Abstract
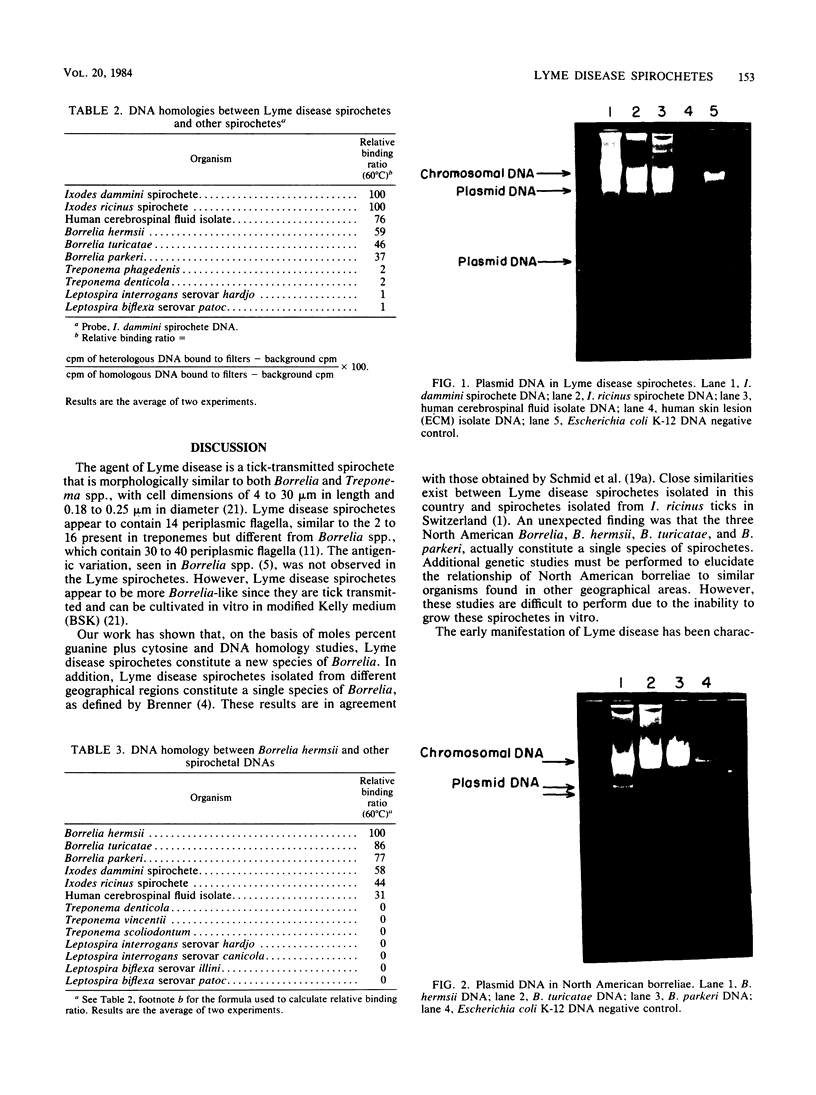
Genetic studies were performed on the following spirochetes: three Lyme disease spirochetes isolated from Ixodes ticks and from human spinal fluid; three species of North American borreliae; four species of Treponema; and two species of Leptospira. The mol% G+C values for Lyme disease spirochetes were 27.3 to 30.5%, similar to values of 28.0 to 30.5% for Borrelia species but different from the values of Leptospira or Treponema species which ranged from 35.3 to 53%. Lyme disease spirochetes represent a new species of Borrelia, with DNA homologies of 31 to 59% with the three North American strains of Borrelia studied. These studies also showed that Lyme disease spirochetes from three sources constituted a single species, with DNA homologies ranging from 76 to 100%. A high degree of relatedness was also seen between the three North American borreliae, with homology varying from 77 to 95%, indicating that these spirochetes represent a single species. Lyme disease spirochetes and Borrelia species exhibited almost no homology with Leptospira and Treponema species (0 to 2%). Plasmids were detected in the three Lyme disease spirochetes and in the three North American borreliae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C. Protein-free and low-protein media for the cultivation of Leptospira. Infect Immun. 1978 Feb;19(2):562–569. doi: 10.1128/iai.19.2.562-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Keirans J. E. Ticks and Lyme disease in the United States. Ann Intern Med. 1983 Jul;99(1):121–121. doi: 10.7326/0003-4819-99-1-121. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Haapala D. K., Rogul M., Evans L. B., Alexander A. D. Deoxyribonucleic acid base composition and homology studies of Leptospira. J Bacteriol. 1969 May;98(2):421–428. doi: 10.1128/jb.98.2.421-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L. Nucleic acid reassociation as a guide to genetic relatedness among bacteria. Curr Top Microbiol Immunol. 1974;64(0):105–128. doi: 10.1007/978-3-642-65848-8_4. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Plasmid DNA in Treponema pallidum (Nichols): potential for antibiotic resistance by syphilis bacteria. Science. 1981 Jul 31;213(4507):553–555. doi: 10.1126/science.6264606. [DOI] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid G. P., Steigerwalt A. G., Johnson S. E., Barbour A. G., Steere A. C., Robinson I. M., Brenner D. J. DNA characterization of the spirochete that causes Lyme disease. J Clin Microbiol. 1984 Aug;20(2):155–158. doi: 10.1128/jcm.20.2.155-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]