Abstract
Individual variation in stable behavioral traits may explain variation in ecologically-relevant behaviors such as foraging, dispersal, anti-predator behavior, and dominance. We investigated behavioral variation in mountain chickadees (Poecile gambeli), a North American parid that lives in dominance-structured winter flocks, using two common measures of behavioral profile: exploration of a novel room and novel object exploration. We related those behavioral traits to dominance status in male chickadees following brief, pair-wise encounters. Low-exploring birds (birds that visited less than four locations in the novel room) were significantly more likely to become dominant in brief, pairwise encounters with high-exploring birds (i.e., birds that visited all perching locations within a novel room). On the other hand, there was no relationship between novel object exploration and dominance. Interestingly, novel room exploration was also not correlated with novel object exploration. These results suggest that behavioral profile may predict the social status of group-living individuals. Moreover, our results contradict the idea that novel object exploration and novel room exploration are always interchangeable measures of individuals' sensitivity to environmental novelty.
Keywords: Behavioral profile, Exploration, Mountain Chickadee, Pocile gambeli, Personality, Social dominance, Temperament
Although individual differences in behavior were long ignored as “noise” around an adaptive mean (Dall et al 2004; Wilson 1998), consistent variation in individual behavioral traits such as exploratory behavior now appears to be associated with variation in many ecologically-relevant behaviors, including dispersal distance, antipredator behavior, aggression toward conspecifics, nest defense and response to social defeat (Koolhaas et al. 1999; Sih et al. 2004, Groothius and Carere 2005; Reale et al. 2007; Hollander 2008). Broadly, exploratory behavior has been shown to correlate with survival and therefore fitness (Smith and Blumstein 2008), although the direction of the association can be context-dependent: in great tits (Parus major), the relationship between exploration and survival is always opposite for males and females, and varies depending on environmental conditions (Dingemanse et al. 2004). Consistent with the terminology of Groothius and Carere (2005), we use the term “behavioral profile” to refer to inter-individual behavioral differences. Commonly used proxies for behavioral profiles in nonhuman animals include escape behavior and exploration of novel objects or environments (Bolhuis et al. 2005; Reale et al. 2000; Verbeek et al. 1994; Hessing et al. 1994; Benus 1991). A number of studies have shown that individual variation in behavioral profile is both repeatable (e.g., Armitage and van Vuren 2003; Schjolden et al. 2005) and at least moderately heritable (Dingemanse et al. 2002; Cockrem 2007), which in conjunction with the fact that behavioral profiles have been shown to influence survival (Dingemanse et al. 2004; Smith and Blumstein 2008), means that such behavioral variation may be susceptible to natural selection.
To fully understand the significance of variation in behavioral profiles, it is crucial to investigate how variation in behavioral profile may affect behaviors directly linked to individual fitness (Smith and Blumstein 2008). In socially living animals, social dominance may strongly influence fitness (e.g., Desrochers 1985; Ekman 1989; Mennill et al. 2004; Otter et al. 2007), and it is thus important to establish whether variation in behavioral profile might be linked to the acquisition of dominance status. Although social interactions are necessary for the formation of dominance hierarchies, individual variation in attributes may also play a significant role in hierarchy formation (Chase et al. 2002). While most work on hierarchy formation has focused on variation in physical attributes, there are reasons to expect that behavioral profiles might also play a substantial role. For example, both aggressiveness and response to social defeat can be determinants of individuals' dominance ranks, and these factors are correlated with exploration-based measures of behavioral profile (Verbeek et al. 1996; Verbeek et al. 1999; Dingemanse and de Goede 2004).
Dominance status can affect both survival (e.g., Piper and Wiley 1990) and reproductive success (e.g., Mennill et al. 2004). Among parids, many of which live in dominance-structured flocks for at least part of the year (Ekman 1989), dominant individuals may benefit from greater access to food resources (Hogstad 1989), access to foraging locations that are safer from predators (Desrochers 1985; Ekman 1989), increased likelihood of siring both within-pair and extrapair offspring (Mennill et al. 2004), greater attractiveness to the opposite sex, and higher mate retention (Otter and Ratcliffe 1996). Recent evidence also shows that subordinate black-capped chickadees (Poecile atricapillus) may be disproportionately affected by food limitation in poor habitats (Otter et al. 2007).
It is also clear that among parids (as well as other birds and mammals), dominants differ both behaviorally and physiologically from subordinates (Verbeek et al. 1999; Barnard and Luo 2002; Pravosudov et al. 2003; Arakawa et al. 2006). Unsurprisingly, dominant black-capped chickadees are more aggressive than subordinates, and are also more likely to approach a feeder when other birds are present (Ficken et al. 1990). In the laboratory, subordinate mountain chickadees (Poecile gambeli) cache less food, are less efficient at cache retrieval, show poorer performance on a memory task and have lower rates of hippocampal cell proliferation than dominants (Pravosudov et al. 2003; Pravosudov & Omanska 2005). Subordinate males also show lower maximal corticosterone levels following acute stress (Pravosudov et al. 2003). However, it is not clear whether these behavioral differences between dominants and subordinates reflect pre-existing variations in behavioral profile or arise as a result of social rank acquisition. Work in mice suggests that the acquisition of dominance status may change exploratory behavior (Arakawa 2006) and learning ability (Barnard and Luo 2002). However, Boogert et al. (2006) demonstrates that in starlings (Sturnus vulgaris), dominant and subordinate birds differ in learning speed prior to the establishment of dominance relationships between birds. Additionally, studies of great tits (Parus major) also demonstrate that individual differences in exploratory behavior and stress responsiveness may predict aggression toward conspecifics (Verbeek et al. 1996), and dominance following pairwise encounters, in aviary groups, and in the wild (Verbeek et al. 1996; Verbeek et al. 1999; Dingemanse and de Goede 2004). Similar results have been found in salmonid fish (Pottinger and Carrick 2001; Schjolden et al. 2005).
The objectives of the current study were to characterize behavioral profiles in a North American parid, the mountain chickadee (Poecile gambeli), and to test whether individual differences in behavioral profile relate to the acquisition of dominance status. We chose the mountain chickadee as a study species because we were specifically interested in how behavioral profile relates to the acquisition of dominance status in a species with fixed flock membership and a rigidly linear dominance hierarchy (by contrast, flocks of great tits, the other parid species in which the relationship between behavioral profile dominance has explicitly been studied, are fluid in both membership and dominance structure, with reversals of dominance status being common; Ekman 1989). Such differences in social structure may be extremely important when considering the effect of behavioral profile on the acquisition of dominance status. In great tits, a species in which challenges to dominants and dominance reversals are common (Ekman 1989), sensitivity to social defeat (which is correlated with exploratory behavior; Verbeek et al. 1999) is crucially important in determining whether individuals are able to maintain their dominance status. In mountain chickadees, the dominance hierarchy appears to be rigid and challenges to dominant birds are exceptionally rare (Ekman 1989), so sensitivity to social defeat seems likely to be far less important in relation to the acquisition and maintenance of dominance status, and thus (assuming that social defeat is generally related to exploration), the relationship between exploration and dominance may be different.
To classify behavioral profiles in mountain chickadees, we chose to use measures of exploratory behavior (both exploration of a novel flight room and behavior toward an unfamiliar object placed in the home cage) because similar measures have been used in studies of dominance in another parid species (Verbeek et al. 1996; Verbeek et al. 1999). Although we relied on measures of exploration collected at a single time point and did not explicitly evaluate the repeatability of exploratory behavior in mountain chickadees, we are confident that exploratory behavior remained reasonably consistent over the time scale of the experiment. No more than three weeks elapsed between testing in the novel room and dominance testing, and a number of studies examining exploration in nonhuman animals have shown that within individuals, exploratory behavior and other indices of “boldness” typically show substantial repeatability over periods of weeks to months, and in many cases much longer (e.g., Verbeek et al. 1994; Dingemanse et al. 2002; Armitage and van Vuren 2003; Schjolden et al. 2005; Quinn and Cresswell 2005).
Behavior in novel environments has been linked to the establishment of dominance relationships in both birds and fish, although the direction of the relationship between exploration of or adjustment to a novel environment and the acquisition of dominance status varies. In great tits, birds that explore a novel environment more quickly generally become dominant in staged dyadic encounters (Verbeek et al. 1996), but are subordinate to slow-exploring birds when housed in multi-individual groups in aviaries (Verbeek et al. 1999). This is thought to be related to the fact that fast-exploring birds are more aggressive, but take longer to recover from social defeat (Verbeek et al. 1999). In rainbow trout (Oncorhynchus mykiss), individuals that acclimate more quickly to a novel tank dominate fish that are slower to acclimate (Schjolden et al. 2005). Among chickadees, dominant individuals are typically more risk-averse than subordinates and prefer to forage in less exposed locations (e.g., Desrochers 1985; Ekman 1989). Assuming that this behavioral difference between dominants and subordinates in fact exists prior to the establishment of dominance relationships, we predicted that mountain chickadees that showed lower levels of exploratory behavior would dominate more exploratory birds, based on the finding that exploration and other indices of what is commonly referred to as “boldness” are generally positively correlated with risk-taking (Sih et al. 2003; van Oers et al. 2004; Quinn and Cresswell 2005; Bell 2005).
Methods
Animals
Subjects were 48 juvenile male mountain chickadees that were captured around Sagehen creek, Tahoe National Forest, California (near Truckee, CA) on September 11-12 2007 using mist nets near feeders. Birds for this study were captured at a network of forty feeders situated at spatially distinct locations spread over a distance of approximately 11 km along two forest roads. Based on extensive observations at these feeders over the last eight years, it is highly unlikely that birds trapped at different feeders on the same day belonged to the same flock (Pravosudov, unpublished data). We captured birds at sixteen feeders (eleven feeders on Sept. 11 and five different feeders that were separated from the original eleven by several km on Sept. 12), and thus the birds that we captured are almost certainly from at least sixteen distinct flocks with non-overlapping membership. Birds were transferred to the laboratory at the University of Nevada, Reno, and were housed individually in wire-mesh cages (60 × 42 × 60 cm) which were visually, though not acoustically, isolated from one another by solid metal partitions between cages. Birds were maintained on a 10h:14h light-dark cycle at a constant 20 C temperature and a mixture of pine nuts, shelled and unshelled sunflower seeds, crushed peanuts, and Roudybush bird pellets (Roudybush Inc., Woodland, CA) was available ad libitum. Each bird was also given 6-10 mealworms daily, and ad libitum water was provided.
After 7 days in captivity, we collected 1 capillary tube (75 μl) of blood from the brachial vein of each bird for genetic sex determination. DNA was extracted from samples using a Qiagen DNEasy kit (Qiagen Inc., Valencia, CA). Sex was determined by amplifying a portion of the sex-linked CHD genes (CHD-W in females only and CHD-Z in both sexes) in a polymerase chain reaction using microsatellite primers P2 and P8 (Griffiths et al. 1998). Sex was confirmed by visual examination of the gonads after birds were sacrificed with an overdose of sodium pentobarbital as part of another experiment. Results of the visual and genetic sexing matched 100%.
Exploration Tests
After birds had been housed in individual cages and visually isolated from other birds for three weeks, each bird was given two tests of exploratory behavior based on the methods of Verbeek et al. (1994). We waited three weeks to administer behavioral tests because our previous research has shown that the level of the stress hormone, corticosterone, returns to its normal, undisturbed level after three weeks in captive settings, suggesting that the birds become habituated to captive housing in approximately three weeks (Pravosudov et al. 2003).
The first test measured exploratory behavior in an unfamiliar room. Each bird was released into an experimental room (325 × 218 × 312 cm) and its behavior observed through a one-way Plexiglas window. The room contained two artificial “trees” with 20 perching sites. Additionally, 36 wood blocks with attached perches were attached by Velcro to two opposite walls (18 perches on each wall). No food was available in the testing room. Every home cage was connected to the experimental room by a door covered by a flap, so that birds could enter the experimental room without handling. The experimenter manipulated the lights and opened the flap so that the bird could enter or leave by flying from the dark to the light. Birds remained in the room until they had visited both trees and at least one perching site on each wall, or for 30 minutes, whichever was sooner. For each bird, we measured the number of perching sites visited during the 30 minute time period. Birds could therefore visit a minimum of 0 sites (if the bird hung from the ceiling or a wall without perches for the entire test period) and a maximum of 4 sites (if the bird visited both “trees” and both walls containing perches). For those birds that visited all four perching sites, we recorded exploration time (the time required for a bird to visit all four perching locations). We also recorded the number of hops a bird made between perches during each visit to a given tree or wall (hereafter “hops per site visit”). Our testing technique differs somewhat from the technique employed by Dingemanse et al. (2004) to assess exploratory behavior in wild-captured great tits. Dingemanse et al. (2004) used the number of flights and hops (which in great tits are known to be tightly correlated with arrival time) during the first two minutes in the novel room as a proxy for exploratory behavior. As we did not know a priori whether activity in the novel room would be correlated with arrival time in mountain chickadees, we also measured the number of sites visited during the testing period as an alternative index of exploration. Birds were considered high-exploring if they visited all four perching locations within 30 min, and low-exploring if they did not. Additionally, although 30 minutes is longer than the time typically allotted for novel-environment tests (Verbeek et al. 1994 allotted 10 min, as did Martins et al. 2007), we argue that the environment was still likely to have been quite novel to the chickadees even after half an hour. It typically takes 2-3 1 h sessions in the flight room for birds to become habituated enough to the room to perform well on memory tasks (e.g., Pravosudov et al. 2003). Additionally, 12 birds failed to visit all four perching locations, suggesting that they may still have been somewhat neophobic.
The second test measured novel object exploration. Birds were videotaped for ten minutes following the introduction of a novel object (a plastic Pink Panther keychain, approximately 8 cm long by 1.5 cm wide) into the home cage. An experimenter hung the novel object from the perch at the front of each bird's home cage, approximately 10 cm from the cage wall. Birds could easily reach the object while standing on the perch. We scored approach behavior toward the novel object during a ten minute time period on a scale of 1 to 4. Birds assigned a score of 1 never landed on the perch with the novel object. Birds assigned a score of 2 landed on the perch with the object but did not approach it. Birds assigned a score of 3 approached to within less than a body length of the novel object, and birds assigned a score of 4 approached the object and made bill contact with it (most birds did so while standing on the perch on which the object was hanging). Timing began immediately after the experimenter placed the object in the cage. To control for the possibility that differences in birds' behavior toward the novel object actually reflected something other than sensitivity to the novel object (e.g., differences in sensitivity to the experimenter's manipulation of the home cage or differences in innate motivation to interact with objects placed in the home cage), birds were first given a control test with a familiar object. A balsa wood toy for small birds (Balsa Buddies Sun, Birdalog.com) was hung from the front wall of each bird's home cage for three days to habituate birds to the object. Following the habituation period, each bird was videotaped for ten minutes after the experimenter hung the familiar object from the front perch of the home cage, and we again scored approach behavior.
To analyze temperament structure in mountain chickadees, we examined the relationship between sites visited in the novel room, novel object approach scores, and average number of hops per perching site visit. We used nonparametric measures because the data were not normally distributed (Shapiro-Wilk test of normality: sites visited, W = 0.56, p < 0.0001; novel object approach score, W = 0.80, p = < 0.0001; hops per visit, W = 0.72, p < 0.0001). P-values ≤ 0.05 were considered to be statistically significant. All tests were 2-tailed. SAS was used for all statistical analyses (SAS Systems, Cary, NC).
Dominance Tests
No more than three weeks after birds were tested in the novel room, pairs of males underwent staged dyadic encounters. Pairs of unfamiliar males taken from known different non-overlapping social groups were introduced into the experimental flight room (the same room used during the initial novel environment exploration tests) to determine within-pair dominance rank. There is some possibility that birds may not have been totally unfamiliar with one another in the lab prior to testing because they were housed in visual but not acoustic isolation and thus may have learned about each other via vocalizations; however, birds used in the dominance tests had never been in visual or physical contact with one another.
Birds were allowed to enter the room without handling, and were observed through the one-way Plexiglas window. We determined dominance rank by recording typical dominance interactions including aggressive interactions (the dominant bird attacked the subordinate while the subordinate bird offered no resistance) and passive displacements (the subordinate bird always gives way to the dominant bird; Lahti et al. 1998; Pravosudov and Lucas 2000; Pravosudov et al. 2003). We always observed birds for at least five minutes (allowing for multiple interactions) before ending the dominance test, despite the fact that dominance in pairs of male mountain chickadees is generally apparent after a single interaction, and reversals are never seen following multiple interactions (Pravosudov and Lucas 2000; Pravosudov et al. 2003). In chickadees, dominance hierarchies are strictly linear without reversals and the relationship between any two given birds reflects their dominance relationship within a social group (Ekman 1989; Hogstad 1989; Lahti et al. 1998; Pravosudov and Lucas 2000; Ratcliffe et al. 2007). After we established the dominance status of each bird, birds were returned to their home cages. Because we recorded which bird behaved in a subordinate fashion (i.e., was chased by the other male or repeatedly yielded perching locations to the other male), the outcomes of these dyadic encounters represent a measure of dominance status, rather than a simple measure of aggression, per se. In fact, in several cases, the dominance relationship appeared to be established (i.e., one bird was repeatedly displaced by the other) with little or no overt aggression toward the subordinate bird on the part of the dominant individual.
We tested 12 pairs of birds consisting of one lower-exploring bird and one higher-exploring bird (i.e., birds that had visited 4 sites were paired with opponents that had visited either 2 or 3 sites). We also staged encounters between thirteen pairs consisting of two high-exploring birds (i.e., birds that had visited all four sites within the novel room) with different exploration times. The outcomes of dyadic encounters between birds of different exploration types and the outcomes of dyadic encounters between high-exploring birds with different exploration times were analyzed separately, and birds were used only once in a given set of dyadic encounters. Birds were at least loosely size-matched by wing length (average difference in wing length between opponents differing in exploration type: 1.33 ± 0.48 mm; average difference in wing length between high-exploring opponents differing in arrival time: 0.45 ± 0.16 mm). Wing lengths of birds tested ranged from 68.5 to 74.5 mm.
Ethical Note
Birds were collected under the U.S. Federal Fish and Wildlife (MB022532) and California State (802017-05) scientific collecting permits. All experiments were performed under Animal Care and Use Protocol #A05/06-39, approved by the University of Nevada, Reno Institutional Animal Care and Use Committee.
Results
Behavioral Traits
All birds visited at least two perching sites in the novel room. Twelve birds failed to visit all four perching locations during the 30 minute observation period and were classified as “low-exploring” (Fig. 1a). Of these 12 low-exploring birds, nine birds visited only two locations in the room, while the remaining three birds visited three perching sites. The remaining 36 birds visited all four perching locations within the room and were classified as “high-exploring.” Among high-exploring birds, latencies to visit all four perching locations within the room (i.e., exploration time) ranged from 4.38 min – 27.3 min, and appeared to follow a roughly bimodal distribution (Fig. 1b).
Figure 1.
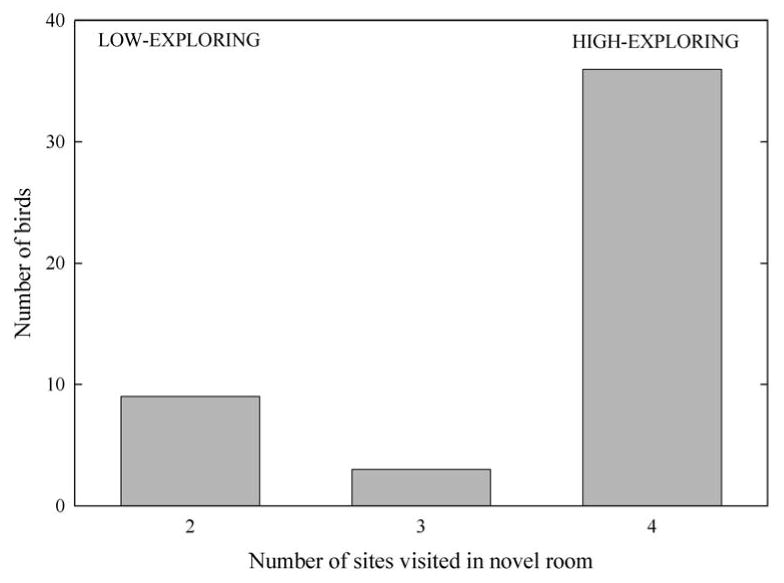
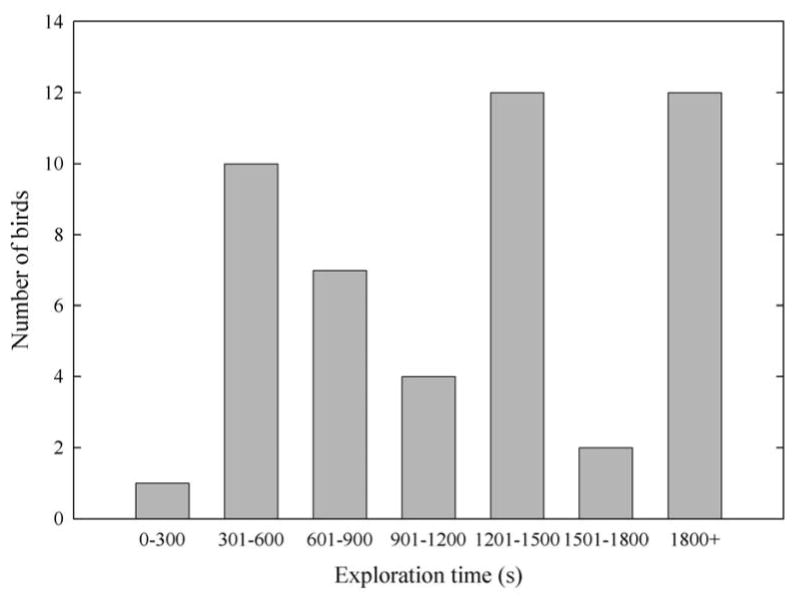
Figure 1a. Distribution of exploration scores for 48 mountain chickadees. Exploration was scored based on the number of perching sites visited in a novel room during a 30 minute time period. There were four possible perching sites. Nine birds visited two sites in the novel room, three birds visited three sites, and 36 birds visited all four possible perching sites.
Figure 1b. Distribution of exploration times for 48 mountain chickadees. Among the 36 high-exploring (i.e., those with exploration times < 1800s, indicating that they visited all four perching sites during the observation period), exploration appeared to follow a roughly bimodal distribution. The remaining 12 birds (low explorers) failed to visit all four perching sites during the observation period, but we can draw no conclusions about the distribution of exploration times for birds with exploration times greater than 30 min.
The association between the number of sites visited in the novel room and the average number of hops that a bird made during a single site visit was not statistically significant, though it did approach significance (Kruskal-Wallis test: 2 d.f., χ2 = 5.19, P = 0.075), with low-exploring birds (those with novel-room scores of 2 and 3 sites visited) tending to make more hops during a single site visit and high-exploring birds tending to make fewer hops during a single site visit (Fig. 2). Two outliers were excluded from this analysis: among 46 of the males tested, the average number of hops per visit ranged from 0.33 – 9.87, while the two outlier males (a high-exploring male and a low-exploring male, respectively) had average numbers of hops per visit of 17.70 and 51.30.
Figure 2.
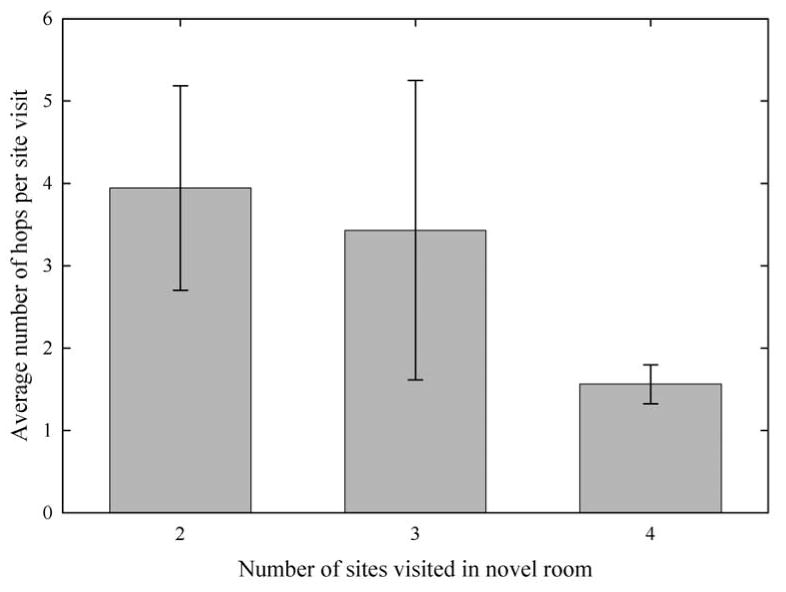
The association between exploration score and the average number of hops per site visit in the novel room approached statistical significance (P = 0.075). Low-exploring birds tended to make more hops per site visit than high-explorers. N = 46 (novel environment score of 2: N = 9; novel environment score of 3: N = 2; novel environment score of 4: N = 35; two extreme outliers were excluded from the analysis).
With regard to behavior toward a novel object, all birds received a score of at least 2, indicating that they, at minimum, landed on the perch with the novel object. Novel object scores were fairly evenly distributed within the population (Fig. 3), with 12 birds receiving a score of 2 (landed on the perch with the object), 19 birds receiving a score of 3 (approached within one body length of the object), and 17 birds receiving a score of 4 (contacted the object with the bill). There was no association between novel object approach score and the number of sites visited in the novel room (Kruskal-Wallis test: 2 d.f., χ2 = 1.60, P = 0.45). There was no difference between birds with novel object approach scores of 2, 3, or 4 with regard to their approach scores when tested with the familiar object (Kruskal-Wallis test: 2 d.f., χ2 = 0.87, P = 0.64), indicating that novel object approach scores were unlikely to be confounded with variation in how birds responded to the experimenter or with differences in innate motivation to explore objects placed in the cage. However, birds were apparently habituated to the familiar object: only 17 birds actually contacted the novel object with their bills, while 34 birds touched the familiar object. This difference was highly significant (χ2 = 12.09, 1 d.f., P = 0.001).
Figure 3.
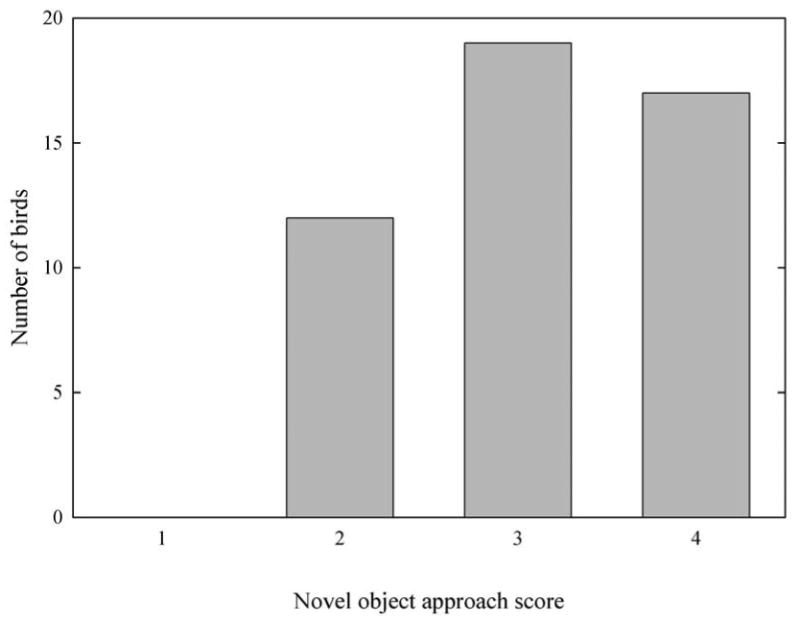
Distribution of novel object approach scores for 48 male mountain chickadees. A novel object was suspended from a perch in each bird's home cage for 10 min, and each bird was scored according to how closely it approached the novel object. A score of 1 indicates the bird never landed on the perch with the object (N = 0), a score of 2 indicates that the bird landed on the perch but failed to approach the object (N = 12), a score of 3 indicates that the bird approached to within one body length of the object (N = 19), and a score of 4 indicates that the bird touched the object with its bill (N = 17).
Behavioral Profile and Dominance
In staged dyadic encounters between birds of different exploration type, birds with lower exploration scores were more likely to become dominant. Birds that became dominant following these encounters had previously visited an average of 2.5 ± 0.23 sites in the novel room, while birds that became subordinate had visited an average of 3.58 ± 0.23 sites in the novel room (Fig. 4). This difference was significant (Wilcoxon two-sample test: N1 = N2 = 12, U = 106.00, P = 0.005). We used a binomial test to test whether, across pairs, low-exploring birds were more likely to win dominance encounters with high-exploring birds. In 10 of 12 dyadic encounters between birds of different exploration score, the lower-exploring bird became dominant, a significantly greater proportion than expected by chance (binomial test: N = 12, x ≥ 10, P = 0.038).
Figure 4.
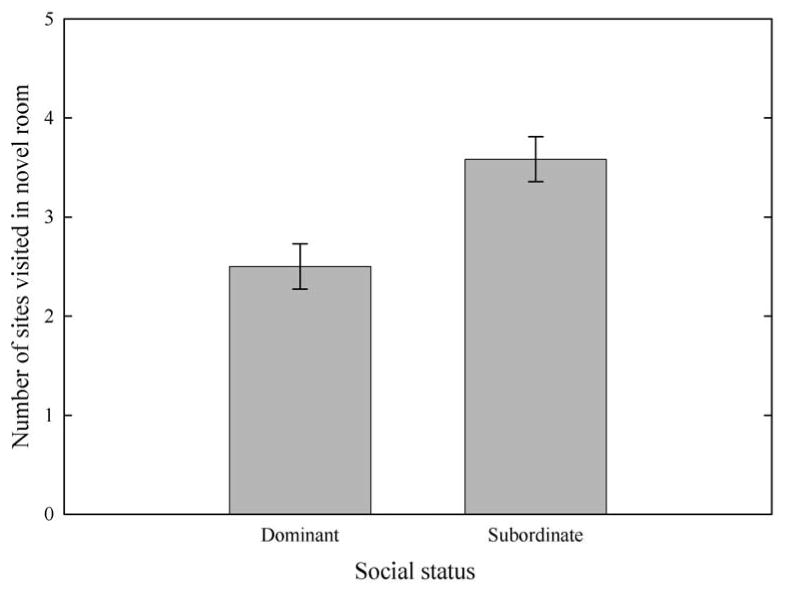
Exploration scores for dominant and subordinate birds in staged dyadic encounters between male mountain chickadees of different exploration types. N = 12 dyads.
While exploration type clearly affected the outcomes of staged dominance encounters between low- and high-exploring birds, variation in exploration time (another commonly used proxy for exploratory behavior; Verbeek et al. 1994) had no significant effect on the outcome of dominance encounters between two high-exploring birds (Wilcoxon two-sample test: N1 = N2 = 13, U = 154.00, P = 0.29). There was no association between the other measures of behavioral profile and the outcome of dominance encounters in either encounters between birds with different exploration scores (Wilcoxon two-sample test: N1 = N2 = 12; hops per site visit, U = 167.00, P = 0.34; novel object approach score, U = 158.00, P = 0.64) or in encounters between high-explorers that differed in exploration time (Wilcoxon two-sample test: N1 = N2 = 13; hops per site visit, U = 157.00, P = 0.36, novel object approach score, U = 158.00, P = 0.35).
Discussion
Characterization of Behavioral Profile in Mountain Chickadees
Mountain chickadees exhibit considerable inter-individual variation in exploratory behavior. Individuals show substantial differences in the extent to which they explore a novel environment (i.e., number of perching sites visited in the novel room), the extent to which they explore individual perching sites within the novel environment (i.e., number of hops per site visit), and their behavior toward novel objects. A relationship that approached significance between number of perching sites visited during the novel room test and the average number of perch hops per site visit suggests that low-exploring birds may explore individual perching sites more thoroughly than high-exploring birds. This finding may be parallel to the results of Verbeek et al. (1994), who showed that great tits that took longer to explore a novel room did so in part because they explored the room more thoroughly. However, we note that in our data, this finding could also simply be an artifact of low-exploring birds visiting fewer sites in the novel room than high-exploring birds, causing their perch hops to be spread among fewer possible sites.
The distribution of exploration types among the 48 males tested, with only three males exhibiting what might be considered an intermediate level of exploration (i.e., visiting three of the possible four locations in the room), parallels the roughly bimodal distribution of exploratory behavior and other measures of “coping style” that have been widely reported in studies of fish (e.g., Schjolden et al. 2005), birds (e.g., Verbeek et al. 1994), and mammals (e.g., Benus et al. 1991). Four times as many males in our laboratory population were classified as high-exploring as were classified as low-exploring. Whether this finding reflects the actual distribution of behavioral profiles within the wild population from which these birds were drawn or merely reflects the fact that high-exploring birds may have been more likely to enter our mist nets is unknown.
Behavior toward novel objects is another measure that is commonly used in studies of behavioral profiles or “personalities” in animals. Novel object approach scores were much more evenly distributed than exploration types among our male mountain chickadees. Moreover, we found no relationship between exploration type and novel object scores: high-exploring birds were no more likely than low-exploring birds to make physical contact with an unfamiliar object placed in their home cage. This finding does not simply reflect a broad-based insensitivity to the novelty of objects: twice as many birds touched the familiar object with their bills as touched the novel object, suggesting that birds were in fact more fearful of the unfamiliar object. Therefore our results suggest that while mountain chickadees are in fact sensitive to whether or not an object is familiar, and while individual birds vary in their willingness to approach unfamiliar objects, this variation is not predicted by exploration type. It seems likely that in mountain chickadees, exploration type and novel object approach score are proxies for two different stable behavioral traits. Martins et al. (2007) found an identical result in zebra finches (Taenopygia guttata), in which the correlation between the scores from novel environment and novel objects tests was not significantly different from zero. Similarly, Moretz et al. (2007) found that in zebrafish (Danio rerio) various measures of “boldness” were not consistently correlated across laboratory strains. Additionally, in another parid species, research has shown that novel environment exploration and novel object exploration may have different genetic bases, though both traits are at least moderately heritable and show some correlation with one another (van Oers et al. 2004). Both our findings and the results of Martins et al. (2007), Moretz et al. (2007), and van Oers et al. (2004) suggest that researchers should be cautious in assuming that various measures of “boldness” or “exploration” are interchangeable – an assumption that seems to be fairly pervasive in reviews and meta-analyses dealing with the ecological implications of behavioral profile or temperament in animals (e.g., Gosling 2001; Reale et al. 2007; Smith and Blumstein 2008).
Behavioral Profiles and Dominance in Mountain Chickadees
While some research has shown that behavioral differences between dominants and subordinates may arise as a result of the establishment of dominance relationships (Arakawa 2006; Barnard and Luo 2002), our results suggest that dominant individuals may also differ behaviorally from subordinates prior to the establishment of social rank. Specifically, birds that were classified as low-exploring prior to the staged dyadic encounters dominated high-exploring birds in almost all cases. Parallel results have been seen in fish, though the direction of the relationship between reactivity to the stress of being in a novel environment and dominance may be different. In juvenile rainbow trout, fish that mount a low plasma cortisol response to being moved to a novel tank are significantly more likely to become dominant in staged dominance encounters with high-responding conspecifics (Schjolden et al. 2005). While we do not know for certain whether differences in exploration type may be associated with differences in stress responsiveness in mountain chickadees, this seems like a reasonable hypothesis: in Japanese quail (Coturnix japonica), chickens (Gallus domesticus), and great tits, birds that behave more fearfully in tests of novel environment exploration typically mount a greater corticosterone response to acute stress (reviewed in Cockrem 2007).
It is also entirely possible that there is no direct causal link between exploration type and the acquisition of dominance status, or even between stress responsiveness and the acquisition of dominance status. Instead, as suggested by Pottinger and Carrick (2001), stress responsiveness and exploratory behavior may simply covary with some other trait that more directly affects competitive ability, such as aggression (e.g., Verbeek et al. 1996; Armitage and van Vuren 2003; Bolhuis et al. 2005) or response to social defeat (Verbeek et al. 1999). In captive-reared great tits, more-exploratory birds are more aggressive and tend to dominate less-exploratory birds in dyadic encounters (Verbeek et al. 1996). However, less-exploratory birds take less time to recover from social defeat and are more likely to acquire high status when housed in larger groups (Verbeek et al. 1999). Dingemanse and de Goede (2004) found similar results in wild great tits: while “fast”-exploring territorial males dominate “slow”-exploring territorial males at feeding trays, among flock-living nonterritorial males, the “slow” males dominate, presumably because they are better able to cope with the stress of living in a large group. Because the social structure of mountain chickadee flocks is so different from that of great tits (Ekman 1989), it is a bit difficult to interpret our results in the context of what has been observed in great tits, though it is certainly possible that low-exploring male mountain chickadees, while they are apparently more reactive to environmental novelty than high-exploring males, may also be better able to cope with social stress and therefore might perform better in dyadic encounters. It is also quite possible that low-exploring chickadees are more aggressive than high-exploring males. Both of these hypotheses warrant further testing. However, what is clear from the results of the present study is that, at least in mountain chickadees, some of the behavioral differences that have been observed between dominant and subordinate birds exist prior to the establishment of dominance relationships. Specifically, dominant chickadees have been shown to be more risk-averse than subordinate birds (Desrochers 1985; Ekman 1989) and it now seems likely that this behavioral difference may actually precede the establishment of social relationships within winter flocks.
It is also worth noting that, at least in terms of the establishment of dominance relationships, difference in exploration type is apparently more salient than are differences in exploration time (i.e., latency to visit all four perching locations) between high-exploring birds, despite the fact that differences in exploration time can be substantial. While exploration type is a significant predictor of dominance in encounters between low- and high- exploring birds, latency to visit all four perching locations in the novel room does not predict dominance status in encounters between two high-exploring birds. This suggests that low explorers and high explorers are more different from one another behaviorally than are high explorers with very different exploration times.
Taken together, the results of the present study suggest that variation in behavioral profile may affect the establishment of dominance relationships and that measurements of exploratory behavior are a potentially useful tool for characterizing behavioral profiles in parids. Additionally, our results strongly suggest that the observed differences in risk sensitivity between dominant and subordinate chickadees may be present before dominance relationships are established. In a broader sense, the present study adds further weight to a substantial body of research that suggests that behavioral profiles or animal “personalities” affect behaviors directly linked to fitness, including the acquisition of dominance status, antipredator behavior, and dispersal (Verbeek et al. 1996; Wilson 1998; Armitage and van Vuren 2003; Dingemanse et al. 2003 Quinn and Cresswell 2005) and that therefore the study of behavioral profiles is important for understanding the evolutionary maintenance of behavioral variation both within and between species and populations, as suggested by Dingemanse and Reale (2005).
Figure 5.
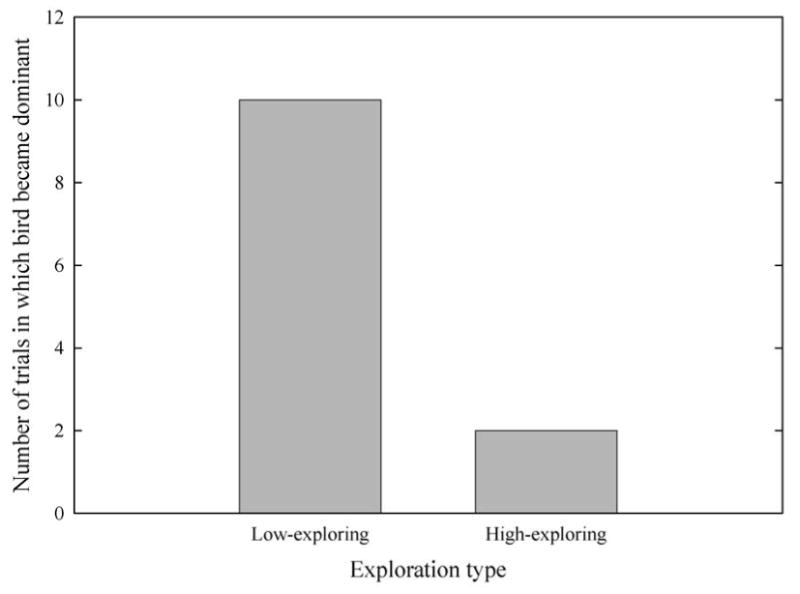
Number of staged dyadic encounters in which the low-exploring bird became dominant (N = 10) versus the number of staged dyadic encounters in which the high-exploring bird became dominant (N = 2). N = 12 dyadic encounters.
Acknowledgments
This study was supported by grants from the National Science Foundation (IOB-0615021) and from the National Institutes of Health (MH079892 and MH076797) to Vladimir Pravosudov. We thank Geniveve Hanson, Kathleen Cornfield and Ashley Rolfe for help in bird maintenance and in running the experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa H. Changes in the pattern of exploratory behavior are associated with the emergence of social dominance relationships in male rats. Developmental Psychobiology. 2006;48:39–47. doi: 10.1002/dev.20114. [DOI] [PubMed] [Google Scholar]
- Armitage KB, Van Vuren DH. Individual differences and reproductive success in yellow-bellied marmots. Ethology, Ecology, and Evolution. 2003;15:207–233. [Google Scholar]
- Barnard CJ, Luo N. Acquisition of dominance status affects maze learning in mice. Behavioural Processes. 2002;60:53–59. doi: 10.1016/s0376-6357(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Bell A. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) Journal of Evolutionary Biology. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;47:1008–1019. doi: 10.1007/BF01923336. [DOI] [PubMed] [Google Scholar]
- Bolhuis JE, Schouten WGP, Schrama JW, Weigant VM. Individual coping characteristics, aggressiveness and fighting strategies in pigs. Animal Behaviour. 2005;69:1085–1091. [Google Scholar]
- Boogert NJ, Reader SM, Laland KN. The relation between social rank, neophobia, and individual learning in starlings. Animal Behaviour. 2006;72:1229–1239. [Google Scholar]
- Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proceedings of the National Academy of Science. 2002;99:5744–5749. doi: 10.1073/pnas.082104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrem JF. Stress, corticosterone responses, and avian personalities. Journal of Ornithology. 2007;148:S169–S178. [Google Scholar]
- Coleman K, Wilson DS. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Animal Behaviour. 1998;56:927–936. doi: 10.1006/anbe.1998.0852. [DOI] [PubMed] [Google Scholar]
- Dall SRX, Houston AI, McNamara JM. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecology Letters. 2004;7:734–739. [Google Scholar]
- Desrochers A. Sex, dominance, and microhabitat use in wintering black-capped chickadees: a field experiment. Ecology. 1985;70:636–645. [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ. Repeatability and heritability of exploratory behaviour in great tits from the wild. Animal Behaviour. 2002;64:929–938. [Google Scholar]
- Dingemanse N, Both C, van Noordjwijk AJ, Rutten AL, Drent PJ. Natal dispersal and personalities in great tits (Parus major) Proceedings of the Royal Society of London, Series B. 2003;270:741–747. doi: 10.1098/rspb.2002.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society of London, Series B. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, de Goede P. The relation between dominance and exploratory behavior is context-dependent in wild great tits. Behavioral Ecology. 2004;15:1023–1030. [Google Scholar]
- Dingemanse NJ, Reale D. Natural selection and animal personality. Behaviour. 2005;142:1165–1190. [Google Scholar]
- Ekman J. Ecology of non-breeding social systems of Parus. Wilson Bulletin. 1989;101:263–288. [Google Scholar]
- Ficken MS, Weise CM, Popp JM. Dominance rank and resource access in winter flocks of black-capped chickadees. Wilson Bulletin. 1990;102:623–633. [Google Scholar]
- Gosling SD. From mice to men: what can we learn about personality from animal research? Psychological Bulletin. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Molecular Ecology. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Groothius TGG, Carere C. Avian personalities: characterization and epigenesis. Neuroscience and Biobehavioral Reviews. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Hessing MJC, Hagelso AM, Schouten WGP, Wiepkema PR, van Beek JAM. Individual behavioral and physiological strategies in pigs. Physiology and Behavior. 1994;55:39–46. doi: 10.1016/0031-9384(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Hogstad O. Social organization and dominance behavior in some Parus species. Wilson Bulletin. 1989;101:254–262. [Google Scholar]
- Hollander FA, van Overveld T, Tokka I, Matthysen E. Personality and nest defence in the great tit (Parus major) Ethology. 2008;114:405–412. [Google Scholar]
- Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, de Jong IC, Ruis W, Blokhuis HJ. Coping styles: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lahti K. Social dominance and survival in flocking passerine birds: a review with an emphasis on the Willow tit Parus montanus. Ornis Fennica. 1998;75:1–17. [Google Scholar]
- Martin JGA, Reale D. Temperament, risk assessment, and habituation to novelty in eastern chipmunks, Tamius striatus. Animal Behaviour. 2007;75:309–318. [Google Scholar]
- Martins TLF, Roberts ML, Giblin I, Huxham R, Evans MR. Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Hormones and Behavior. 2007;52:445–453. doi: 10.1016/j.yhbeh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Mennill DJ, Ramsay SM, Boag PT, Ratcliffe LM. Patterns of extrapair mating in relation to male dominance status and female nest placement in black-capped chickadees. Behavioral Ecology. 2004;5:757–765. [Google Scholar]
- Moretz JA, Martins EP, Robison BD. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behavioral Ecology. 2007;18:556–562. [Google Scholar]
- Otter K, Ratcliffe L. Female initiated divorce in a monogamous songbird: abandoning mates for males of higher quality. Proceedings of the Royal Society of London, Series B. 1996;263:351–355. [Google Scholar]
- Otter KA, Atherton SE, van Oort H. Female food solicitation calling, hunger levels, and habitat differences in the black-capped chickadee. Animal Behaviour. 2007;74:847–853. [Google Scholar]
- Piper WH, Wiley RH. The relationship between social dominance, subcutaneous fat, and annual survival in wintering white-throated sparrows. Behavioral Ecology and Sociobiology. 1990;26:201–208. [Google Scholar]
- Pottinger TG, Carrick TR. Stress responsiveness affects dominant-subordinate relationships in rainbow trout. Hormones and Behavior. 2001;40:419–427. doi: 10.1006/hbeh.2001.1707. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Lucas JR. The effect of social dominance on fattening and food-caching behaviour in Carolina chickadees. Animal Behaviour. 2000;60:483–493. doi: 10.1006/anbe.2000.1506. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Mendoza SP, Clayton NS. The relationship between dominance, corticosterone, memory, and food-caching in mountain chickadees (Poecile gambeli) Hormones and Behavior. 2003;44:93–102. doi: 10.1016/s0018-506x(03)00119-3. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Omanska A. Dominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. Journal of Neurobiology. 2005;62:31–41. doi: 10.1002/neu.20065. [DOI] [PubMed] [Google Scholar]
- Quinn JL, Cresswell W. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour. 2005;142:1377–1402. [Google Scholar]
- Ratcliffe L, Mennill DJ, Schubert KA. Social dominance and fitness in black-capped chickadees. In: Otter KA, editor. Ecology and Behavior of Chickadees and Titmice, an Integrated Approach. Oxford, UK: Oxford University Press; 2007. pp. 131–146. [Google Scholar]
- Reale D, Gallant BY, Leblanc M, Festa-Bianchet M. Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Animal Behaviour. 2000;60:589–597. doi: 10.1006/anbe.2000.1530. [DOI] [PubMed] [Google Scholar]
- Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament with ecology and evolution. Biological Reviews. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Schubert KA, Mennill DJ, Ramsay SM, Otter KA, Boag PT, Ratcliffe LM. Variation in social rank acquisition influences lifetime reproductive success in black-capped chickadees. Biological Journal of the Linnean Society. 2007;90:85–95. [Google Scholar]
- Schjolden J, Stoskhus A, Winberg S. Does individual variation in stress responses and agonistic behavior reflect divergent stress coping strategies in juvenile rainbow trout? Physiological and Biochemical Zoology. 2005;78:715–723. doi: 10.1086/432153. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RM. Behavioural syndromes: an integrative overview. Quarterly Review of Biology. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: a meta-analysis. Behavioral Ecology. 2008;19:448–455. [Google Scholar]
- Smith SM. Social influences on the dynamics of a northeastern black-capped chickadee population. Ecology. 1994;75:2043–2051. [Google Scholar]
- van Oers K, Drent PJ, de Jong G, van Noordwijk AJ. Additive and nonadditive variation in avian personality traits. Heredity. 2004;93:496–503. doi: 10.1038/sj.hdy.6800530. [DOI] [PubMed] [Google Scholar]
- van Oers K, Drent PJ, de Goede P, van Noordwijk AJ. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proceedings of the Royal Society of London, Series B. 2004;271:65–73. doi: 10.1098/rspb.2003.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort H, Otter KA, Fort KT, Holschuh CI. Habitat quality, social dominance, and dawn chorus song output in black-capped chickadees. Ethology. 2006;112:772–778. [Google Scholar]
- Verbeek MEM, Drent PJ, Wiepkema PR. Consistent individual differences in early exploratory behaviour of male great tits. Animal Behaviour. 1994;48:1113–1121. [Google Scholar]
- Verbeek ME, Boon A, Drent PJ. Exploration, aggressive behaviour, and dominance in pair-wise confrontations of juvenile male great tits. Behaviour. 1996;133:23–48. [Google Scholar]
- Verbeek ME, DeGoede P, Drent PJ, Wiepkema PR. Individual behavioral characteristics and dominance in aviary groups of Great Tits. Behaviour. 1999;136:23–48. [Google Scholar]
- Wilson DS. Adaptive individual differences within single populations. Philosophical Transactions of the Royal Society of London, Series B. 1998;353:199–205. [Google Scholar]


