Abstract
The genus Anaplasma (Rickettsiales: Anaplasmataceae) includes obligate tick-transmitted intracellular organisms, Anaplasma phagocytophilum and Anaplasma marginale that multiply in both vertebrate and tick host cells. Recently, we showed that A. marginale affects the expression of tick genes that are involved in tick survival and pathogen infection and multiplication. However, the gene expression profile in A. phagocytophilum-infected tick cells is currently poorly characterized. The objectives of this study were to characterize tick gene expression profile in Ixodes scapularis ticks and cultured ISE6 cells in response to infection with A. phagocypthilum and to compare tick gene expression responses in A. phagocytophilum- and A. marginale-infected tick cells by microarray and real-time RT-PCR analyses. The results of these studies demonstrated modulation of tick gene expression by A. phagocytophilum and provided evidence of different gene expression responses in tick cells infected with A. phagocytophilum and A. marginale. These differences in Anaplasma-tick interactions may reflect differences in pathogen life cycle in the tick cells.
1. Introduction
Ticks transmit pathogens that greatly impact both human and animal health [1]. The genus Anaplasma (Rickettsiales: Anaplasmataceae) includes obligate tick-transmitted intracellular organisms found exclusively within membrane-bound inclusions or vacuoles in the cytoplasm of both vertebrate and tick host cells [2, 3]. A. marginale infects cattle and wild ruminants and causes bovine anaplasmosis [2]. A. phagocytophilum infects humans and wild and domesticated animals [2, 4, 5] and is the causative agent of human, equine and canine granulocytic anaplasmosis and tick-borne fever of ruminants [5, 6]. In the United States, A. phagocytophilum is transmitted by Ixodes scapularis and I. pacificus [2, 4].
The ticks and the pathogens they transmit have evolved molecular interactions that affect their survival and life cycle [3]. The A. phagocytophilum outer membrane proteins that are involved in interactions with tick cells have been identified and partially characterized [7, 8]. Recently, we identified and characterized tick molecules that are involved in A. marginale-tick interactions, demonstrating that A. marginale affects the expression of tick genes essential for tick survival and pathogen infection and multiplication [9]. However, tick molecules that are affected by and participate in A. phagocytophilum infection and multiplication are currently poorly characterized [10, 11].
The objectives of this study were the characterization of tick gene expression profiles in I. scapularis ticks and cultured tick cells in response to infection with A. phagocytophilum and to compare tick gene expression responses in A. phagocytophilum- and A. marginale-infected cultured tick cells by microarray and real-time RT-PCR analyses. The results reported herein demonstrated modulation of tick gene expression by A. phagocytophilum and identified differentially expressed genes that are relevant for the understanding of basic biological questions of A. phagocytophilum life cycle in I. scapularis. The results also provided evidence of differences in tick gene expression in response to infection with A. phagocytophilum or A. marginale.
2. Materials and Methods
2.1. Uninfected and Anaplasma-Infected Ticks and Tick Cells
The I. scapularis nymphs uninfected and infected with A. phagocytophilum (Gaillard and Dawson strains) were obtained from a laboratory colony reared at the Centers for Disease Control and Prevention, Atlanta, Ga, USA. Tick larvae were fed on uninfected or infected mice, collected after feeding, and allowed to molt to nymphs. Animals were housed with the approval and supervision of the Institutional Animal Care and Use Committee.
The tick cell line ISE6, derived from I. scapularis embryos (provided by U.G. Munderloh, University of Minnesota, USA), was cultured in L15B medium as described previously for IDE8 cells [12], but the osmotic pressure was lowered by the addition of one-fourth sterile water by volume after Munderloh et al. [13]. The ISE6 cells were first inoculated with A. phagocytophilum- (NY18 isolate) infected HL-60 cells and maintained according to Munderloh et al. [13] until infection was established and routinely passaged. Infected ISE6 cells were frozen in liquid nitrogen and served as inoculum for uninfected cells. The ISE6 cells were initially infected with A. marginale (Virginia isolate) from a frozen inoculum of infected bovine erythrocytes. Following infection and routine passage, infected ISE6 cells were frozen in liquid nitrogen and served to infect uninfected ISE6 cells. For the current study each inoculum of infected cells was thawed and centrifuged, and the pellet was resuspended in culture medium and put on the ISE6 cells. When the infection reached approximately 80% of the tick cells, the monolayer was passaged onto uninfected ISE6 monolayers and maintained in L15B medium as described above. Monolayers of infected ISE6 cells were collected at different time points as described above. Uninfected cells were cultured in the same way but adding 1 mL of culture medium instead of infected cells. Collected cells were centrifuged at 10 000 × g for 3 minutes, and cell pellets were frozen in liquid N until used for RNA extraction.
The infection of ticks and tick cells with A. phagocytophilum or A. marginale was corroborated by major surface protein 4 (msp4) PCR [14, 15].
2.2. Microarray Analysis
Infected tick ISE6 cells were sampled at 6 days postinfection (dpi) with approximately 70% infected cells (separate cell cultures grown under similar conditions had >90% cells infected at 8 dpi). Uninfected cells were sampled at the same time point as infected cells to account for culture time effects. Total RNA was extracted from three A. marginale-infected, three A. phagocytophilum-infected, and three uninfected ISE6 cell cultures using the RNeasy Mini Kit (Qiagen) including the on-column DNA digestion with the RNase-free DNase set following manufacturer's instructions. RNA quality was checked by gel electrophoresis to verify the integrity of RNA preparations. Total RNAs (5 μg) were labeled using the 3DNA Array900 kit with Alexa Fluor dyes (Genisphere, Hatfield, Pa, USA), Superscript II (Invitrogen, Carlsbad, Calif, USA), the supplied formamide-based hybridization buffer and 24 × 60 mm LifterSlips (Erie Scientific, Portmouth, NH, USA) according to the manufacturer's (Genisphere) instructions. The microarray was constructed with 768 random I. scapularis sequences enriched for genes differentially expressed after subolesin knockdown as previously described [16] (NCBI Gene Expression Omnibus (GEO) platform accession number GPL6394 and series number GSE10222). Eight pools of 12 clones each from an unsubtracted I. scapularis cDNA library and subolesin cDNA were also arrayed and used to validate normalization. Hybridization signals were measured using a ScanArray Express (PerkinElmer, Boston, Mass, USA), and the images were processed using GenePix Pro version 4.0 (Axon, Union City, Calif, USA). Ratios were calculated as Anaplasma-infected cells versus uninfected control cells. Preprocessing of data was accomplished using R-project statistical environment (http://www.r-project.org) and Bioconductor (http://www.bioconductor.org) and the LIMMA package as previously described [17]. This included (1) removal of data points where signal was less than the background plus two standard deviations in both channels, (2) removal of data points where signal was less than 200 RFU in both channels, (3) removal of poor quality spots flagged during image processing, (4) removal of spots with less than 50% valid biological and technical replicates, (5) log transformation of the background subtracted mean signal ratios, and (6) normalization using global Lowess intensity-dependent normalization. Normalized ratio values obtained for each probe were averaged across 3 biological replicates, and four technical replicates and significant differences were defined as P-value ≤ .05 and displaying an expression fold change greater than 2-fold in either A. phagocytophilum or A. marginale infected cells.
2.3. Sequence Analysis and Database Search
Partial sequences were determined for cDNA sequences identified as differentially expressed in the microarray analysis. Multiple sequence alignment was performed using the program AlignX (Vector NTI Suite V 8.0, InforMax, Invitrogen, Carisbad, Calif, USA) to exclude vector sequences and to identify redundant (not unique) sequences. Searches for sequence similarity were performed with the BLASTX program (http://www.ncbi.nlm.nih.gov/BLAST) against the nonredundant sequence database (nr) and databases of tick specific sequences (http://www.vectorbase.org/index.php; http://compbio.dfci.harvard.edu/tgi/).
2.4. Real-Time Reverse Transcription (RT)-PCR Analysis
Total RNA was extracted from uninfected and A. phagocytophilum-infected and A. marginale-infected ISE6 cells (three cultures each) and A. phagocytophilum-infected I. scapularis whole unfed nymphs (three groups of uninfected ticks, three groups of ticks infected with the Gaillard strain, and three groups of ticks infected with the Dawson strain with 10 nymphs each) using the RNeasy Mini Kit (Qiagen) including the on-column DNA digestion with the RNase-free DNase set following manufacturer's instructions. Infected tick cells were sampled at a single time point at 3 dpi with approximately 40% infected cells (companion cultures were terminal at 8 dpi) or at 2, 5, and 8 dpi. When included in the analysis, uninfected cells were sampled at the same time point as infected cells to account for culture time effects. Two primers were synthesized based on the sequences determined for candidate differentially expressed genes for real-time RT-PCR analysis. Real-time RT-PCR was done using the QuantiTec SYBR Green RT-PCR kit (Qiagen, Valencia, Calif, USA) and a Bio-Rad iQ5 thermal cycler (Hercules, Calif, USA) following manufacturer's recommendations. Reactions were done for 40 cycles and 30 seconds annealing using oligonucleotide primers and annealing temperatures described in Table 1. Negative controls included reactions without RNA. mRNA levels were normalized against tick β-actin or 16S rRNA using the comparative Ct method and compared between infected and uninfected tick cells and ticks or between A. phagocytophilum- and A. marginale-infected tick cells by Student's t-test (P = .05).
Table 1.
RT-PCR oligonucleotide primers and conditions for the characterization of the expression profiles of differentially expressed tick genes.
| Gene ID(a) | Upstream/downstream primer sequences | PCR annealing temperature |
|---|---|---|
| 1I1H6 | GGTACATGGAATCCGACTGC | 54°C |
| GTCCCCTTTTGCTTCGACTT | ||
| 1I3A8 | GACGCAAAACTTCCTTCGAG | 54°C |
| GCACTTTCCAAGAGCCTGAC | ||
| 1I3F5 | GCTTTCACGTTTTCGATGGT | 50°C |
| GGCAAAGATCCAAGACAAGG | ||
| 1I3H6 | GCCTAGGGAGGACGTCGTAG | 50°C |
| ACGTGGAACACATCGAGTCA | ||
| 1I4C6 | AATGCGAGACACTGGAGGAC | 50°C |
| AATCCAGGAATGTTGCCAAG | ||
| 1I4G12 | GACGGACCTTGTCCGACTAC | 53°C |
| ATTCCCTCCTTGTCCTGGAT | ||
| 1I5B9 | CGTCCCCTTCTGTGGAATTA | 53°C |
| TCATCGTTGTTCTGGTCTCG | ||
| 2I1C2 | GAGACCATCAAGTGGCTGGA | 53°C |
| CTTGGTGATGATGGGGTTG | ||
| 2I1F6 | CAACCCCAAGATCGTCAACT | 53°C |
| ACGCGTCCTTACGTTTCACT | ||
| 2IP10 | TCTTGCCGGTCAGAGTCTTT | 53°C |
| GAAGGCGAAAATTCAGGACA | ||
| 2I3A3 | TAAAACCCCTTTCCCCACTT | 53°C |
| GCACTCGAACCTAGCAAACC | ||
| 2I3A7 | TCGACTCTGTTCAGGAGGAAG | 53°C |
| GGTCCAAATGGCAGAGCAT | ||
| 2I3G1 | AGGAAGTGCACGATGATGG | 54°C |
| GGTTGGTTATCCTCTGGGAGA | ||
| 2I4F6 | CTTTCTTGCCGTGCTTCTTT | 53°C |
| GCTCAACTTCCTCGTCGTTC | ||
| UP8 | CCTCCCTCGCTAACCTCTCT | 54°C |
| ATCGTCACGGTCGAAGTAGC | ||
| U2A8 | GCTCATCGTCGCCAACAT | 54°C |
| GAGTTCCTCCGTCCAGCTC | ||
| C2E6 | GTAAAGCCCGCTCTCAAGAA | 53°C |
| CATTCGGGTTTGTCCACAG | ||
| C3B2 | GAGTAGTGCCCGTCTTCGAC | 53°C |
| AGGTGATGCTGCCCTTGTAG | ||
| C4G3 | AACTGCCTTGGAGTTGCAGT | 53°C |
| CTTGTGTCCCAGGTGGAAGT | ||
| C4B10 | GTTCTTCTAACGGCCACTGC | 53°C |
| AGTCTTTGGTGCAAGCGAGT | ||
| R1E12 | ATGTGAAGCTGAGGCCAAAC | 53°C |
| GGAATTCGATTAGCGTGGTC | ||
| R4G5 | CCTTCCCTGCAATGTCAAAT | 53°C |
| CACAAGTGGGCAATCAACAC | ||
| Beta actin | GAGAAGATGACCCAGATCA | 50°C |
| (AF426178) | GTTGCCGATGGTGATCACC | |
| 16S rRNA | GACAAGAAGACCCTA | 42°C |
| (L34293) | ATCCAACATCGAGGT |
(a)IDs for I. scapularis genes are described herein and in de la Fuente et al. [9].
The A. phagocytophilum and A. marginale infection levels were evaluated in tick ISE6 cells at 2, 5, and 8 dpi by real-time PCR of msp4 and normalizing against tick 16S rDNA sequences using the QuantiTec SYBR Green PCR kit (Qiagen) in an iQ5 thermal cycler (Bio-Rad) as described above. Known amounts of the full length A. phagocytophilum and A. marginale msp4 PCR product were used to construct a standard for quantitation of pathogens per cell.
2.5. Nucleotide Sequence Accession Numbers
The nucleotide sequences of the ESTs reported in this paper have been deposited in the GenBank database under accession numbers FL685631-FL685658.
3. Results
3.1. A. phagocytophilum Modulates Gene Expression in Infected I. scapularis Nymphs and Tick ISE6 Cells
The infection with A. marginale has been shown to modulate tick gene expression [9]. However, the effect of A. phagocytophilum infection on tick gene expression is unknown. Here, two experimental approaches were used to characterize gene expression profiles in tick cells infected with A. phagocytophilum. In the first approach, tick gene expression was characterized by microarray analysis of RNA from infected and uninfected tick ISE6 cells. In the second approach, genes identified as differentially expressed in tick IDE8 cells and ticks infected with A. marginale were used to characterize the effect of A. phagocytophilum on tick gene expression in infected I. scapularis nymphs and tick ISE6 cells by real-time RT-PCR.
The microarray analysis showed in A. phagocytophilum-infected tick ISE6 cells the upregulation of genes C4B10 with homology to von Willebrand factor and R1E12 with homology to ribosomal protein L32, C4A10, C3C3, C4A1, and C3D9 with unknown function and the downregulation of genes C3B2 with homology to an aspartic protease and R2A12, C3A7, R2G1, R2D6, C3C11, and R3D4 with unknown function (Table 2). The expression of other genes with homology to troponin I (C2E6), putative secreted salivary protein (C4G3), and ML domain-containing protein (R4G5) did not change after infection of tick ISE6 cells with A. phagocytophilum (Table 2).
Table 2.
Microarray analysis of gene expression profile in A. marginale- and A. phagocytophilum-infected and -uninfected tick ISE6 cells.
| Probe ID(a) | Description(b) | A. marginale infection versus control | A. phagocytophilum infection versus control | ||
| Fold change(c) | SD(d) | Fold change(c) | SD(d) | ||
|
| |||||
| C4A10 | No homolog found | 6.249 | 0.000 | 2.208 | 0.294 |
| R1A6 | No homolog found | 2.539 | 0.162 | 1.049 | 0.346 |
| C3C5 | [Genbank:L22271] internal transcribed spacer 1 (Ixodes dammini) | 2.406 | 1.211 | 1.383 | 0.000 |
| C4A8 | No homolog found | 2.239 | 0.165 | 1.188 | 0.442 |
| R3A7 | No homolog found | 2.209 | 0.805 | −1.384 | 0.562 |
| C4G3 | [Genbank:AAY66629] putative secreted salivary protein (Ixodes scapularis) | 2.167 | 0.326 | 1.037 | 0.614 |
| C2E6 | [Genbank:ABB89211] troponin I protein (Rhipicephalus haemaphysaloides) | 2.040 | 0.400 | −1.068 | 0.442 |
| C3C3 | No homolog found | 1.916 | 0.559 | 3.422 | 1.037 |
| C4A1 | No homolog found | 1.857 | 0.000 | 2.121 | 0.517 |
| R2A12 | No homolog found | 1.199 | 0.232 | −2.219 | 0.450 |
| C3A7 | No homolog found | 1.135 | 0.282 | −3.028 | 0.141 |
| C3D9 | [Genbank:XP_791420] hypothetical protein (Strongylocentrotus purpuratus) | 1.076 | 0.335 | 4.875 | 2.069 |
| C3B2 | [Genbank:BAE53722] aspartic protease (Haemaphysalis longicornis) | −1.311 | 0.330 | −6.986 | 0.379 |
| R2G1 | No homolog found | −1.497 | 0.495 | −2.086 | 0.826 |
| R2D6 | No homolog found | −1.538 | 0.309 | −2.440 | 0.563 |
| C3C11 | No homolog found | −2.053 | 0.401 | −2.477 | 0.488 |
| C4D12 | No homolog found | −2.066 | 0.547 | −1.022 | 0.533 |
| R4G5 | [Genbank:AAP84098] ML domain-containing protein (Ixodes ricinus) | −2.066 | 0.161 | 1.020 | 0.266 |
| C4C9 | [Genbank:AAH56007] H13-prov protein (Xenopus laevis) | −2.070 | 0.270 | 1.081 | 0.689 |
| C4G11 | [Genbank:EAA09467] ENSANGP00000010016 (Anopheles gambiae) | −2.093 | 0.550 | −1.193 | 0.598 |
| C4G9 | No homolog found | −2.095 | 0.402 | −1.598 | 0.888 |
| R3F5 | [Genbank:AAY66764] putative secreted salivary protein (Ixodes scapularis) | −2.118 | 0.310 | −1.037 | 0.320 |
| R3G4 | No homolog found | −2.292 | 0.259 | 1.090 | 0.415 |
| R1F3 | No homolog found | −2.339 | 0.570 | −1.344 | 0.855 |
| R1E12 | [Genbank:NP_001119682] ribosomal protein L32 (Acyrthosiphon pisum) | −2.379 | 0.000 | 2.488 | 0.000 |
| C3F10 | [Genbank:AAM93633] putative secreted protein (Ixodes scapularis) | −2.386 | 0.545 | −1.647 | 0.315 |
| R3D4 | No homolog found | −2.529 | 1.046 | −2.377 | 0.518 |
| C4D2 | No homolog found | −2.702 | 0.860 | 1.043 | 0.972 |
| R3F4 | No homolog found | −2.928 | 0.298 | 1.174 | 0.396 |
| C1H10 | No homolog found | −3.341 | 0.307 | −1.057 | 0.000 |
| C4A4 | No homolog found | −3.678 | 1.181 | −1.430 | 0.331 |
| C4E12 | [Genbank:AAY66942] ribosomal protein S17 (Ixodes scapularis) | −3.964 | 0.822 | 1.430 | 0.993 |
| C4B10 | [Genbank:AAQ01562] von Willebrand factor (Ixodes ricinus) | −4.422 | 0.000 | 2.413 | 0.000 |
(a)Probe ID (library plate and well) identifies sample (clone) in stock plates.
(b)Description of the probe based on top (best) BLASTX alignment.
(c)Fold change is the fold change of Lowess intensity-dependent normalized log2 ratio of valid background-corrected means averaged between valid replicates. Only entries displaying an expression change greater than 2-fold and P < .05 in either A. phagocytophilum or A. marginale infected cells are shown. Positive and negative values correspond to genes upregulated and downregulated in infected cells, respectively.
(d)SD is the standard deviation determined from the normalized average log2 ratio but determined on data from valid spots only.
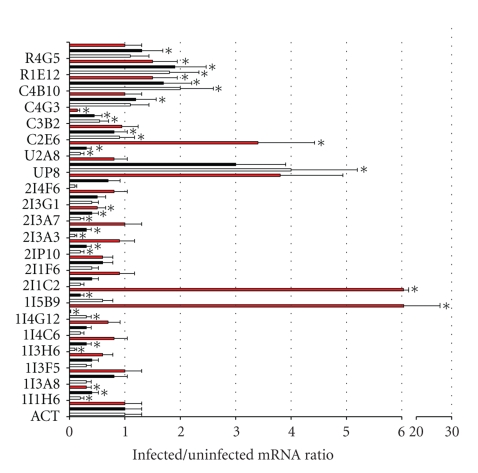
The mRNA levels of selected genes differentially expressed in A. phagocytophilum-infected tick ISE6 cells were evaluated by real-time RT-PCR in infected and uninfected cells (Figure 1). Similar to microarray hybridization results, the analysis of mRNA levels by real-time RT-PCR showed significant upregulation of C4B10 (von Willebrand factor) and R1E12 (ribosomal protein L32) and downregulation of C3B2 (aspartic protease) in tick ISE6 cells infected with A. phagocytophilum (Figure 1). The mRNA levels of genes identified previously as differentially expressed in tick IDE8 cells and ticks infected with A. marginale [9] were also evaluated by real-time RT-PCR in infected and uninfected tick ISE6 cells. The mRNA levels of genes differentially expressed in A. marginale-infected ISE6 cells were similar to those reported previously in infected IDE8 cells [9] and data not shown. The results in A. phagocytophilum-infected ISE6 cells showed that pathogen infection significantly upregulated the expression of U2A8 (signal sequence receptor delta), 1I5B9 (ixodegrin-2A RGD containing protein), and 1I4G12 (unknown function) and downregulated the expression of 2I3A7 (NADH-ubiquinoe oxidoreductase) and 1I1H6 (glutathione S-transferase (GST)) in tick ISE6 cells (Figure 1).
Figure 1.
Differential gene expression in A. phagocytophilum-infected I. scapularis ticks and cultured tick ISE6 cells. Real-time RT-PCR was done on uninfected and infected I. scapularis nymphs (three groups each of uninfected ticks, infected ticks (Gaillard strain; black bars) and infected ticks (Dawson strain; white bars) with 10 nymphs each) and uninfected and NY18 isolate-infected tick ISE6 cells (three independent cultures each; red bars). Bars represent the ratio between infected normalized Ct values/uninfected average normalized Ct values (+SD). The mRNA levels were normalized against tick β-actin (ACT) and compared between infected and uninfected ticks and tick cells by Student's t-test (*P ≤ .05).
The expression of selected genes was also analyzed in I. scapularis nymphs infected with two different A. phagocytophilum strains (Figure 1). In I. scapularis nymphs, the expression of C4G3 (putative secreted salivary protein), C4B10 (von Willebrand factor), R1E12 (ribosomal protein L32), and R4G5 (ML domain-containing protein) was significantly upregulated, and the expression of U2A8 (signal sequence receptor delta), UP8 (ferritin), 2I3A7 (NADH-ubiquinoe oxidoreductase), 2I3A3 (gamma actin-like protein), 2IP10 (ubiquitin C variant 5-like), 1I5B9 (ixodegrin-2A RGD containing protein), 1I1H6 (GST), C2E6 (troponin I), C3B2 (aspartic protease), and 1I4G12 and 1I3H6 with unknown function was significantly downregulated. Interestingly, the mRNA levels were similar in I. scapularis nymphs infected with two different strains of A. phagocytophilum but differed from those obtained in infected ISE6 cells for some genes such as U2A8, 1I5B9, 1I4G12, C2E6, C4G3 and R4G5 (Figure 1).
3.2. Differential Gene Expression in A. phagocytophilum-Infected I. scapularis Nymphs and ISE6 Cells Differed from That Observed after A. marginale Infection
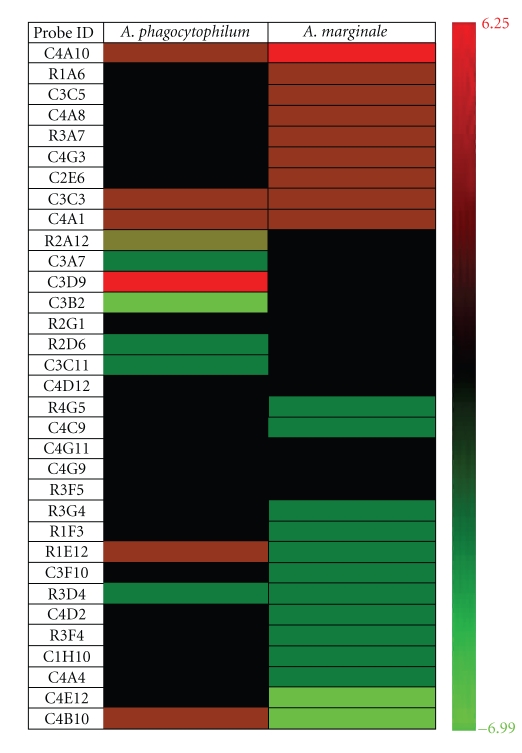
The results reported herein showed that gene expression profiles were different for A. phagocytophilum- and A. marginale-infected tick cells (Figures 2 and 3). The microarray analysis in infected and uninfected tick ISE6 cells showed that the expression of genes with homology to internal transcribed spacer 1 (probe C3C5), putative secreted salivary protein (C4G3), troponin I (C2E6), aspartic protease (C3B2), ML domain-containing protein (R4G5), H13-prov protein (C4C9), ribosomal protein L32 (R1E12), putative secreted protein (C3F10), ribosomal protein S17 (C4E12), von Willebrand factor (C4B10), and sequences with unknown function (R1A6, C4A8, R3A7, R2A12, C3A7, C3D9, R2D6, C3C11, R3G4, R1F3, C4D2, R3F4, C1H10, C4A4) was different between A. phagocytophilum- and A. marginale-infected cells (Figure 2). The expression of other genes changed in a similar way after infection of tick ISE6 cells with A. marginale or A. phagocytophilum (C4A10, C3C3, C4A1, R2G1, C4D12, C4G11, C4G9, R3F5, R3D4; Figure 2).
Figure 2.
Effect of A. phagocytophilum and A. marginale infection on tick ISE6 cells gene expression. Total RNA was extracted from three A. marginale-infected, three A. phagocytophilum-infected, and three uninfected ISE6 cell cultures. The expression fold change was determined by microarray hybridization at 6 days postinfection (dpi) (approximately 70% infected cells; companion cultures were terminal at 8 dpi). Uninfected cells were sampled at the same time point as infected cells to account for culture time effects. Ratios were calculated as Anaplasma-infected cells versus uninfected control cells. Normalized ratio values obtained for each probe were averaged across 3 biological replicates and four technical replicates and only entries displaying a significant (P ≤ .05) expression fold change >2 in either A. phagocytophilum- or A. marginale-infected cells are shown. Clone ID (library plate and well) are shown. The graph was constructed with the HCE software (http://www.cs.umd.edu/hcil/hce/hce3.html).
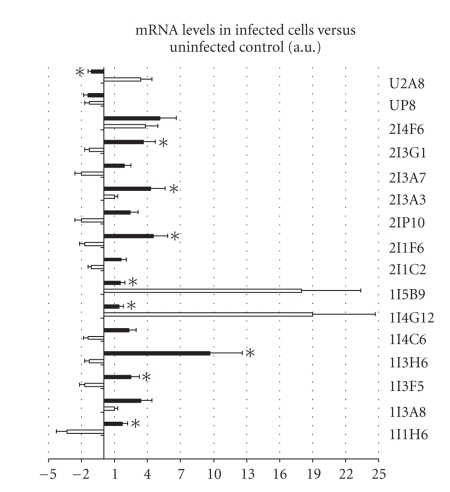
Figure 3.
Effect of A. phagocytophilum and A. marginale infection on tick ISE6 cells gene expression. The mRNA levels were compared between A. phagocytophilum- (white bars) and A. marginale- (black bars) infected tick ISE6 cells by real-time RT-PCR. Bars represent the ratio between infected normalized Ct values and uninfected average normalized Ct values (+SD). The mRNA levels were normalized against tick 16S rRNA and compared between A. phagocytophilum- and A. marginale-infected tick cells by Student's t-test (*P ≤ .05). Positive and negative values denote upregulation and downregulation, respectively, with respect to uninfected controls.
By real-time RT-PCR, the mRNA levels of U2A8 (signal sequence receptor delta), 2I3G1 (proteasome 26S subunit, non-ATPase), 2I3A3 (gamma actin-like protein), 2I1F6 (hematopoietic stem/progenitor cells protein-like), 1I5B9 (ixodegrin-2A RGD containing protein), 1I4G12 (unknown function), 1I3H6 (unknown function), 1I3F5 (ubiquitin), and 1I1H6 (GST) were significantly different between A. phagocytophilum- and A. marginale-infected ISE6 cells collected at 3 dpi with approximately 40% infected cells (Figure 3). Except for U2A8, 1I5B9, 1I4G12, C2E6, C4G3, and R4G5 which had different mRNA levels in A. phagocytophilum-infected nymphs and tick ISE6 cells (Figure 1), the mRNA levels of the studied genes were also different between A. phagocytophilum-infected I. scapularis nymphs and A. marginale-infected tick cells (data not shown).
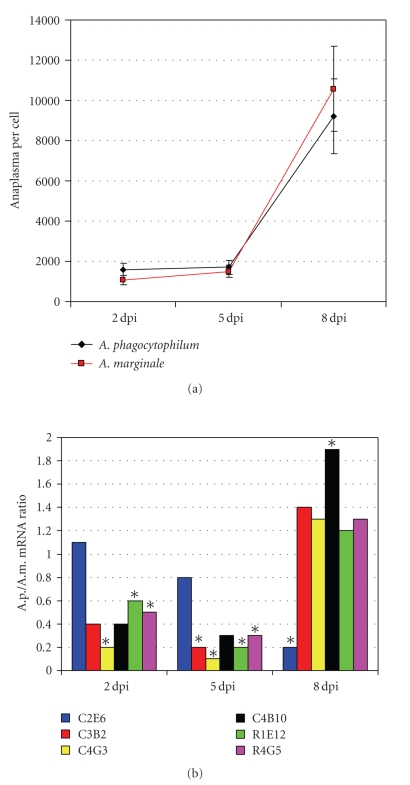
Because the kinetics of differentially expressed genes may vary with Anaplasma infection levels, the expression of selected genes was compared by real-time RT-PCR in A. phagocytophilum- and A. marginale-infected ISE6 cells collected at 2, 5, and 8 dpi (Figure 4(a)). The results showed time-dependent variation in the mRNA ratios of studied genes between A. phagocytophilum- and A. marginale-infected cells (Figure 4(b)). However, significant differences were observed between A. phagocytophilum and A. marginale infection at all time points, thus suggesting that differences in gene expression profiles elicited by these pathogens are present throughout the infection cycle in tick ISE6 cells.
Figure 4.
Comparison between differential gene expression in A. phagocytophilum- and A. marginale-infected tick ISE6 cells at different time points after infection. Studies were done on A. phagocytophilum- (A.p.-) and A. marginale- (A.m.-) infected tick ISE6 cells (two independent cultures each) at 2, 5, and 8 days postinfection (dpi) with approximately 30%–40%, 60%–70%, and >90% infected cells, respectively. (a) The A.p. and A.m. infection levels were evaluated by real-time PCR of msp4 and normalized against tick 16S rDNA. Known amounts of the full length A.p. and A.m. msp4 PCR product were used to construct a standard curve for quantitation of pathogens per cell. Data represent average ± SD. (b) The mRNA levels of selected genes were evaluated by real-time RT-PCR and normalized against tick 16S rRNA. Bars represent the ratio between average Ct values in A.p.-infected cells/average Ct values in A.m.-infected cells. The mRNA levels were compared between A.p.- and A.m.-infected tick cells by Student's t-test (*P ≤ .05). Identical mRNA levels in A. phagocytophilum and A. marginale infected cells equal one.
4. Discussion
We have shown previously that A. marginale modulates gene expression in infected ticks and tick cells [9]. In the experiment reported herein we hypothesized that A. marginale and A. phagocytophilum may elicit similar gene expression responses in infected cultured tick cells. To test this hypothesis, gene expression profiles were compared between A. phagocytophilum and A. marginale in infected tick ISE6 cells. The results showed that A. phagocytophilum modulates gene expression in infected I. scapularis nymphs and cultured tick ISE6 cells but with different gene expression profiles when compared with A. marginale. These results suggested that A. marginale and A. phagocytophilum produce different differential gene expression profiles in infected tick cells. These differences in Anaplasma-tick interactions may reflect differences in pathogen developmental cycle in the tick cells. Alternatively, differences in gene expression profiles between A. phagocytophilum- and A. marginale-infected tick ISE6 cells may be due to the fact that I. scapularis is not a natural vector of A. marginale. However, I. scapularis-cultured cells have shown to provide functionally relevant data for the study of tick-A. marginale interactions [9]. The differences in gene expression between A. marginale- and A. phagocytophilum-infected tick cells could be attributed to nonspecific responses to the presence of bacterial components that differ between the two Anaplasma species While this explanation is potentially possible, it is more likely that gene expression profiles resulted from Anaplasma intracellular infection because at least for some genes differential expression persisted until 8 dpi, when >90% cells were infected. Taken together the results reported here consistently provided differences in gene expression profiles between A. phagocytophilum- and A. marginale-infected tick cells. Importantly, sampling time points during Anaplasma infection of tick ISE6 cells may be important to characterize the expression of particular genes. Although not addressed in this study, these differences may be also present during tick feeding and development.
The genes differentially expressed in I. scapularis nymphs and tick ISE6 cells infected with A. phagocytophilum included some genes such as GST and ferritin shown previously to affect A. marginale infection and/or multiplication in ticks and/or tick cells [9]. However, while GST and ferritin were upregulated and downregulated after A. marginale infection, respectively, they were regulated in the opposite direction in A. phagocytophilum-infected ticks and tick cells. GST, ferritin, and aspartic protease (C3B2), also found to be differentially expressed in A. phagocytophilum-infected ISE6 cells, have been reported to be regulated by tick feeding or infection with other pathogens [12–18]. Other genes differentially expressed after A. phagocytophilum infection such as U2A8 (signal sequence receptor delta), 1I5B9 (ixodegrin-2A RGD containing protein), 2I3A7 (NADH-ubiquinone oxidoreductase), 2IP10 (ubiquitin C variant 5-like), 2I3A3 (gamma actin-like protein), C4B10 (von Willebrand factor), C2E6 (troponin I), and R1E12 (ribosomal protein L32) constitute new findings and may be involved in infection and/or multiplication of the pathogen in ticks or are part of tick cell immune response to moderate infection levels. As shown previously for A. marginale [9], RNA interference experiments may help to characterize the function of differentially expressed genes during A. phagocytophilum infection of ticks and tick ISE6 cells.
The expression of selected genes was analyzed in I. scapularis ISE6 cells and nymphs infected with A. phagocytophilum. These experiments allowed us to compare the results of gene expression in vitro and in vivo. The nymphs were selected for analysis because this stage plays an important role during pathogen transmission to humans [5]. The ISE6 cell line was obtained from embryos of I. scapularis, one of the natural vectors of A. phagocytophilum [19]. However, this cell line is heterogeneous in the cell types represented [19], which may have different susceptibility and response to pathogen infection. Although cultured tick cells have been shown to be a good model for the study of tick-Anaplasma interactions [19–21], these results demonstrated that differences may exist between I. scapularis-cultured tick cells and nymphs in the mRNA levels of certain genes, at least under the experimental conditions used herein. These differences may account for differences in gene expression between infected ISE6 cells and tick whole nymph tissues and/or due to differences in the infection levels in both systems. An additional source of potential differences in gene expression between I. scapularis ISE6 cells and nymph tissues could be attributed to the fact that different cells types may be infected in both systems resulting in different responses to infection. Finally, although less likely, these differences may be related to differences between the NY18 isolate used to infect ISE6 cells and the Gaillard and Dawson strains used to infect I. scapularis nymphs.
Recent studies have characterized A. marginale and A. phagocytophilum proteins that are involved in interactions with tick cells [7, 8, 22]. However, tick-Anaplasma coevolution also involves genetic traits of the vector as demonstrated recently in studies on the role of tick proteins in the infection and transmission of A. marginale [9, 11] and A. phagocytophilum [10, 11]. Furthermore, genetic factors have been associated with intraspecific variation in vector competence for a variety of vector-borne pathogens, including A. phagocytophilum [23] and A. marginale [24, 25].
5. Conclusions
In summary, we have characterized the gene expression profile in A. phagocytophilum-infected I. scapularis nymphs and cultured ISE6 cells. Interestingly, differential gene expression seems to differ between A. marginale and A. phagocytophilum infected cultured tick cells. Future experiments would provide detailed information on the role of these genes during A. phagocytophilum life cycle in ticks. These results provide fundamental information toward understanding tick-Anaplasma interactions and may lead to formulations of new interventions for the prevention of the transmission of tick-borne pathogens.
OMB Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the United States Department of Health and Human Services.
Acknowledgments
The authors thank Patricia Ayoubi (Department of Biochemistry and Molecular Biology, Oklahoma State University, USA) for technical assistance. This research was supported by the Ministerio de Ciencia e Innovación, Spain (project BFU2008-01244/BMC), the CSIC intramural project 200830I249 and PA1002025 to JF, the Oklahoma Agricultural Experiment Station (project 1669), and the Sitlington Endowed Chair for Food Animal Research (K. M. Kocan, Oklahoma State University). V. Naranjo was founded by the European Social Fund and the Junta de Comunidades de Castilla-La Mancha (Program FSE 2007-2013), Spain. Raúl Manzano-Roman was funded by Ministerio de Educación y Ciencia, Spain.
References
- 1.de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Frontiers in Bioscience. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Kocan KM, de la Fuente J, Blouin EF, Garcia-Garcia JC. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology. 2004;129(supplement):S285–S300. doi: 10.1017/s0031182003004700. [DOI] [PubMed] [Google Scholar]
- 3.Kocan KM, de la Fuente J, Blouin EF. Advances toward understanding the molecular biology of the Anaplasma-tick interface. Frontiers in Bioscience. 2008;13:7032–7045. doi: 10.2741/3208. [DOI] [PubMed] [Google Scholar]
- 4.Dumler JS, Barbet AF, Bekker CPJ, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila . International Journal of Systematic and Evolutionary Microbiology. 2001;51(6):2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 5.Dumler JS, Choi K-S, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum . Emerging Infectious Diseases. 2005;11(12):1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlyon JA, Fikrig E. Invasion and survival strategies of Anaplasma phagocytophilum . Cellular Microbiology. 2003;5(11):743–754. doi: 10.1046/j.1462-5822.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 7.Hotopp JC, Lin M, Madupu R, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genetics. 2006;2(2, article e21) doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson CM, Herron MJ, Felsheim RF, et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics. 2008;9, article 364 doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Fuente J, Blouin EF, Manzano-Roman R, et al. Functional genomic studies of tick cells in response to infection with the cattle pathogen, Anaplasma marginale . Genomics. 2007;90(6):712–722. doi: 10.1016/j.ygeno.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Sukumaran B, Narasimhan S, Anderson JF, et al. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. Journal of Experimental Medicine. 2006;203(6):1507–1517. doi: 10.1084/jem.20060208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente J, Almazán C, Blouin EF, Naranjo V, Kocan KM. Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin. Parasitology Research. 2006;100(1):85–91. doi: 10.1007/s00436-006-0244-6. [DOI] [PubMed] [Google Scholar]
- 12.Blouin EF, Saliki JT, de la Fuente J, Garcia-Garcia JC, Kocan KM. Antibodies to Anaplasma marginale major surface proteins 1a and 1b inhibit infectivity for cultured tick cells. Veterinary Parasitology. 2003;111(2-3):247–260. doi: 10.1016/s0304-4017(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 13.Munderloh UG, Jauron SD, Fingerle V, et al. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. Journal of Clinical Microbiology. 1999;37(8):2518–2524. doi: 10.1128/jcm.37.8.2518-2524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Fuente J, Vicente J, Hofle U, et al. Anaplasma infection in free-ranging Iberian red deer in the region of Castilla-La Mancha, Spain. Veterinary Microbiology. 2004;100(3-4):163–173. doi: 10.1016/j.vetmic.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.de la Fuente J, Massung RF, Wong SJ, et al. Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. Journal of Clinical Microbiology. 2005;43(3):1309–1317. doi: 10.1128/JCM.43.3.1309-1317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Fuente J, Maritz-Olivier C, Naranjo V, et al. Evidence of the role of tick subolesin in gene expression. BMC Genomics. 2008;9, article 372 doi: 10.1186/1471-2164-9-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Fuente J, Ayoubi P, Blouin EF, Almazán C, Naranjo V, Kocan KM. Gene expression profiling of human promyelocytic cells in response to infection with Anaplasma phagocytophilum . Cellular Microbiology. 2005;7(4):549–559. doi: 10.1111/j.1462-5822.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 18.Macaluso KR, Mulenga A, Simser JA, Azad AF. Interactions between rickettsiae and Dermacentor variabilis ticks: analysis of gene expression. Annals of the New York Academy of Sciences. 2003;990:568–572. doi: 10.1111/j.1749-6632.2003.tb07428.x. [DOI] [PubMed] [Google Scholar]
- 19.Mulenga A, Simser JA, Macaluso KR, Azad AF. Stress and transcriptional regulation of tick ferritin HC. Insect Molecular Biology. 2004;13(4):423–433. doi: 10.1111/j.0962-1075.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudenko N, Golovchenko M, Edwards MJ, Grubhoffer L. Differential expression of Ixodes ricinus tick genes induced by blood feeding or Borrelia burgdorferi infection. Journal of Medical Entomology. 2005;42(1):36–41. doi: 10.1093/jmedent/42.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Dreher-Lesnick SM, Mulenga A, Simser JA, Azad AF. Differential expression of two glutathione S-transferases identified from the American dog tick, Dermacentor variabilis . Insect Molecular Biology. 2006;15(4):445–453. doi: 10.1111/j.1365-2583.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro JMC, Alarcon-Chaidez F, Francischetti IM, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochemistry and Molecular Biology. 2006;36(2):111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Boldbaatar D, Sikalizyo Sikasunge C, Battsetseg B, Xuan X, Fujisaki K. Molecular cloning and functional characterization of an aspartic protease from the hard tick Haemaphysalis longicornis . Insect Biochemistry and Molecular Biology. 2006;36(1):25–36. doi: 10.1016/j.ibmb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Nascimento-Silva MCL, Leal AT, Daffre S, et al. BYC, an atypical aspartic endopeptidase from Rhipicephalus (Boophilus) microplus eggs. Comparative Biochemistry and Physiology, Part B. 2008;149(4):599–607. doi: 10.1016/j.cbpb.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Bell-Sakyi L, Zweygarth E, Blouin EF, Gould EA, Jongejan F. Tick cell lines: tools for tick and tick-borne disease research. Trends in Parasitology. 2007;23(9):450–457. doi: 10.1016/j.pt.2007.07.009. [DOI] [PubMed] [Google Scholar]