Abstract
Infection with HIV-1 causes degeneration of neurons leading to motor and cognitive dysfunction in AIDS patients. One of the key viral regulatory proteins, Tat, which is released by infected cells, can be taken up by various uninfected cells including neurons and by dysregulating several biological events induces cell injury and death. In earlier studies, we demonstrated that treatment of neuronal cells with Tat affects the nerve growth factor (NGF) signaling pathway involving MAPK/ERK. Here we demonstrate that a decrease in the level of Egr-1, one of the targets for MAPK, by Tat has a negative impact on the level of p35 expression in NGF-treated neural cells. Further, we demonstrate a reduced level of Egr-1 association with the p35 promoter sequence in NGF-treated cells expressing Tat. As p35, by associating with Cdk5, phosphorylates several neuronal proteins including neurofilaments and plays a role in neuronal differentiation and survival, we examined kinase activity of p35 complexes obtained from cells expressing Tat. Results from H1 kinase assays showed reduced activity of the p35 complex from Tat-expressing cells in comparison to that from control cells. Accordingly, the level of phosphorylated neurofilaments was diminished in Tat-expressing cells. Similarly, treatment of PC12 cells with Tat protein or supernatant from HIV-1 infected cells decreased kinase activity of p35 in these cells. These observations ascribe a role for Tat in altering p35 expression and its activity that affects phosphorylation of proteins involved in neuronal cell survival.
HIV-1 proteins may induce neuronal apoptosis in AIDS patients with neurological disorders by dysregulating the regulatory events responsible for cell growth, survival, and programmed cell death (Adle-Biassette et al., 1995; New et al., 1997; Macho et al., 1999; Kaul et al., 2001, 2005; Xu et al., 2004; Kaul and Lipton, 2006; King et al., 2006). Upon entry into the CNS, HIV-1 productively infects brain macrophages and microglia leading to production of large amounts of viral proteins including Tat, which, in turn, can induce neuronal damage. Tat is a viral transcriptional activator that plays a critical role in the replication of HIV-1 and has the ability to interact with several important cellular regulatory proteins and alter their function (Dingwall et al., 1989; Jeang et al., 1993; Cujec et al., 1997; Greenberg et al., 1997; Hottiger and Nabel, 1998; Wei et al., 1998; Sawaya et al., 2000; Liu et al., 2002). It is thought that Tat possesses neurotoxic activity through its unusual ability to be released by infected cells and be taken up in a biologically active form by the neighboring uninfected cells including neurons (Frankel and Pabo, 1988; Ensoli et al., 1993; Philippon et al., 1994; Li et al., 1995; Ma and Nath, 1997). Although the mechanisms whereby Tat induces neuronal damage are not fully understood, earlier studies revealed that activation of glycogen synthase kinase 3β (GSK3β) by Tat via PI3 kinase and Akt may contribute to neurotoxicity of this protein (Maggirwar et al., 1999; Sui et al., 2006a,b). It has also been found that the ability of HIV-1 Tat to induce apoptosis involves Tat binding to microtubules and delaying tubulin depolymerization (Chen et al., 2002; Epie et al., 2005; de Mareuil et al., 2005). In rat embryonic cortical neurons, tubulin-mediated binding of Tat to the cytoskeleton caused a proteasomal-dependent degradation of microtubule-associated protein 2 (MAP2) and neuronal damage (Aprea et al., 2006). In a previous study, we found that mitogen-activated protein kinase (MAPK), which plays a critical role in the NGF network and its control of neuronal cell differentiation and survival (Tsuruda et al., 2004), was a target for dysregulation of signaling pathways by Tat. Activation of the NGF signaling pathway stimulates transcription of p35, a neuron-specific activator of Cdk5 (Tsai et al., 1994; Harada et al., 2001). The induction of p35/Cdk5 kinase activity is critical for neurite outgrowth and survival (Cheung and Ip, 2004; Song et al., 2005; Fu et al., 2007). In earlier studies, it was demonstrated that the induction of Egr-1 through MAPK leads to the up-regulation of p35 in PC12 cells (Harada et al., 2001). This induction appeared to be mediated by binding of Egr-1 to a specific region of the p35 regulatory region encompassing GC-rich nucleotides. Here we demonstrate that by blocking expression of Egr-1 in response to NGF, Tat impairs the ability of Egr-1 to stimulate p35 in neuronal cells. As a result, the reduced activity of p35 reduces the phosphorylation of neurofilament protein in the cells.
Materials and Methods
Plasmids and synthetic oligonucleotides
GST-Tat was cloned by PCR amplification of a BglII–EcoRI fragment containing the HIV-1 Tat gene (1–86 amino acids), and ligation with BamHI–EcoRI-digested pGEX-2T. p35-1116 (−1,116 to +54 of p35 promoter) was kindly provided by Dr. Julie Cook (Harvard Medical School) (Ross et al., 2002). pCMV-Egr-1 plasmid which expresses full length Egr-1 was a gift from Dr. Dona Lee Wong (Harvard Medical School). The sequence of all plasmids was verified by DNA sequencing using an ABI automatic sequencer. Oligonucleotides were prepared commercially by Integrated DNA Technologies (Coralville, IA). The following oligonucleotides were used in the ChIP assay (putative Egr-1 binding NGFIA GC-rich repeated sequences are underlined): ChIP-p35 primer (−720): CGCGTTCCCGCCGCCCGCGCCGTGT. ChIP-p35 primer (−477): GACGCCGCGGCTCCGCCCCC. The DNA sequence for the Egr-1 oligonucleotide used for band-shift was: GGATCCAGCGGGGGCGAGCGGGGGCGA. The 21-nucleotide sequence of Egr-1 siRNA was derived from the human Egr-1 mRNA sequence (gi:31317226) and was targeted to the coding region 1,510–1,529 of Egr-1 gene corresponding to the 503–509 amino acid region of Egr-1 protein. The sequence of the Egr-1 siRNA was CCGCAAGUGGAUCUUGGUAUGUU. The non-targeting control siRNA was obtained from Dharmacon Research (Lafayette, CO).
Purification of recombinant Tat protein
GST-Tat recombinant protein was overexpressed in E. coli and purified according to the methods described previously (Darbinian et al., 2001) followed by thrombin cleavage. The integrity and purity of the GST-Tat fusion protein was verified by SDS–PAGE followed by Coomassie Blue staining. Protease treatment was carried out by incubation of GST-Tat with 1 U/μg of thrombin protease, insoluble enzyme attached to 4% cross-linked beaded agarose (Sigma, St. Louis, MO) for 15 min at 37°C. Samples were then pelleted by centrifugation at 14,000 rpm for 1 min at 4°C.
Cell culture, transfection, luciferase assay
Human neuroblastoma SK-N-MC cells were obtained from the American Type Culture Collection (ATCC; HTB-10) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Rat PC12 cells were obtained from American Type Culture Collection (ATCC: CRL-1721) and cultured in F-12 medium supplemented with 15% horse serum and 2.5% fetal bovine serum. Cells were maintained at 37°C in a humidified incubator containing 7% CO2.
SK-N-MC cell lines expressing CFP-Tat and CFP (cyan fluorescent protein) were developed by stably transfecting SK-N-MC cells with CFP-Tat or pECFP-C1 (Clontech, Mountain View, CA) and clonal expression as described previously (Darbinian-Sarkissian et al., 2006).
Transfections were carried out by the calcium phosphate technique as described (Graham and van der Eb, 1973), using 5 μg of p35-luciferase reporter construct (Ross et al., 2002). Thirty-six hours post-transfection, protein extracts were prepared and equal amounts of proteins (approximately 4 μg) were assayed for Luciferase activity according to procedures described by the manufacturer (Promega, Madison, WI). NGF treatment was done for 3 h prior to harvesting cell for luciferase assay. Each transfection was repeated a minimum of three times. Transfection efficiency was monitored using pGLEGFP. In general, transfection efficiency was about 50% in SK-N-MC cells. To inhibit expression of Egr-1, cells were transiently transfected with Egr-1-siRNA (Dharmacon Research) using oligofectamine reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA), and after 72 h, cells were harvested and used for further experiments.
Cell treatment, lysis, immunoprecipitation, and Western blotting
SK-N-MC cells (0.5 × 106/dish) were seeded in 60-mm tissue culture dishes and cultured for 2 days. Cells were starved in serum-free DMEM for 24 h prior to NGF stimulation (100 ng/ml) for 3 h. PC12 cells were plated onto collagen-coated 60 mm tissue culture dishes at a density of 0.5 × 106 and cultured for 2 days. Cells were starved in serum free F-12 medium for 24 h prior to NGF stimulation (100 ng/ml) for 5 days for the study of neurite outgrowth.
Cells were washed with cold phosphate-buffered saline (PBS) and solubilized in lysis buffer [50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% Nonidet P-40, and 1% protease inhibitors cocktail for use with mammalian cell and tissue extracts (Sigma)]. Cell debris was removed by centrifugation for 5 min at 4°C. Fifty micrograms of proteins were eluted with Laemmli sample buffer, heated at 95°C for 10 min, and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE).
Immunoprecipitation of complexes was carried out by incubating approximately 500 μg of precleared cell lysates with either 5 μl of anti-Egr-1 antibody, anti-p35 or normal rabbit serum for 16 h at 4°C. Then, immune complexes were precipitated with 30 μl of protein A-Sepharose for 2 h at 4°C, washed, and analyzed in kinase or ChIP assays.
For Western blot analysis, protein samples were resolved by SDS–PAGE and transferred to nitrocellulose membranes as described previously (Darbinian-Sarkissian et al., 2006).
Antibodies to Egr-1, p35, neurofilament (NF-H) and actin were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA), Grb2 from BD Biosciences (San Jose, CA), phospho-Neurofilament mouse monoclonal antibody cocktail from Sternberger Monoclonals Incorporated (Lutherville, MD), and CFP Living Colors full length polyclonal antibody from Clontech. The HIV-1 BH10 Tat antiserum R705 polyclonal antibody against Tat was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Band-shift assay
A double-stranded DNA fragment containing the Egr-1 binding site (Santa Cruz Biotechnologies) was end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (Roche Applied Sciences, Indianapolis, IN). DNA-protein binding assays were performed as described previously (Darbinian-Sarkissian et al., 2006). Complexes were resolved by 6% native polyacrylamide gel electrophoresis in 0.5 × TBE at 180 V for 3–4 h at 4°C, and detected by autoradiography.
Chromatin immunoprecipitation assay (ChIP)
Cells were serum starved for 24 h followed by treatment with NGF for 3 h and ChIP assay was performed as described previously (Darbinian-Sarkissian et al., 2006). The precleared supernatants were immunoprecipitated with anti-Egr-1 antibody or normal rabbit serum (4°C for 16 h). DNA samples were used for PCR amplification with p35 primers corresponding to the promoter region of p35 spanning nucleotides −741 to −425 relative to the transcription start site.
In vitro p35:Cdk5 kinase assay
The kinase assay was performed on SK-N-MC cells expressing CFP-Tat or CFP, NGF stimulated for 3 h or in the case of PC12 cells incubated with Tat or conditioned media from HIV-1 infected cells. The p35 immunoprecipitated complexes were washed three times with kinase buffer (25 mM Tris–HCl, 10 mM MgCl2, 2 mM dithiothreitol) and used to assay Histone-1 (H1) phosphorylation by incubating with 5 μCi [γ-32P]ATP (PerkinElmer, Shelton, CT) in 50 μl of kinase buffer at 30°C for 45 min. The phosphorylated H1 was subjected to 10% SDS–PAGE and p35 activation was assayed by autoradiography and quantification by densitometry.
HIV-1 infection of U-937 cells
Approximately 2 × 106 U-937 cells were grown in RPMI with 2% fetal bovine serum and were infected with the HIV-1 strain JF-RL. Five days post-infection, conditioned media from HIV-1 infected or uninfected cells were collected and used for the treatment of PC12 cells.
All statistical analyses have been done by STDEV and AVERAGE programs.
Results and Discussion
In the first set of experiments, we utilized the human neuroblastoma cell line, SK-N-MC, which responds to NGF, as a culture model to investigate the effect of Tat on NGF signaling pathways. As an extension of our previous study, we utilized cell lines that express either CFP or CFP-Tat, and demonstrated that treatment of the control cells expressing CFP with NGF increased the level of Egr-1 (Harada et al., 2001). This induction was not detected in cells expressing CFP-Tat (Fig. 1A). Egr-1 is a DNA binding protein that recognizes specific DNA motifs containing CACCCCCGC or GCGGGGGCG nucleotide composition. In earlier studies, it was shown that the induction of Egr-1 through MAPK activation in PC12 cells leads to the upregulation of p35, and that this event may interfere with Egr-1 interaction with its target DNA element positioned in the p35 promoter. Examination of p35 levels in SK-N-MC cells showed induction of p35 upon NGF treatment in the control but not Tat-expressing cells (Fig. 1B). Tat has a peculiar characteristic that allows its efficient uptake, in a biologically active form, by a variety of eukaryotic cells (Frankel and Pabo, 1988; Ensoli et al., 1993; Philippon et al., 1994; Li et al., 1995; Ma and Nath, 1997). Thus, in an alternative approach to assess the impact of Tat on p35, we treated the neural cell line PC12 with NGF in the absence or presence of exogenously added Tat in the culture media. As seen in Figure 1C, treatment of PC12 cells with NGF elevated expression of Egr-1 and enhanced the level of p35 expression. Treatment of the cells with Tat interfered with NGF-mediated induction of Egr-1 and p35 expression, suggesting that Tat can suppress expression of p35, at least in part, via the Egr-1 pathway (Kawai-Kowase et al., 1999; Thiel and Cibelli, 2002; Yang et al., 2002; Rolli-Derkinderen et al., 2003; Virolle et al., 2003; Al-Sarraj et al., 2005).
Fig. 1.
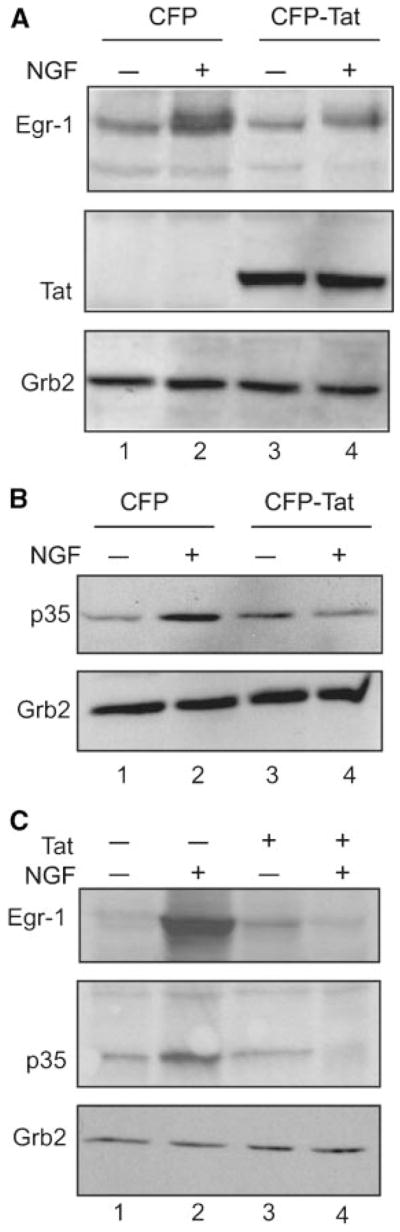
The effect of Tat and NGF on Egr-1 and p35 protein expression. A: Western blot analysis for Egr-1 expression in Tat-producing and control SK-N-MC cells, with and without NGF treatment for 3 h. Anti-Tat antibody was used to confirm the presence of the Tat protein in Tat-producing cells. Grb2 was used as a control for protein loading. B: (Top) Western blot analysis for the detection of p35 protein in SK-N-MC cells after exposure of the cells to NGF (lanes 1–2). (Bottom) Western blot analysis for the detection of Grb2 as a loading control. C: Western blot analysis for the detection of Egr-1 and p35 proteins in PC12 cells upon NGF treatment, in the presence or absence of exogenously added Tat. The level of Grb2 expression serves as a control for protein loading.
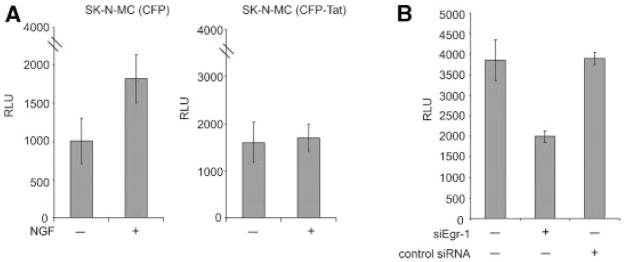
To more directly assess the impact of Tat on Egr-1 mediated activation of p35 expression, SK-N-MC cells were transfected with a luciferase reporter construct containing the p35 promoter in the absence and presence of NGF treatment. As seen in Figure 2A, treatment of cells with NGF enhanced the level of transcription from the p35 promoter in SK-N-MC cells. In the cells that express Tat, no significant increase in the transcriptional activity of the p35 promoter was observed upon NGF treatment (Fig. 2A). The impact of Egr-1 on transcription was further examined by utilizing siRNA that specifically targets Egr-1 expression. As seen in Figure 2B, Egr-1-specific siRNA, but not control siRNA, decreased the level of p35 promoter activity in SK-N-MC cells. Thus, this observation suggests that expression of p35 is regulated by Egr-1 protein.
Fig. 2.
Effect of Tat, NGF, and Egr-1 siRNA on transcription of the p35 promoter. A: Effect of NGF on transcription of the p35 promoter in SK-N-MC (CFP) and SK-N-MC (CFP-Tat) cells. Luciferase assay of p35 promoter activity upon NGF treatment of the SK-N-MC (CFP) and SK-N-MC (CFP-Tat) cells was performed as described in Materials and Methods Section. B: Effect of Egr-1 silencing on the activity of the p35 promoter in SK-N-MC cells. Cells were co-transfected with the p35-luciferase reporter plasmid and the Egr-1 expression plasmid, and were then treated with NGF for 3 h in the presence of Egr-1-specific siRNA or a control non-targeting siRNA for 72 h. Cells were harvested and protein extracts were analyzed by luciferase assay to determine p35 promoter activity. The histograms show mean relative light units representing luciferase activity and the error bars show the standard deviation for the experiments, which were repeated in duplicate.
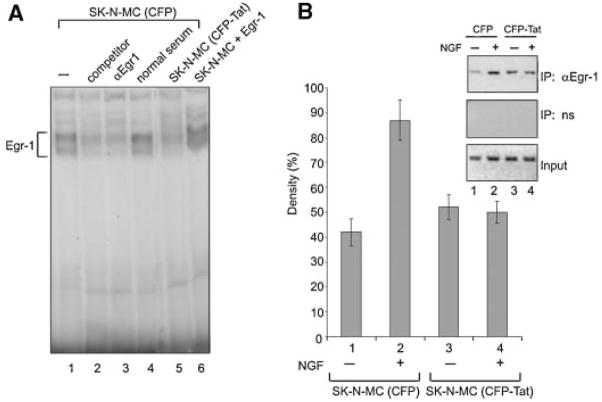
Examination of the promoter sequence of p35 revealed the presence of multiple predicted Egr-1 binding sites designated NGF1A, NGF2A. and NGF3S at nucleotides −720/−687, −558/−524, and −511/−477, respectively (Ohshima et al., 1996; Harada et al., 2001; Ross et al., 2002; Chen et al., 2004; Lee and Kim, 2004, 2007). To examine the ability of Egr-1 to associate with the DNA sequence, we performed band-shift assay using an oligonucleotide spanning the NGF1A DNA motif. As seen in Figure 3A, incubation of nuclear extract from SK-N-MC with [32P]-labeled DNA resulted in the formation of a DNA protein complex (depicted by a bracket) whose intensity was decreased upon the addition of unlabeled competitor DNA or anti-Egr-1 antibody, but not normal control sera, to the binding reaction (compare lane 1 with lanes 2, 3, and 4, respectively). The level of interaction of Egr-1 with the DNA was reduced in extract from cells expressing Tat (compare lanes 1 and 5). As expected, ectopic expression of Egr-1 in SK-N-MC cells enhanced the formation of an Egr-1:DNA complex (lane 6) and served as a control for positioning Egr-1 complexes in our gel. In an alternate strategy, we used chromatin immunoprecipitation (ChIP) assay and the result (Fig. 3B), after normalizing to the input DNA, showed an increase in the in vivo association of Egr-1 with p35 DNA sequence following NGF treatment in Tat negative SK-N-MC, but not Tat-producing cells. The resulting PCR product [−720/−477] includes all 3 NGF1A Egr-1 binding sites within the p35 promoter, when the first nucleotide of the ATG initiation codon of the p35 gene is designated +1 (Ohshima et al., 1996; Harada et al., 2001), and also in accordance with previous studies indicating that the major p35 transcription start site lies 411 bp upstream of the ATG (Ross et al., 2002; Chen et al., 2004; Lee and Kim, 2004, 2007). These observations indicate that Egr-1 can associate with the p35 promoter and that the level of its association is reduced, most likely due to reduced levels of Egr-1 protein, in Tat-expressing SK-N-MC cells.
Fig. 3.
Effect of Taton Egr-1 binding to the p35 promoter. A:Band-shift assay analysis of protein extracts from Tat-CFP and CFP-producing cells in the presence of NGF. The association of Egr-1 with the DNA results in the formation of DNA-protein complexes (shown by a bracket). The addition of unlabeled competitor (lane2) oranti-Egr-1 antibody (lane3) in CFP-producing cells or the effect of Tat (lane5) was examined. Addition of normal serum as a negative control has no effect on the formation of the Egr-1:DNA complex (lane 4). B: ChIP assay to detect the association of Egr-1 with the p35 promoter in Tat-CFP and CFP-producing cells. SK-N-MC stable cell lines producing CFP or CFP-Tat that express Egr-1 were utilized in ChIP assay as described in Materials and Methods Section. Top part: Immunoprecipitation with antibody to Egr-1, indicating binding of Egr-1 to the p35 promoter region spanning −720 to −477 nt. Middle part: Immunoprecipitation with normal rabbit serum (negative control). Bottom part: Input DNA (positive control). The density of bands (top part) was quantified and is presented as a histogram.
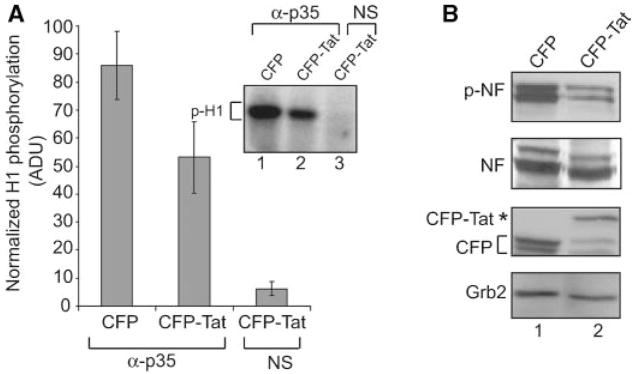
p35 is a partner of Cdk5 (Tsai et al., 1994; Ohshima et al., 1996; Terada et al., 1998; Harada et al., 2001; Nguyen et al., 2002; Ross et al., 2002; Chen et al., 2004; Lee et al., 2007), a neuronal-specific kinase whose activity is critical for neurite outgrowth and neuronal survival. The reduced level of p35 in cells expressing Tat prompted us to determine the kinase activity of p35-associated kinase in the cells. To this end, cytoplasmic extracts from SK-N-MC cells with or without Tat expression were prepared and after treatment with anti-p35 antibody, the kinase activity of the immunocomplexes was assessed using a histone H1 kinase assay (Chapman and Wolgemuth, 1994; Matsushime et al., 1994; Zhang et al., 1997; Harada et al., 2001). Results from the kinase assay showed reduced phosphorylation (nearly 40%) of H1 by p35/Cdk5 immunocomplexes from non-Tat producing cells versus those from cells producing Tat (Fig. 4A). As anticipated, the complex pulled down by normal serum had very little kinase activity demonstrating the specificity of this activity. Neurofilament proteins are among the critical targets for phosphorylation by p35:Cdk5 (Lees et al., 1988; Shetty et al., 1993; Sharma et al., 1999; Tanaka et al., 2001; Kesavapany et al., 2003; Smith, 2003; Shea et al., 2004), an event that is important for neuronal cell growth, differentiation, and survival. Thus, we examined the total level of neurofilament and its phosphorylation in the control and Tat-producing cells. Results from immunoblot analysis showed a noticeable decrease in the intensity of the phosphorylated forms of neurofilaments in Tat-expressing cells in comparison with that from control cells (Fig. 4B). Examination of the total level of neurofilaments showed no significant differences in their levels in these cells suggesting that the reduction in the level of p35 as a result of a decrease in Egr-1 activation of p35 by Tat reduced the phosphorylation of p35:Cdk5 targets such as neurofilament in SK-N-MC cells.
Fig. 4.
Effect of Tat on p35/Cdk5 kinase activity reduces phosphorylation of neurofilaments. A: Suppression of p35/Cdk5-associated phosphorylation of H1 by Tat in NGF treated Tat-producing cells. SK-N-MC cells were treated for 3 h in the presence of NGF, and protein extracts were prepared and immunoprecipitated with p35 antibody and analyzed by kinase assay using purified histone H1 as a substrate. A decrease in the intensity of the band corresponding to the phosphorylated H1 was observed in NGF stimulated cells in the presence of Tat (lane 2). Lane 3 represents the negative control, non-immune mouse serum (NS) used in the precipitation step. Densitometric analysis data for p35 kinase assay are presented. The kinas eassay was performed twice, and the results after normalizing to input H1 are presented in arbitrary units of 1–100.B:Western blot analysis for evaluating levels of phosphorylated NF (top part) in SK-N-MC cells that express either CFP or CFP-Tat grown after serum starvation for 24 h and after NGF treatment of cells for 3 h. Anti-CFP antibody was used to confirm the presence of CFP-Tat protein upon NGF treatment in Tat-producing cells. Expression of Grb2 served as a loading control.
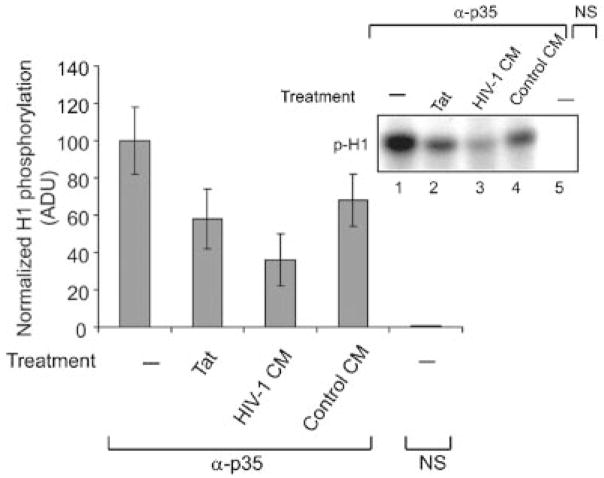
In connection with our earlier study (shown in Fig. 1C) demonstrating suppression of p35 expression upon treatment of PC12 cells with Tat, in the next study we evaluated the kinase activity of the p35:Cdk5 complex from PC12 cells before and after addition of exogenous Tat to culture media. In parallel, PC12 cells were also treated with conditioned media from uninfected or HIV-1 infected macrophages. Results from the H1 kinase assay showed a decrease in the kinase activity of p35 immunocomplexes from Tat-treated cells compared to that from mock treated control cells (Fig. 5). Moreover, a substantial decrease in the kinase activity of p35 immunocomplexes was observed upon treatment of PC12 cells with conditioned media from HIV-1 infected cells compared to the control samples (Fig. 5).
Fig. 5.
Treatment of PC12 cells with exogenous Tat and HIV-1 infected culture media inhibits p35/Cdk5 kinase activity. Approximately 2 × 106 U-937 cells grown in RPMI with 2% fetal bovine serum were infected with HIV-1 JF-RL and after 5 days post-infection, supernatants were collected and used for incubation with PC12 cells. Approximately 2 × 106 PC12 cells maintained in media containing NGF were treated with exogenous Tat protein or conditioned media obtained from infected U-937 cells. Protein extracts were harvested, immunoprecipitated with p35 antibody, and analyzed by kinase assay using purified histone H1 as a substrate. A decrease in the intensity of the band corresponding to the phosphorylated H1 was observed in Tat-treated PC12 cells (lane 2) and after treatment of cells with conditioned media from HIV-1 infected macrophages (lane 3). Lane 4 represents PC12 cells treated with conditioned media from uninfected monocytes. Lane 5 represents the negative control, non-immune mouse serum (NS) used in the precipitation step. Densitometric analysis data for the p35 kinase assay are presented. The kinase assay was performed twice and the results were normalized to total H1 input and presented in arbitrary units.
Altogether, our results provide new evidence supporting a model consistent with a negative impact of endogenous (as examined in SK-N-MC) or exogenous Tat (as demonstrated in PC12 cells) on Egr-1 expression. As a result, expression of p35 and its activity upon proteins that are important for neurogenesis are affected by HIV-1 Tat.
The Tat protein of HIV-1 has been shown to be involved in several biological processes including cell proliferation, apoptosis, and differentiation; all of which may contribute to the pathogenesis of HIV-1 associated disorders. The finding that Tat is released by infected cells via an endolytic process has suggested that some of the bystander pathologic features exerted by HIV-1 infection may be triggered by Tat. Here we demonstrate that expression of Tat in cells of neuronal origin such as SK-N-MC cells affects the level of cellular responses to NGF. In a previous study we demonstrated that expression of Tat reduces NGF-induced Egr-1 levels in SK-N-MC cells (Darbinian-Sarkissian et al., 2006). In an extension of those observations, we now demonstrate that the failure of NGF to stimulate Egr-1 in Tat-expressing cells is transmitted to the level of expression of p35. Our results show a decrease in the level of p35 promoter activation upon NGF treatment, where no increase in association of Egr-1 with the p35 promoter was observed. Finally, our studies illustrate the reduced kinase activity of p35 and a decrease in the phosphorylation of neurofilament, one of the substrates for p35, upon treatment of Tat-expressing cells with NGF. Thus, the picture that emerges from this and the previous studies suggests that Tat by altering the activity of MAPK and Egr-1, dysregulates the NGF signaling pathway by suppressing p35 expression and its activity. In addition to its impact upon Egr-1 expression, Tat can affect Egr-1 activity by associating with this protein (unpublished results) (see Yang et al., 2002). The interaction of Egr-1 and Tat may potentiate Egr-1 activity. In fact, our results showed a higher baseline of p35 promoter activity in Tat-expressing SK-N-MC cells compared to that from the control cells in the untreated cells. Accordingly, results from ChIP assay showed that in the absence of NGF, the interaction of Egr-1 with the p35 promoter is slightly higher in Tat-producing cells compared to that from the control cells (Fig. 3, compare lanes 1 and 3), suggesting that the interaction of Tat with Egr-1 may enhance Egr-1 binding to the p35 promoter and its activity. Treatment with NGF, which triggers the MAPK signaling pathway, had no impact on the cytoplasmic events that promote Egr-1 and p35 activation in Tat-expressing cells, suggesting that Tat can interfere with the NGF signaling pathway. Our results also demonstrated that treatment of PC12 cells with conditioned media from HIV-1 infected cells, where Tat released by the infected cells is present, decreased the kinase activity of p35, further supporting the earlier model that Tat from the infected cells can have an impact on neuronal cells by disturbing several biological events including the NGF-mediated p35 pathway.
Acknowledgments
The authors wish to thank past and present members of the Department of Neuroscience for their support, and sharing of ideas and reagents. We especially thank Dr. Martyn White and Dr. Bassel E. Sawaya for their helpful advice and suggestions during the preparation of this manuscript. We also thank C. Schriver for editorial assistance. This work was made possible by grants awarded by NIH to F.P. and S.A.
Contract grant sponsor: NIH; Contract grant numbers: P01 NS43980, R01 MH71162.
Literature Cited
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Al-Sarraj A, Day RM, Thiel G. Specificity of transcriptional regulation by the zinc finger transcription factors Sp1, Sp3, and Egr-1. J Cell Biochem. 2005;94:153–167. doi: 10.1002/jcb.20305. [DOI] [PubMed] [Google Scholar]
- Aprea S, Del Valle L, Mameli G, Sawaya BE, Khalili K, Peruzzi F. Tubulin-mediated binding of human immunodeficiency virus-1 Tat to the cytoskeleton causes proteasomal-dependent degradation of microtubule-associated protein 2 and neuronal damage. J Neurosci. 2006;26:4054–4062. doi: 10.1523/JNEUROSCI.0603-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Wolgemuth DJ. Regulation of M-phase promoting factor activity during development of mouse male germ cells. Dev Biol. 1994;165:500–506. doi: 10.1006/dbio.1994.1270. [DOI] [PubMed] [Google Scholar]
- Chen D, Wang M, Zhou S, Zhou Q. HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J. 2002;21:6801–6810. doi: 10.1093/emboj/cdf683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wang Q, Wang X, Studzinski GP. Up-regulation of Egr-1 by 1,25-dihydroxy vitamin D3 contributes to increased expression of p35 activator of cyclin-dependent kinase 5 and consequent onset of the terminal phase of HL60 cell differentiation. Cancer Res. 2004;64:5425–5433. doi: 10.1158/0008-5472.CAN-04-0806. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. Cdk5: Mediator of neuronal death and survival. Neurosci Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
- Cujec TP, Cho H, Maldonado E, Meyer J, Reinberg D, Peterlin BM. The human immunodeficiency virus transactivator Tat interacts with the RNA polymerase II holoenzyme. Mol Cell Biol. 1997;17:1817–1823. doi: 10.1128/mcb.17.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbinian N, Sawaya BE, Khalili K, Jaffe N, Wortman B, Giordano A, Amini S. Functional interaction between cyclin T1/cdk9 and Puralpha determines the level of TNFalpha promoter activation by Tat in glial cells. J Neuroimmunol. 2001;121:3–11. doi: 10.1016/s0165-5728(01)00372-1. [DOI] [PubMed] [Google Scholar]
- Darbinian-Sarkissian N, Czernik M, Peruzzi F, Gordon J, Rappaport J, Reiss K, Khalili K, Amini S. Dysregulation of NGF-signaling and Egr-1 expression by Tat in neuronal cell culture. J Cell Physiol. 2006;208:506–515. doi: 10.1002/jcp.20675. [DOI] [PubMed] [Google Scholar]
- de Mareuil J, Carre M, Barbier P, Campbell GR, Lancelot S, Opi S, Esquieu D, Watkins JD, Prevot C, Braguer D, Peyrot V, Loret EP. HIV-1 Tat protein enhances microtubule polymerization. Retrovirology. 2005;2:5. doi: 10.1186/1742-4690-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Ernberg I, Gait MJ, Green SM, Heaphy S, Karn J, Lowe AD, Singh M, Skinner MA, Valerio R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci USA. 1989;86:6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epie N, Ammosova T, Sapir T, Voloshin Y, Lane WS, Turner W, Reiner O, Nekhai S. HIV-1 Tat interacts with LIS1 protein. Retrovirology. 2005;2:6. doi: 10.1186/1742-4690-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, Lai KO, Yung WH, Fu AK, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Ostapenko DA, Mathews MB. Potentiation of human immunodeficiency virus type 1 Tat by human cellular proteins. J Virol. 1997;71:7140–7144. doi: 10.1128/jvi.71.9.7140-7144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr-1. Nature Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- Hottiger MO, Nabel GJ. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Chun R, Lin NH, Gatignol A, Glabe CG, Fan H. In vitro and in vivo binding of human immunodeficiency virus type 1 Tat protein and Sp1 transcription factor. J Virol. 1993;67:6224–6233. doi: 10.1128/jvi.67.10.6224-6233.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: Consequences for the central nervous system. Cell Death Differ. 2005;12:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kawai-Kowase K, Kurabayashi M, Hoshino Y, Ohyama Y, Nagai R. Transcriptional activation of the zinc finger transcription factor BTEB2 gene by Egr-1 through mitogen-activated protein kinae pathways in vascular smooth muscle cells. Circ Res. 1999;85:787–795. doi: 10.1161/01.res.85.9.787. [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Li BS, Pant HC. Cyclin-dependent kinase 5 in neurofilament function and regulation. Neurosignals. 2003;12:252–264. doi: 10.1159/000074627. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim KT. Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells. J Neurochem. 2004;91:634–647. doi: 10.1111/j.1471-4159.2004.02770.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim KT. Regulation of cyclin-dependent kinase 5 and p53 by Erk1/2 pathway in the DNA damage-induced neuronal death. J Cell Physiol. 2007;210:784–797. doi: 10.1002/jcp.20899. [DOI] [PubMed] [Google Scholar]
- Lees JF, Shneidman PS, Skuntz SF, Carden MJ, Lazzarini RA. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988;7:1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li J, Kim BO, Pace BS, He JJ. HIV-1 Tat protein-mediated transactivation of the HIV-1 long terminal repeat promoter is potentiated by a novel nuclear Tat-interacting protein of 110 kDa, Tip110. J Biol Chem. 2002;277:23854–23863. doi: 10.1074/jbc.M200773200. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A, Calzado MA, Jimenez-Reina L, Ceballos E, Leon J, Munoz E. Susceptibility of HIV-1-TAT transfected cells to undergo apoptosis. Biochemical mechanisms. Oncogene. 1999;18:7543–7551. doi: 10.1038/sj.onc.1203095. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat mediated activation of glycogen synthase kinase-3b contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP. Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 2002;9:1294–1306. doi: 10.1038/sj.cdd.4401108. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Kizak CA, Nagle JW, Pant HC, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse gene encoding cycline-dependent kinase 5 regulatory subunit p35. Genomics. 1996;35:372–375. doi: 10.1006/geno.1996.0370. [DOI] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: Involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Rolli-Derkinderen M, Machavoine F, Baraban JM, Grolleau A, Beretta L, Dy M. ERK and p38 inhibit the expression of 4E-BP1 repressor of translation through induction of Egr-1. J Biol Chem. 2003;278:18859–18867. doi: 10.1074/jbc.M211696200. [DOI] [PubMed] [Google Scholar]
- Ross S, Tienhaara A, Lee MS, Tsai LH, Gill G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J Biol Chem. 2002;277:4455–4464. doi: 10.1074/jbc.M110771200. [DOI] [PubMed] [Google Scholar]
- Sawaya BE, Khalili K, Gordon J, Taube R, Amini S. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J Biol Chem. 2000;275:35209–35214. doi: 10.1074/jbc.M005197200. [DOI] [PubMed] [Google Scholar]
- Sharma M, Sharma P, Pant HC. CDK-5-mediated neurofilament phosphorylation in SHSY5Y human neuroblastoma cells. J Neurochem. 1999;73:79–86. doi: 10.1046/j.1471-4159.1999.0730079.x. [DOI] [PubMed] [Google Scholar]
- Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004;29:933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: Isolation and characterization. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. Cdk5 in neuroskeletal dynamics. Neurosignals. 2003;12:239–251. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- Song JH, Wang CX, Song DK, Wang P, Shuaib A, Hao C. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem. 2005;280:12896–12901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- Sui Z, Kovacs AD, Maggirwar SB. Recruitment of active glycogen synthase kinase-3 into neuronal lipid rafts. Biochem Biophys Res Commun. 2006a;345:1643–1648. doi: 10.1016/j.bbrc.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Sui Z, Sniderhan LF, Fan S, Kazmierczak K, Reisinger E, Kovacs AD, Potash MJ, Dewhurst S, Gelbard HA, Maggirwar SB. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3beta to antagonize nuclear factor-kappaB survival pathway in neurons. Eur J Neurosci. 2006b;23:2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Veeranna S, Ohshima T, Rajan P, Amin ND, Cho A, Sreenath T, Pant HC, Brady RO, Kulkarni AB. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M, Yasuda H, Kogawa S, Maeda K, Haneda M, Hidaka H, Khashiwagi A, Kikkawa R. Expression and activity of cyclin dependent kinase 5/p35 in adult rat peripheral nervous system. J Neurochem. 1998;71:2600–2606. doi: 10.1046/j.1471-4159.1998.71062600.x. [DOI] [PubMed] [Google Scholar]
- Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor, Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Tsuruda A, Suzuki S, Maekawa T, Oka S. Constitutively active Src facilitates NGF-induced phosphorylation of TrkA and causes enhancement of the MAPK signaling in SK-N-MC cells. FEBS Lett. 2004;560:215–220. doi: 10.1016/S0014-5793(04)00115-2. [DOI] [PubMed] [Google Scholar]
- Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D. Egr-1 promotes growth and survival of prostate cancer cells. Identification of novel Egr-1 target genes. J Biol Chem. 2003;278:11802–11810. doi: 10.1074/jbc.M210279200. [DOI] [PubMed] [Google Scholar]
- Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kulkosky J, Acheampong E, Ninnari G, Sullivan J, Pomerantz RJ. HIV-1-mediated apoptosis of neuronal cells: Proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc Natl Acad Sci USA. 2004;101:7070–7075. doi: 10.1073/pnas.0304859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Dong B, Mittelstadt PR, Xiao H, Ashwell JD. HIV Tat binds Egr proteins and enhances Egr-dependent transactivation of the Fas ligand promoter. J Biol Chem. 2002;277:19482–19487. doi: 10.1074/jbc.M201687200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ahuja HS, Zakeri ZF, Wolgemuth DJ. Cyclin-dependent kinase 5 is associated with apoptotic cell death during development and tissue remodeling. Dev Biol. 1997;183:222–233. doi: 10.1006/dbio.1996.8494. [DOI] [PubMed] [Google Scholar]