amplification of a partial cDNA of Spa-daf-12. RNA was prepared from young progeny of parasitic and free-living adults. Reverse transcriptase (RTase) was omitted in the negative control. The identity of the amplified fragments was verified by DNA sequencing (data not shown)
While many nematodes are obligate parasites with limited access for experimental studies, some species retain a free-living life cycle in addition to the parasitic life cycle. One such example is Strongyloides papillosus, a parasite of ruminants that can be cultured in rabbits under laboratory conditions [40]. Phylogenetically, Strongyloides is an outgroup to Caenorhabditis and Pristionchus and represents a clade IV nematode according to the phylogeny of Blaxter et al (1998), whereas Caenorhabditis, Pristionchus and the hookworm Ancylostoma are all clade V nematodes (Fig. 1C) [32, 33]. S. papillosus has a complex life history in which parthenogenetic females of S. papillosus in the small intestine of the host produce eggs that are released with the feces (Fig. 1B). These eggs can either become infective female larvae (Fig. 1D) developing into parasitic females or become free-living males and females, which produce offspring that become parasites and are all females [41, 42].
To test if DAs control infective larvae formation of S. papillosus, we collected young larvae from feces of a rabbit infected with parasitic females. When we grew these larvae on agar plates containing Δ4-DA or Δ7-DA, we observed a complete suppression of the formation of infective larvae by Δ7-DA (p<0.01) (Fig. 3B). In contrast, the same concentration of Δ4-DA did not show any significant effect (p=0.90). These results indicate that Δ7-DA can completely inhibit the development of parasitic females from feces-derived larvae under laboratory conditions.
Next we asked if the progeny of free-living adults, all of which usually form infective larvae, could be prevented from developing into infective larvae. We found that administration of Δ7-DA completely prevented infective larvae formation (p<0.01) (Fig. 3C). Instead, the Δ7-DA treated animals developed into individuals with a rhabdiform esophagus characteristic of free-living animals, and a mid-body vulva characteristic of adult females (Fig. 1E-G). No males were found in the Δ7-DA treated progeny of free-living adults. However female adults obtained by the Δ7-DA treatment could mate with the male progeny of parasitic females, and laid eggs that again developed to a free-living adult upon treatment with Δ7-DA (data not shown). These results suggest that Δ7-DA negatively regulates the formation of infective larvae in S. papillosus and that exogenous administration of Δ7-DA redirects the development to an additional free-living life cycle. Consistently, we could amplify cDNA sequences encoding putative Spa-daf-12 from the transcripts of young progeny of parasitic and free-living adults by RT-PCR (Figs. S4B,C; 3D).
The functional conservation of the small molecule ligand Δ7-DA has several evolutionary and practical implications. First, DA-DAF-12 represents an endocrine signaling module of a kind that is a common feature in the regulation of adaptive phenotypic plasticity [43, 44]. Endocrine signaling was suggested as a useful mechanism for controlling phenotypic plasticity because it allows organisms to modulate gene expression in response to environmental fluctuation in a fast and systemic manner. In addition, susceptibility of endocrine systems to both environmental and genetic perturbation may produce phenotypic variations, facilitating evolutionary change. While many studies suggested the conservation of developmental regulation by steroid hormones within insects and within vertebrates [44], no such conserved mechanism has been known to exist in nematodes. This study provides evidence for a conserved steroid signaling in nematodes and builds a platform on which to study the evolution of life histories and its genetic and environmental regulation.
Second, the similar effect of Δ7-DA on C. elegans [21], P. pacificus and S. papillosus supports a common origin of dauer and infective larvae in these species and leads to hypotheses on the evolution of parasitism. Nematode parasitism has evolved multiple times from free-living ancestors [33]. Parasitic nematodes often infect their hosts as specialized infective larvae and their morphological similarities to dauer larvae led to the suggestion that infective larvae might have evolved from dauer larvae of free-living species [1-3]. The conservation of Δ7-DA function suggests that the dauer and infective larvae indeed share a common regulatory basis for cell fate specification, and is consistent with the notion that infective larvae evolved from dauer larvae. The evolution of the parasitic life cycle in S. papillosus involved the diversification of the post-dauer development from the post-L3 development to produce highly specialized parasitic and free-living adults, respectively (Fig. 1B). We hypothesize that the gradual evolution of parasitism has been facilitated by phenotypic plasticity [44] enabling the retention of a bacteria-feeding free-living life cycle while already shaping an alternative feeding strategy. Many other parasitic nematodes infect their hosts as dauer-like arrested larvae [1]. In most cases, development to such infective larvae is obligatory. Although the regulatory mechanisms and origin of infective larvae in these species need to be verified individually, specialization of post-dauer development to produce parasitic morphs and the subsequent loss of ancestral free-living life cycle, could serve as a useful working hypothesis for the evolution of parasitism. Finally our data suggest that a DAF-12 ligand with a 3-keto-5α-7-ene (Δ7-DA) rather than a 3-keto-4-ene (Δ4-DA) ring structure [21] might be more strongly conserved. The deep conservation of this small molecule ligand has obvious pharmacological implications for the prevention of problems caused by nematodes.
Supplementary Material
Supplementary Data include Experimental Procedures, three figures and one table.
Acknowledgments
We thank E. J. Corey for providing us Δ7-DA, T. Kawano for advice on pheromone purification, J. Wollam for help in DA assays, A. Eberhardt and L. Nemetschke for help in experiments with S. papillosus, M. Riebesell for help in microscopy, I. Dinkelacker, H. Witte, W. Röseler, H. Haussmann and A. Walther for technical assistance, K, Bomblies, M. Harris, R. Hong, B. Schlager, G. Bento, and M. Herrmann for critically reading the manuscript, and other members of Sommer laboratory for discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee DL, editor. The Biology of Nematodes. New York: Taylor and Francis; 2002. [Google Scholar]
- 2.Anderson RC. The Origins of Zooparasitic Nematodes. Canadian Journal of Zoology. 1984;62:317–328. [Google Scholar]
- 3.Poulin R. Evolutionary Ecology of Parasites. 2. Princeton: Princeton University Press; 2007. [Google Scholar]
- 4.Estevez M, Attisano L, Wrana JL, Albert PS, Massague J, Riddle DL. The Daf-4 Gene Encodes a Bone Morphogenetic Protein-Receptor Controlling C. elegans Dauer Larva Development. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- 5.Hu PJ. Dauer. WormBook; 2007. pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 7.Kimura KD, Tissenbaum HA, Liu YX, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 8.Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 9.Viney ME, Thompson FJ, Crook M. TGF-beta and the evolution of nematode parasitism. Int J Parasitol. 2005;35:1473–1475. doi: 10.1016/j.ijpara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Brand AM, Varghese G, Majewski W, Hawdon JM. Identification of a DAF-7 ortholog from the hookworm Ancylostoma caninum. Int J Parasitol. 2005;35:1489–1498. doi: 10.1016/j.ijpara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Crook M, Thompson FJ, Grant WN, Viney ME. daf-7 and the development of Strongyloides ratti and Parastrongyloides trichosuri. Mol Biochem Parasitol. 2005;139:213–223. doi: 10.1016/j.molbiopara.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Freitas TC, Arasu P. Cloning and characterisation of genes encoding two transforming growth factor-beta-like ligands from the hookworm, Ancylostoma caninum. Int J Parasitol. 2005;35:1477–1487. doi: 10.1016/j.ijpara.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massey HC, Castelletto ML, Bhopale VM, Schad GA, Lok JB. Sst-tgh-1 from Strongyloides stercoralis encodes a proposed ortholog of daf-7 in Caenorhabditis elegans. Mol Biochem Parasitol. 2005;142:116–120. doi: 10.1016/j.molbiopara.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 16.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 17.Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 18.Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 20.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 21.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Giroux S, Corey EJ. Stereocontrolled synthesis of dafachronic acid A, the ligand for the DAF-12 nuclear receptor of Caenorhabditis elegans. J Am Chem Soc. 2007;129:9866–9867. doi: 10.1021/ja074306i. [DOI] [PubMed] [Google Scholar]
- 23.Antebi A, Culotti JG, Hedgecock EM. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development. 1998;125:1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- 24.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 25.Snow MI, Larsen PL. Structure and expression of daf-12: a nuclear hormone receptor with three isoforms that are involved in development and aging in Caenorhabditis elegans. Biochim Biophys Acta. 2000;1494:104–116. doi: 10.1016/s0167-4781(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 26.Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 29.Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue T, Ailion M, Poon S, Kim HK, Thomas JH, Sternberg PW. Genetic Analysis of Dauer Formation in Caenorhabditis briggsae. Genetics. 2007;177:809–818. doi: 10.1534/genetics.107.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong RL, Sommer RJ. Pristionchus pacificus: a well-rounded nematode. Bioessays. 2006;28:651–659. doi: 10.1002/bies.20404. [DOI] [PubMed] [Google Scholar]
- 32.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 33.Holterman M, van der Wurff A, van den Elsen S, van Megen H, Bongers T, Holovachov O, Bakker J, Helder J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown Clades. Mol Biol Evol. 2006;23:1792–1800. doi: 10.1093/molbev/msl044. [DOI] [PubMed] [Google Scholar]
- 34.Dieterich C, Clifton SW, Schuster LN, Chinwalla A, Delehaunty K, Dinkelacker I, Fulton L, Fulton R, Godfrey J, Minx P, Mitreva M, Roeseler W, Tian H, Witte H, Yang SP, Wilson RK, Sommer RJ. The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet. 2008;40:1193–1198. doi: 10.1038/ng.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, Sommer RJ. The Nematode Pristionchus pacificus (Nematoda: Diplogastridae) Is Associated with the Oriental Beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zoolog Sci. 2007;24:883–889. doi: 10.2108/zsj.24.883. [DOI] [PubMed] [Google Scholar]
- 36.Kawano T, Kataoka N, Abe S, Ohtani M, Honda Y, Honda S, Kimura Y. Lifespan extending activity of substances secreted by the nematode Caenorhabditis elegans that include the dauer-inducing pheromone. Biosci Biotechnol Biochem. 2005;69:2479–2481. doi: 10.1271/bbb.69.2479. [DOI] [PubMed] [Google Scholar]
- 37.Matyash V, Entchev EV, Mende F, Wilsch-Brauninger M, Thiele C, Schmidt AW, Knolker HJ, Ward S, Kurzchalia TV. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2:e280. doi: 10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perlmann T, Rangarajan PN, Umesono K, Evans RM. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993;7:1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- 39.Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 40.Eberhardt AG, Mayer WE, Streit A. The free-living generation of the nematode Strongyloides papillosus undergoes sexual reproduction. Int J Parasitol. 2007;37:989–1000. doi: 10.1016/j.ijpara.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Streit A. Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology. 2008;135:285–294. doi: 10.1017/S003118200700399X. [DOI] [PubMed] [Google Scholar]
- 42.Viney ME, Lok JB. Strongyloides spp. WormBook; 2007. pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nijhout HF. Development and evolution of adaptive polyphenisms. Evolution and Development. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- 44.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data include Experimental Procedures, three figures and one table.