Abstract
OBJECTIVE
There is evidence of gut barrier and immune system dysfunction in some patients with type 1 diabetes, possibly linked with exposure to dietary wheat polypeptides (WP). However, questions arise regarding the frequency of abnormal immune responses to wheat and their nature, and it remains unclear whether such responses are diabetes specific.
RESEARCH DESIGN AND METHODS
In type 1 diabetic patients and healthy control subjects, the immune response of peripheral CD3+ T-cells to WPs, ovalbumin, gliadin, α-gliadin 33-mer peptide, tetanus toxoid, and phytohemagglutinin was measured using a carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assay. T–helper cell type 1 (Th1), Th2, and Th17 cytokines were analyzed in WP-stimulated peripheral blood mononuclear cell (PBMNC) supernatants, and HLA was analyzed by PCR.
RESULTS
Of 42 patients, 20 displayed increased CD3+ T-cell proliferation to WPs and were classified as responders; proliferative responses to other dietary antigens were less pronounced. WP-stimulated PBMNCs from patients showed a mixed proinflammatory cytokine response with large amounts of IFN-γ, IL-17A, and increased TNF. HLA-DQ2, the major celiac disease risk gene, was not significantly different. Nearly all responders carried the diabetes risk gene HLA-DR4. Anti-DR antibodies blocked the WP response and inhibited secretion of Th1 and Th17 cytokines. High amounts of WP-stimulated IL-6 were not blocked.
CONCLUSIONS
T-cell reactivity to WPs was frequently present in type 1 diabetic patients and associated with HLA-DR4 but not HLA-DQ2. The presence of an HLA-DR–restricted Th1 and Th17 response to WPs in a subset of patients indicates a diabetes-related inflammatory state in the gut immune tissues associated with defective oral tolerance and possibly gut barrier dysfunction.
The gastrointestinal tract contains the largest collection of immune cells in the body. In healthy individuals, the gut immune system does not normally mount an immune response against molecules from foods and commensal bacteria, preferring a default state of immune unresponsiveness called oral tolerance (1).
When oral tolerance is broken, an immune imbalance results that can lead to increased gut permeability, inflammation, and tissue damage. The best understood example of this is celiac disease, which is the classic food-induced autoimmune disorder and the only autoimmune disease for which the autoantigen (tissue transglutaminase) and the inciting environmental factors (gluten proteins) are known (2). In celiac disease, specific wheat gliadin peptides undergo deamidation by gut mucosal tissue transglutaminase and are presented to T-cells on HLA-DQ2 or HLA-DQ8 molecules, resulting in the stimulation of a T–helper cell type 1 (Th1)-biased proinflammatory attack that causes villous atrophy (2). It has also been proposed that the gut and dietary antigens play an important role in human type 1 diabetes, based on animal studies, epidemiological reports, and a small number of studies on human tissue (3–5).
The gut barrier and immune system in diabetes-prone rodents display abnormalities similar to those of celiac disease. For example, there are signs of enteropathy in BBdp rats (6) and NOD mice (7) and inflammatory cytokines in the gut are increased (8,9), as is permeability before islet inflammation (10–12). Closing gut-tight junctions prevents diabetes in the rat (12), there is increased antibody and T-cell response to dietary antigens (13,14), and wheat-based diets are major promoters of diabetes in rats and mice (4). Diabetes appearance can be partly inhibited by early neonatal feeding of small amounts of wheat proteins to BBdp rats by dampening the proinflammatory state of the gut (8). There are also indications that a gluten-free diet can enhance islet mass in BB rats (15). High-risk children on a gluten-free diet for 6 months showed enhanced first-phase insulin response during an intravenous glucose tolerance test, which could be an indication of increased β-cell mass and/or function (16,17). Thus, wheat is one external factor that could influence the development of diabetes.
Normal regulation of the gut immune system depends on maintaining the integrity of the gut barrier (18). There are now several reports of gut inflammation and signs of gut damage or leakage in humans with type 1 diabetes (19–24). T-cells from human diabetic pancreas display gut mucosal homing properties (25), and T-cells reactive against the diabetes autoantigen GAD express the gut-associated homing receptor α4β7-integrin (26). In two prospective analyses of high-risk children, early exposure to cereals including wheat increased the risk of islet autoimmunity (27,28). Another study showed increased T-cell proliferation in response to high concentrations of wheat gluten in 24% of patients (29). Auricchio et al. (30) reported inflammation and increased immune response to gliadin in jejunal biopsies from patients. Approximately 2–6% of patients with type 1 diabetes have celiac disease, a rate that is several times higher than in the general population, and a recent report indicated that celiac disease patients on a gluten-free diet were protected from later development of type 1 diabetes (31). These findings point to both a loss of barrier integrity and dysregulation in the gut immune system.
Thus, questions arise regarding the frequency of abnormal immune responses to wheat and their nature, and it remains unclear whether such responses are diabetes related, reflect a separate gut dysfunction, or are simply due to shared celiac risk genes, such as HLA-DQ2, HLA-DQ8, and HLA-DR3. The number of studies examining the response of immune cells to wheat proteins in type 1 diabetic patients is limited (20,29,30), and it remains unclear whether there is a genetically determined abnormal immune response to wheat in humans at risk for type 1 diabetes. We favor the view that there is a diabetes-specific abnormal immune response to wheat in some patients that is not explained by shared celiac disease risk genes.
We hypothesize that in some type 1 diabetic patients, excessive amounts of wheat proteins/polypeptides enter the body through a leaky gut barrier and promote an abnormal immune response that breaks oral tolerance and stimulates immune cells that are involved in type 1 diabetes pathogenesis (4). As a first step, it is important to clarify what proportion of patients with type 1 diabetes display abnormal immune reactivity to dietary wheat polypeptides (WPs) and to characterize this abnormal response. The objectives of the present study were to determine whether patients with type 1 diabetes display increased T-cell proliferation in response to a mixture of WPs, to analyze the pattern of cytokines produced, and to determine whether this reactivity is associated with specific HLA alleles.
RESEARCH DESIGN AND METHODS
Type 1 diabetic patients were recruited through physicians at The Ottawa Hospital and the Children's Hospital of Eastern Ontario, Ottawa, Canada. All patients have clinically proven type 1 diabetes. The majority of the 42 subjects were young adults of both sexes, with one child included. A total of 22 unrelated control subjects of a similar age range and ethnic group (Caucasian) without acute infection or autoimmune disease were recruited (Table 1). All individuals included in the study were negative for tissue transglutaminase antibody, as measured by the Ottawa Hospital Clinical Laboratory. Blood was obtained by venepuncture from patients and healthy control subjects with informed consent. The local ethics committees approved the study.
TABLE 1.
Description of control subjects and patients with type 1 diabetes
| Group | n | Sex ratio (female/male) | Age | Duration of diabetes (years) |
|---|---|---|---|---|
| Type 1 diabetic patients | 42 | 28/14 | 26.7 (8–41) | 11.2 ± 7 |
| Healthy control subjects | 22 | 11/11 | 24.5 (18–32) | NA |
Data are means (range) or means ± SD unless otherwise indicated. NA, not applicable.
Isolation of mononuclear cells, tissue culture, and antigen response.
Peripheral blood mononuclear cells (PBMNCs) were isolated by density gradient centrifugation over Ficoll (Histopaque 1.077; Sigma Aldrich, Oakville, ON, Canada). Cells were washed twice with Hank's buffer (Invitrogen, Burlington, ON, Canada) containing 20 mmol/l HEPES (Invitrogen). PBMNCs (20 × 106/ml) were labeled with 2 mmol/l carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) for 20 min and incubated at 37°C in 5% CO2. Cells were washed twice with Hank's buffer containing 5% pooled human AB+ serum (Bioreclamation, Hicksville, NY) and finally diluted in RPMI-1640 (Sigma Aldrich) containing 5% human AB+ serum, 2 mmol/l l-glutamine (Invitrogen), 25 mmol/l HEPES (Invitrogen), 50 μmol/l β-mercaptoethanol (Sigma Aldrich), and 1% antibiotic/antimycotic (Invitrogen). Cells were cultured with various concentrations of wheat protein/peptides (chymotrypsin-treated, heat-inactivated WP; ICN Biochemicals, Cleveland, OH [(32)]) (3.1–12.4 μg/ml), 10 μg/ml gliadin (Sigma Aldrich), 10 μg/ml α-gliadin 33-mer peptide (a generous gift from Dr. Chaitan Khosla, Stanford University, Stanford, CA, and Dr. Hubert Kolb, German Diabetes Center, Düsseldorf, Germany), 10 μg/ml insulin (Sigma Aldrich), 1 μmol ovalbumin (Sigma Aldrich), 2.7 LF/ml tetanus toxoid (Connaught Laboratory, Toronto, ON, Canada), or 5 μg/ml phytohemagglutinin (Sigma Aldrich). 1.2 × 106 cells in 1 ml/well were cultured in 24-well plates (Falcon; VWR, Mississauga, ON, Canada). After 3 days of culture, 10 IU/ml recombinant human interleukin (IL)-2 (PeproTech, Rocky Hill, NJ) was added to each well. On day 8, supernatants were harvested and cell proliferation was assessed using a CFSE-based, flow cytometric assay with results expressed as the cell division index (CDI) (defined as the number of CD3+ CFSEdim cells cultured with antigen/number of CD3+ CFSEdim cells without antigen; the number of CFSEdim events was the number corresponding to 5,000 CFSEbright cells) (33).
Wheat protein preparations were analyzed for lipopolysaccharide (LPS). The concentration was low and comparable with other recombinant proteins. The addition of the LPS inhibitor polymyxin B (Sigma) had no effect on WP-induced T-cell proliferation but blocked LPS-induced T-cell proliferation (supplementary Fig. S1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1579/DC1).
Blocking of HLA-DR with anti-DR antibody.
Inhibition of the proliferation of PBMNCs to wheat protein was studied by adding monoclonal anti-DR antibodies (clone G46-6 and mouse IgG2a,κ; BD Biosciences, Mississauga, ON, Canada) to the culture 30 min before adding wheat protein to the cell culture wells. The mouse IgG2a,κ isotype antibody (BD Biosciences) was used as a control. Both antibodies were used at a concentration of 5 μg/ml.
Cytokine evaluation in culture supernatants.
Cytokines including IL-4, IL-6, IL-10, tumor necrosis factor (TNF), and γ-interferon (IFN-γ) were quantified simultaneously using a human Th1/Th2 cytokine cytometric bead array (CBA) kit. The CBA kit and CBA software were purchased from BD Biosciences. All assays were performed according to the manufacturer's protocol, and samples were read in a Coulter FC500 flow cytometer after appropriate calibration. Quantification of cytokine levels was performed by comparison with standards provided in the kit using CBA software. IL-17A was analyzed in supernatants of PBMNC from a subset of patients and healthy control subjects in the presence or absence of anti-DR antibodies using an enzyme-linked immunosorbent assay (ELISA) kit purchased from eBioscience (San Francisco, CA).
HLA typing.
HLA-DR and -DQ haplotypes of subjects were characterized by PCR-based HLA class II tissue typing using low resolution Olerup SSP typing kits for HLA-DR and -DQ (Genovision, West Chester, PA) or by the Ottawa Hospital Clinical Laboratory.
Statistical analysis.
Differences in CDI among patients and control subjects were analyzed by the nonparametric Mann-Whitney U test using GraphPad Prism (version 4.03 for Windows; GraphPad Software, San Diego, CA). Significance of differences in frequencies was evaluated using Fisher's exact two-tailed test. Spearman's correlation was used to analyze the correlation between WP T-cell response and the other dietary or autoantigens using STATISTICA (version 6; Statsoft, Tulsa, OK).
RESULTS
CD3+ T-cell response to WPs in control and type 1 diabetic subjects.
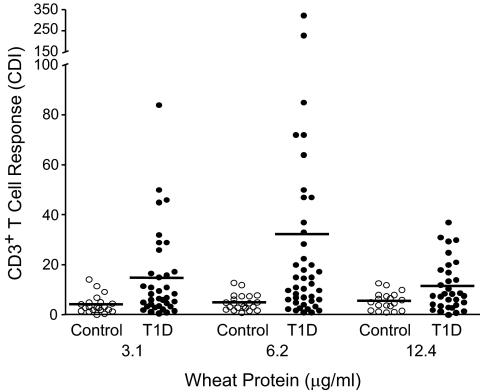
The CFSE assay permitted evaluation of T-cell proliferation to low, nontoxic concentrations of WP (Fig. 1). CD3+ T-cell proliferation in the presence of 3.1 and 6.2 μg/ml WP was significantly higher in the type 1 diabetic patient group compared with control subjects (Fig. 1). At 12.4 μg/ml, CDI was still higher in the type 1 diabetic patient group but the response was less than at 6.2 μg/ml, suggesting inhibition. Therefore, 6.2 μg/ml was chosen as the optimum concentration. To identify patients with a positive proliferation response to WP, the mean + 3 SD of the control group CDI (CDI ≥14.6) was chosen as the cutoff. Of the 42 patients with type 1 diabetes, 20 (47%) had a positive proliferation response to WP. In an expanded analysis of two responders, the proliferative response to WP was mainly from CD4+ T-cells with only a weak CD8+ T-cell response (data not shown). The distribution of CDI to WP was significantly different between patients (n = 42) and control subjects (n = 22); for patients, the median was 11.8 (range 1.0–323) and for control subjects 4.5 (0.8–12.8) (P = 0.0004; Mann-Whitney U test) (Fig. 1).
FIG. 1.
Antigen-specific CD3+ T-cell proliferation. 1.2 × 106 CFSE-labeled PBMNCs from patients with type 1 diabetes (T1D) or healthy control subjects were cultured for 8 days in the absence or presence of different concentrations of WPs. On day 8, cells were stained with Cy-chrome conjugated anti-CD3 monoclonal antibody. CDI was calculated based on a fixed number of 5,000 CD3+ CFSEbright cells using the following formula: CDI = number of CD3+, CFSEdim cells with antigen/number of CD3+, CFSEdim cells without antigen (medium). The horizontal line indicates the mean.
The mean ± SD CDI was 32 ± 60 for patients and 4.9 ± 3.2 for control subjects. WP response was not correlated with duration of disease, but there was a moderate inverse correlation with age (r = −0.35, P = 0.025; data not shown). When a subgroup of six control subjects was matched with 19 patients for HLA-DR4, age, and sex, the increased T-cell proliferation in response to WP in the type 1 diabetic patient group remained significant (P = 0.038) (supplementary Fig. S2).
T-cell responses to other dietary antigens moderately increased in type 1 diabetic patients.
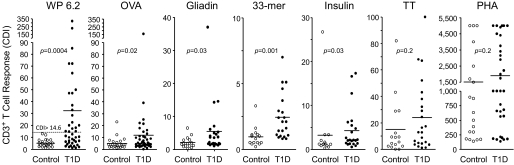
Some patients displayed increased T-cell responses to other dietary antigens including ovalbumin, an irrelevant dietary antigen, and to the celiac-related antigens (wheat gliadin and α-gliadin 33-mer peptide) as well as the type 1 diabetes autoantigen insulin (P = 0.02, P = 0.03, P = 0.001, and P = 0.03, respectively) (Fig. 2). We did not find significant differences between type 1 diabetic patients and control subjects in response to the recall antigen, tetanus toxoid, or the T-cell mitogen phytohemagglutinin (P = 0.2) (Fig. 2). There was a positive correlation between WP T-cell responses and T-cell responses to ovalbumin, gliadin, and α-gliadin 33-mer peptide in type 1 diabetic patients (P = 0.01, P = 0.002, and P = 0.0001; data not shown). In contrast, we detected a weak, nonsignificant correlation between WP T-cell response and insulin T-cell response in type 1 diabetic patients (P = 0.06; data not shown).
FIG. 2.
T-cell proliferation in response to WPs and other antigens or mitogen. 1.2 × 106 CFSE-labeled PBMNCs from patients with type 1 diabetes (T1D) or healthy control subjects were cultured for 8 days in the absence or presence of WP, ovalbumin (OVA), gliadin, 33-mer, insulin, tetanus toxoid (TT), or phytohemagglutinin (PHA) (see research design and methods for details). On day 8, cells were stained with Cy-chrome–conjugated anti-CD3 monoclonal antibody. CDI was calculated. A CDI value greater than the control mean + 3 SD (CDI >14.6) was used to define a positive response to WP. P values indicate statistical difference compared with control subjects. The horizontal lines indicate the means.
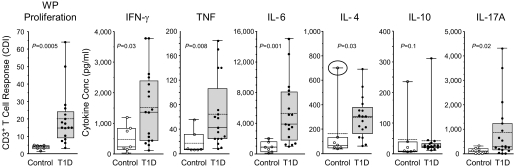
High concentration of IFN-γ, IL-6, and IL-17A in supernatants of WP-stimulated PBMNC.
Cytokines secreted by WP-stimulated PBMNC were analyzed in the culture supernatant using flow CBAs or ELISA (IL-17A) (Fig. 3). The concentration of the proinflammatory cytokines IFN-γ, TNF, and IL-6 was higher in WP-stimulated PBMNC from type 1 diabetic patients (P = 0.03, P = 0.008, and P = 0.001, respectively), whereas the counter inflammatory cytokines IL-4 and IL-10 were not different. Removing one control subject from the IL-4 group analysis (circled symbol, Fig. 3) revealed significantly higher IL-4 concentration in the type 1 diabetic patient supernatants. However, the concentration of IL-4 was much lower than that of IFN-γ. The concentration of IL-17A in WP-stimulated PBMNC culture supernatants from patients was increased compared with control subjects (P = 0.02) (Fig. 3).
FIG. 3.
WP-stimulated cytokine secretion by PBMNC at day 8 of culture. Box and Whisker plots with individual values for control and diabetic subjects. IFN-γ, TNF, IL-6, IL-4, and IL-10 were measured by flow CBA. IL-17A was measured by ELISA. Each plot includes the mean (dashed line), median (solid line), distribution, and range. (For IL-4, the difference between control and type 1 diabetic [T1D] subjects is significant when the circled outlier value in the control group is removed [P = 0.03].)
Association of HLA-DR4 risk alleles for type 1 diabetes with immunity to WPs.
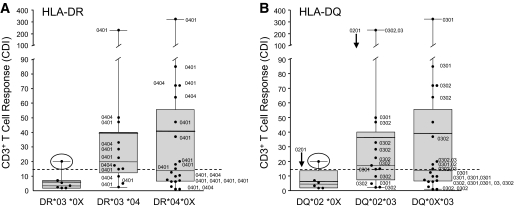
We analyzed HLA-DR and -DQ haplotypes in patients with type 1 diabetes and control subjects. The frequency of HLA haplotypes in the control group was similar to previously published reference populations (34) (data not shown). HLA-DRB1*03 and -DRB1*04, which are associated with type 1 diabetes, were more frequent in type 1 diabetic patients compared with control subjects. Heterozygous DR3/DR4 individuals represented 29% of our type 1 diabetic patients, but none of our control subjects was heterozygous for HLA-DR3/DR4 (data not shown). In addition, we compared the frequency of risk alleles in type 1 diabetic patients who were either responders or nonresponders to WP (Table 2). Type 1 diabetic responders to WP carried HLA-DRB1*04 in 95% of cases, which was significantly higher than in the nonresponders (59%; P = 0.01) (Table 2). Distribution of T-cell proliferation (CDI) to WP in type 1 diabetic patients with HLA-DRB1*03 or HLA-DRB1*04 or in those heterozygous for HLA-DRB1*03/*04 is shown in Fig. 4. One of seven type 1 diabetic patients with HLA-DRB1*03+/*04− showed positive responses to WP. In contrast, 10 of 20 type 1 diabetic patients who carried one risk allele HLA-DRB1*04 or 9 of 12 patients who carried both risk genes HLA-DRB1*03/*04 showed positive reactivity to WP.
TABLE 2.
HLA-DR and -DQ in type 1 diabetic responders and nonresponders
| n | DR3 | DR4 | DR3/DR4 | DQ2 | DQ3 | |
|---|---|---|---|---|---|---|
| Nonresponders | 22 | 41 | 59 | 14 | 41 | 64 |
| Responders | 20 | 50 | 95 (P = 0.01) | 45 (P = 0.04) | 50 (P = 0.75) | 95 (P = 0.02) |
Data are % unless otherwise indicated. T-cell proliferation (CDI) to WP was evaluated in type 1 diabetic patients. Patients with a CDI value greater than mean + 3 SD of the control group (CDI >14.6) were classified as responders. Those below were classified as nonresponders. The percentage of individuals with the indicated HLA haplotypes in these groups was calculated.
FIG. 4.
Graphic representation of the relationship between wheat-induced T-cell response and high-resolution HLA diabetes risk alleles. Box and Whisker plots with individual values for diabetic subjects. Each plot includes the mean (bold line), median (solid thin line), distribution, and range. A: Distribution of T-cell proliferation (CDI) to WP in type 1 diabetic patients with HLA-DRB1*03 and HLA-DRB1*04 or in those heterozygous for HLA-DRB1*03/*04. A CDI value greater than mean + 3 SD of the control group (CDI >14.6, dashed line) was used to define a positive proliferation response. One of the type 1 diabetic patients with HLA-DRB1*03+/*04− showed positive responses to WP (○). Type 1 diabetic patients who carried one risk allele HLA-DRB1*04 (10 of 20) or patients who carried both risk genes HLA-DRB1*03/*04 (9 of 12) showed positive reactivity to WP. High-resolution results of HLA-DR are labeled beside each individual. B: Distribution of T-cell proliferation (CDI) to WP in type 1 diabetic patients with HLA-DQB1*02 and HLA-DQB1*03 or in those heterozygous for HLA-DQB1*02/*03. High-resolution analyses for HLA-DQB1*02 and *03 are labeled beside each symbol. ↓ (beside 0201) means all individuals in the column carry HLA-DQB1*0201. (Three of the 42 type 1 diabetic individuals are neither DR3 nor DR4, and therefore they are not included in this figure. One of the seven individuals with DR3/non-DR4 in the left panel is heterozygous for DQ*02/*03 and was therefore placed in the middle column in the right panel [DQ*02*03]. Differences in high-resolution haplotypes between responders and nonresponders were not evaluated statistically because of the small sample size.)
The frequency of HLA-DQ2, the major celiac disease–associated allele, was not significantly different between type 1 diabetic patients and control subjects (P = 0.6; data not shown). Importantly, the difference for HLA-DQ2 between responders and nonresponders was not significant (P = 0.75) (Table 2). We also evaluated the HLA-DR and -DQ haplotypes by high-resolution analysis in type 1 diabetic patients. A positive response to WP in patients was more frequently associated with the HLA-DR4 (DRB1*0401/4) DQB1*0301/2 haplotype, and HLA-DR3/DQB1*0201 appeared to magnify the response (Fig. 4). Patients who were HLA-DQ3+ were also more likely to be responders (Table 2).
T-cell responses to WP inhibited by monoclonal anti-DR antibodies.
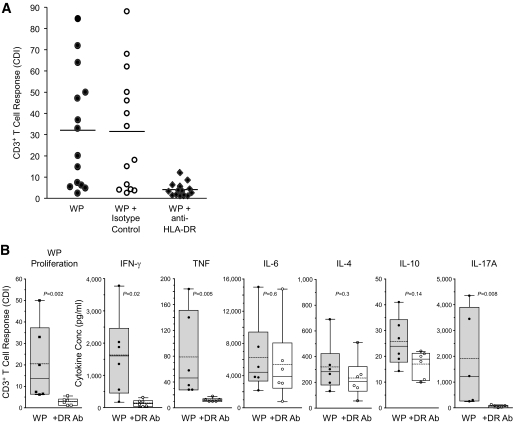
To confirm the role of HLA-DR in the T-cell response to WP, we evaluated the blocking effect of anti–HLA-DR monoclonal antibodies on PBMNC of 14 type 1 diabetic patients. Monoclonal anti-DR antibodies blocked T-cell responses to WPs in type 1 diabetic patients, whereas isotype control antibody did not prevent the T-cell response (Fig. 5A). Anti–HLA-DR4 antibody had only a small inhibitory effect on T-cell response to tetanus toxoid (supplementary Fig. S3). CBA cytokine analysis of the culture supernatants from WP-stimulated PBMNC of type 1 diabetic patients showed that monoclonal anti-DR antibody significantly inhibited secretion of Th1 cytokines but not Th2 cytokines (Fig. 5B). WP-induced production of IL-17A was also blocked by the addition of anti-DR (P = 0.008) (Fig. 5B).
FIG. 5.
Role of HLA-DR in the type 1 diabetes T-cell and cytokine response to WPs. A: The T-cell response to wheat protein (6.2 μg/ml) was calculated as CDI in 14 patients and compared with cells cultured in the presence of anti–HLA-DR monoclonal antibody (5 μg/ml) or with isotype control antibody. B: Cytokine profiles of supernatants from WP-stimulated PBMNC of patients were evaluated in the absence and presence of monoclonal HLA-DR antibodies using Th1/Th2 CBAs for IFN-γ, IL-4, TNF, IL-10, and IL-6 or an ELISA kit for IL-17A.
DISCUSSION
The foods we eat contain numerous nonself molecules that do not normally stimulate a proinflammatory immune response in healthy individuals (35). In some diabetes-prone rodents (6,7,10) and humans (19,21–23), there is evidence that the gut mucosa is mildly inflamed and the epithelial barrier is leaky, providing a potential entry point for nonself antigens in the context of a proinflammatory cytokine imbalance that could promote autoimmunity (4,18). The present results support this view. Although the origin of the antigens and immune cells that initiate or drive the β-cell–specific autoimmune response is not known, diet is an important factor influencing diabetes outcome, possibly by supplying a constant source of stimulatory antigens to the gut immune system (4).
There was moderately increased T-cell proliferative response to other dietary antigens such as ovalbumin, gliadin, and the celiac toxic gliadin 33-mer peptide (Fig. 2), but this was less pronounced than the response to WP. These data suggest a general impairment of oral tolerance in some type 1 diabetic patients (36), with the strongest and most frequent abnormal proliferative response induced by the mixture of WPs.
We are aware of only one other study of WP response in patient PBMNCs (29). Klemetti et al. (29) showed an increased cell-mediated immune response to high concentrations of wheat gluten (400 μg/ml) in 24% of newly diagnosed type 1 diabetic patients. The proliferative responses in this study were low compared with those in the present study, possibly as a result of the high polypeptide concentration, different gluten fractions, culture conditions, proliferation assay, and/or different genetic background of the subjects as well as different duration of diabetes. In keeping with reports that high concentrations of gliadin are cytotoxic (37), we found that 25 μg/ml of wheat protein extract inhibited response of PBMNC, whereas 6.2 μg/ml was optimum for our assay (29). The CFSE assay used here not only permits identification of individual cell populations by flow cytometry but is also more sensitive than the thymidine assay (33). Therefore, the present analysis permitted us to detect wheat-specific CD3+ T-cell proliferation using a low, noninhibitory dose of WP.
Additional evidence linking the development of diabetes in humans to wheat comes from epidemiological studies (27,28) and reports of immune responses of patient tissues to WP (29,30). Anti-gliadin antibodies have been reported in newly diagnosed children with type 1 diabetes (38), and prospective studies of infants at high risk indicate that early exposure to cereals, particularly wheat, was linked to appearance of islet autoantibodies (27,28). Furthermore, a significant subset of type 1 diabetic patients displays celiac disease autoantibodies (39). CD3+ lamina propria and intestinal epithelial lymphocytes were increased in 20% of type 1 diabetic patients receiving rectally instilled gliadin (40). Immunohistochemical evaluation and culture of jejunal biopsies from tissue transglutaminase antibody–negative type 1 diabetic children with WP increased frequency of activated CD25+ cells in the lamina propria as well as expression of HLA-DR in the crypts in association with enhanced infiltration of the epithelium by CD3+ cells (30). Therefore, the present results are consistent with those of Auricchio et al. (30) showing that a subset of type 1 diabetic patients displayed signs of inflamed gut mucosa and increased immune reactivity to WPs.
Type 1 diabetes has been traditionally thought of as a T-cell–mediated disease associated with high levels of the Th1 cytokine IFN-γ. It now seems likely that type 1 diabetes is the result of dysregulation within a broader network of immune cell types (41). For example, the role of the recently discovered Th17 cells and the extent to which there is a dysregulation of communication between Th1, Th2, and Th17 cells in human diabetes remain unclear. We observed WP-induced T-cell proliferation in nearly half of our type 1 diabetic patients but not in healthy control subjects. This response was mixed in nature, accompanied by increased production of proinflammatory cytokines IFN-γ, TNF, IL-6, and IL-17A as well as the counterinflammatory Th2 cytokine IL-4. The high concentrations of IFN-γ and IL-17A suggest that Th1 and Th17 cytokine–producing, WP-responsive CD3+ cells were activated in association with a proinflammatory condition in the gut (6,30). IL-6 was present in WP culture supernatants at very high levels, four to six times those of IFN-γ. TNF was also increased but to a much lesser extent. IL-6 and TNF are proinflammatory cytokines that promote the secretion of IL-17 by Th17 cells and block the production of Foxp3+ regulatory T-cells (42). We did not observe a significant increase in IL-10 in response to WP, making it unlikely that IL-17 originated from Th17 regulatory cells that produce both IL-10 and IL-17. It seems unlikely that such high amounts of IL-17 could originate from CD8+ T-cells because expansion of WP-responsive T-cells was predominantly from CD4+ cells and IL-17A production was blocked by anti-DR antibody.
High levels of IFN-γ were produced in response to WP, whereas the concentration of the Th2 cytokine IL-4, although significantly increased, was only one-third that of IFN-γ, suggesting a predominance of Th1 over Th2 cells. Although both of these cytokines can inhibit development of Th17 cells from naive precursors (42), it has been suggested that committed Th17 cells are not affected (41). Therefore, we favor the interpretation that WP stimulates the production of IL-17A from previously committed Th17 cells. While IL-17A can be produced under certain circumstances by CD8+ T-cells and members of the innate immune system (γδT-cells and natural killer T-cells), Th17 cells are the major producers of IL-17A. Thus, the overall cytokine pattern observed in WP-stimulated PBMNC from type 1 diabetic patients suggests a predominant Th1 and Th17 proinflammatory state consistent with the speculation that autoimmunity can occur when there is inappropriate cross-regulation between Th1 and Th17 cytokine networks (42).
The role of IL-6 in type 1 diabetes remains controversial (43). In the present study, patient PBMNC cultured with WP secreted large amounts of IL-6, the origin of which is presently unclear. Nonetheless, some points are worth noting: WP response was mainly from CD4+ and not CD8+ cells, most responders were HLA-DR4+, and treatment with anti-DR did not block production of IL-6. These results suggest that IL-6 originated from non–T-cells. Others report that IL-6 gene expression was upregulated threefold in monocytes from adult-onset type 1 diabetic subjects (44). Furthermore, overexpression of IL-6 in pancreas was correlated with islet inflammation, which could contribute to the development of autoimmune disease (43). The high concentration of WP-induced IL-6 further supports our previous proposal of a potential mechanism by which wheat can promote the development of diabetes involving induction of proinflammatory cytokines such as IL-6, IFN-γ, and TNF-α by wheat antigens (4,9,13,45).
The HLA genes are the major genetic determinant of type 1 diabetes. More than 90% of Caucasian type 1 diabetic patients carry either HLA-DR3 or HLA-DR4 haplotype, and a synergistic effect on type 1 diabetes risk is observed in HLA-DR3/4 heterozygous individuals (46). Patients with type 1 diabetes and celiac disease share some HLA-associated risk genes such as HLA-DQ2 (HLA-DQB1*02) and HLA-DQ8 (HLA-DQB1*0302). The frequency of HLA risk genes is different between the two diseases: 90–95% of celiac disease patients are HLA-DQ2 and 5–10% are HLA-DQ8 (47), whereas up to 50% of diabetic patients are HLA-DQ8/DQ2 heterozygous (48). In our type 1 diabetic population, increased immune response to WPs was not explained by shared genetic risk for celiac disease. The frequency of HLA-DQB1*02 was not significantly different between type 1 diabetic patients and control subjects (P = 0.6), and we did not detect any significant differences for HLA-DQB1*02 between WP responders and nonresponders (P = 0.75) (Table 2). We also evaluated the HLA-DR and -DQ haplotype by high-resolution analysis in type 1 diabetic patients. A positive response to WP in patients was more frequently associated with the HLA-DR4 (DRB1*0401/4) DQB1*0301/2 haplotype, and HLA-DR3/DQB1*0201 appeared to magnify the response (Table 2, Fig. 4).
A genetic basis for WP response in type 1 diabetic patients is suggested by the finding that T-cell proliferation and inflammatory cytokine production were blocked by anti-DR antibodies and responders were nearly all HLA-DR4+. Others have observed enhanced expression of HLA-DR in the intestinal mucosa of children with type 1 diabetes (20,30), and gluten-induced T-cell proliferation was blocked by anti-DR antibodies in two patients in a previous study (29). Importantly, WP-induced T-cell proliferation was not explained by an overrepresentation of the major celiac disease risk gene HLA-DQ2. HLA-DQ2 prevalence was not different between patients and control subjects or between WP responder and nonresponder patient groups, and the T-cell response was not attributable to the presence of subclinical celiac disease given that all our patients with type 1 diabetes were negative for antibodies against the pathognomic celiac disease autoantigen, tissue transglutaminase. These findings are consistent with other reports showing that the gut inflammation observed in type 1 diabetic patients is different from that seen in patients with celiac disease (20,49) and not necessarily related to HLA-DQ2 or HLA-DQ8 (30). Therefore, the tolerogenic function of the gut immune system with respect to WPs was compromised in a large subset of patients with type 1 diabetes in an HLA-DR–restricted, diabetes-specific manner.
In summary, almost half of our type 1 diabetic patients displayed increased proliferation when PBMNCs were cultured in vitro with nontoxic concentrations of WP. The cytokine pattern was mixed having characteristics of a predominant Th1 and Th17 response with a lesser contribution of the Th2 cytokine IL-4. The WP proliferative response occurred mainly in HLA-DR4 individuals and was blocked with anti-DR antibodies but was not due to the major celiac disease gene HLA-DQ2. This demonstrated the presence of a mixed proinflammatory Th1/Th17 response to dietary WPs that appeared to be diabetes specific.
Supplementary Material
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation and Canadian Institutes of Health Research.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 65th Scientific Sessions of the American Diabetes Association, San Diego, California, 10–14 June 2005, and at the First Nordic Meeting on Genetics and Pathogenesis of Immunological Diseases, Helsinki, Finland, 4–5 September 2006.
We are indebted to the volunteers and the following physicians: Drs. Rene Wong and Janine Malcolm (The Ottawa Hospital) and Dr. Sarah Lawrence (the Children's Hospital of Eastern Ontario). We thank Nancy Hampton (The Ottawa Hospital) for HLA typing and helpful discussions. Dr. Chaitan Khosla (Stanford University) and Dr. Hubert Kolb (German Diabetes Center, Düsseldorf, Germany) provided the α-gliadin 33-mer peptide. We thank Drs. Hubert Kolb and Nanette Schloot (German Diabetes Center, Düsseldorf, Germany) and Dr. William Cameron (the Ottawa Hospital Research Institute) for reviewing the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1723.
REFERENCES
- 1.Solly NR, Honeyman MC, Harrison LC: The mucosal interface between ‘self’ and ‘non-self’ determines the impact of environment on autoimmune diabetes. Curr Dir Autoimmun 2001; 4: 68– 90 [DOI] [PubMed] [Google Scholar]
- 2.Sollid LM: Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2: 647– 655 [DOI] [PubMed] [Google Scholar]
- 3.Courtois P, Nsimba G, Jijakli H, Sener A, Scott FW, Malaisse WJ: Gut permeability and intestinal mucins, invertase and peroxidase in control and diabetes-prone BB rats fed either a protective or diabetogenic diet. Dig Dis Sci 2005; 50: 266– 275 [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre DE, Powell KL, Strom A, Scott FW: Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu Rev Nutr 2006; 26: 175– 202 [DOI] [PubMed] [Google Scholar]
- 5.Vaarala O, Atkinson MA, Neu J: The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008; 57: 2555– 2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM: Enteropathy precedes type 1 diabetes in the BB rat. Gut 2004; 53: 1437– 1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurano F, Mazzarella G, Luongo D, Stefanile R, D'Arienzo R, Rossi M, Auricchio S, Troncone R: Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia 2005; 48: 931– 937 [DOI] [PubMed] [Google Scholar]
- 8.Scott FW, Rowsell P, Wang GS, Burghardt K, Kolb H, Flohe S: Oral exposure to diabetes-promoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes 2002; 51: 73– 78 [DOI] [PubMed] [Google Scholar]
- 9.Flohe SB, Wasmuth HE, Kerad JB, Beales PE, Pozzilli P, Elliott RB, Hill JP, Scott FW, Kolb H: A wheat-based, diabetes-promoting diet induces a Th1-type cytokine bias in the gut of NOD mice. Cytokine 2003; 21: 149– 154 [DOI] [PubMed] [Google Scholar]
- 10.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG: Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol 1999; 276: G951– G957 [DOI] [PubMed] [Google Scholar]
- 11.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, Li N, Caicedo RA, Schatz DA, Atkinson M: Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr 2005; 40: 589– 595 [DOI] [PubMed] [Google Scholar]
- 12.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A: Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 2005; 102: 2916– 2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakir H, Lefebvre DE, Wang H, Caraher E, Scott FW: Wheat protein-induced proinflammatory T helper 1 bias in mesenteric lymph nodes of young diabetes-prone rats. Diabetologia 2005; 48: 1576– 1584 [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane AJ, Burghardt KM, Kelly J, Simell T, Simell O, Altosaar I, Scott FW: A type 1 diabetes-related protein from wheat (Triticum aestivum). cDNA clone of a wheat storage globulin, Glb1, linked to islet damage. J Biol Chem 2003; 278: 54– 63 [DOI] [PubMed] [Google Scholar]
- 15.Wang GS, Kauri L, Wang G-S, Patrick C, Scott FW: Enhanced islet neogenesis and beta cell proliferation in pre-insulitic diabetes-prone rats fed a hydrolyzed casein diet. FASEB J 2007; 21: A771 [Google Scholar]
- 16.Pastore MR, Bazzigaluppi E, Belloni C, Arcovio C, Bonifacio E, Bosi E: Six months of gluten-free diet do not influence autoantibody titers, but improve insulin secretion in subjects at high risk for type 1 diabetes. J Clin Endocrinol Metab 2003; 88: 162– 165 [DOI] [PubMed] [Google Scholar]
- 17.Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM: An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab 2008; 10( Suppl.4): 63– 76 [DOI] [PubMed] [Google Scholar]
- 18.Fasano A, Shea-Donohue T: Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol 2005; 2: 416– 422 [DOI] [PubMed] [Google Scholar]
- 19.Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL: Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 1986; 29: 221– 224 [DOI] [PubMed] [Google Scholar]
- 20.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E: Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 2003; 52: 2287– 2295 [DOI] [PubMed] [Google Scholar]
- 21.Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Carteni M, Prisco F: Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr 1999; 28: 264– 269 [DOI] [PubMed] [Google Scholar]
- 22.Damci T, Nuhoglu I, Devranoglu G, Osar Z, Demir M, Ilkova H: Increased intestinal permeability as a cause of fluctuating postprandial blood glucose levels in Type 1 diabetic patients. Eur J Clin Invest 2003; 33: 397– 401 [DOI] [PubMed] [Google Scholar]
- 23.Secondulfo M, Iafusco D, Carratu R, deMagistris L, Sapone A, Generoso M, Mezzogiomo A, Sasso FC, Carteni M, De Rosa R, Prisco F, Esposito V: Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis 2004; 36: 35– 45 [DOI] [PubMed] [Google Scholar]
- 24.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A: Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006; 55: 1443– 1449 [DOI] [PubMed] [Google Scholar]
- 25.Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O: Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest 1992; 90: 1901– 1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paronen J, Klemetti P, Kantele JM, Savilahti E, Perheentupa J, Akerblom HK, Vaarala O: Glutamate decarboxylase-reactive peripheral blood lymphocytes from patients with IDDM express gut-specific homing receptor α4β7- integrin. Diabetes 1997; 46: 583– 588 [DOI] [PubMed] [Google Scholar]
- 27.Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M: Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003; 290: 1713– 1720 [DOI] [PubMed] [Google Scholar]
- 28.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E: Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003; 290: 1721– 1728 [DOI] [PubMed] [Google Scholar]
- 29.Klemetti P, Savilahti E, Ilonen J, Akerblom HK, Vaarala O: T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand J Immunol 1998; 47: 48– 53 [DOI] [PubMed] [Google Scholar]
- 30.Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R: In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004; 53: 1680– 1683 [DOI] [PubMed] [Google Scholar]
- 31.Cosnes J, Cellier C, Viola S, Colombel JF, Michaud L, Sarles J, Hugot JP, Ginies JL, Dabadie A, Mouterde O, Allez M, Nion-Larmurier I: Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol 2008; 6: 753– 758 [DOI] [PubMed] [Google Scholar]
- 32.Arentz-Hansen EH, McAdam SN, Molberg O, Kristianson C, Sollid LM: Production of a panel of recombinant gliadins for the characterisation of T cell reactivity in coeliac disease. Gut 2000; 46: 46– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannering SI, Morris JS, Jensen KP, Purcell AW, Honeyman MC, van Endert PM, Harrison LC: A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J Immunol Methods 2003; 283: 173– 183 [DOI] [PubMed] [Google Scholar]
- 34.Svejgaard A, Platz P, Ryder LP: HLA and disease 1982—a survey. Immunol Rev 1983; 70: 193– 218 [DOI] [PubMed] [Google Scholar]
- 35.Mowat AM: The regulation of immune responses to dietary antigens. Immunol Today 1987; 8: 93– 98 [DOI] [PubMed] [Google Scholar]
- 36.Atkinson MA, Bowman MA, Kao KJ, Campbell L, Dush PJ, Shah SC, Simell O, Maclaren NK: Lack of immune responsiveness to bovine serum albumin in insulin- dependent diabetes. N Engl J Med 1993; 329: 1853– 1858 [DOI] [PubMed] [Google Scholar]
- 37.Elli L, Dolfini E, Bardella MT: Gliadin cytotoxicity and in vitro cell cultures. Toxicol Lett 2003; 146: 1– 8 [DOI] [PubMed] [Google Scholar]
- 38.Catassi C, Guerrieri A, Bartolotta E, Coppa GV, Giorgi PL: Antigliadin antibodies at onset of diabetes in children. Lancet 1987; 2: 158. [DOI] [PubMed] [Google Scholar]
- 39.Lampasona V, Bonfanti R, Bazzigaluppi E, Venerando A, Chiumello G, Bosi E, Bonifacio E: Antibodies to tissue transglutaminase C in type I diabetes. Diabetologia 1999; 42: 1195– 1198 [DOI] [PubMed] [Google Scholar]
- 40.Troncone R, Franzese A, Mazzarella G, Paparo F, Auricchio R, Coto I, Mayer M, Greco L: Gluten sensitivity in a subset of children with insulin dependent diabetes mellitus. Am J Gastroenterol 2003; 98: 590– 595 [DOI] [PubMed] [Google Scholar]
- 41.Nikoopour E, Schwartz JA, Singh B: Therapeutic benefits of regulating inflammation in autoimmunity. Inflamm Allergy Drug Targets 2008; 7: 203– 210 [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, Oukka M, Kuchroo VK: T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007; 8: 345– 350 [DOI] [PubMed] [Google Scholar]
- 43.Kristiansen OP, Mandrup-Poulsen T: Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 2005; 54( Suppl.2): S114– S124 [DOI] [PubMed] [Google Scholar]
- 44.Padmos RC, Schloot NC, Beyan H, Ruwhof C, Staal FJ, de Ridder D, Aanstoot HJ, Lam-Tse WK, de Wit H, de Herder C, Drexhage RC, Menart B, Leslie RD, Drexhage HA: Distinct monocyte gene-expression profiles in autoimmune diabetes. Diabetes 2008; 57: 2768– 2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikulina M, Habich C, Flohe SB, Scott FW, Kolb H: Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol 2004; 173: 1925– 1933 [DOI] [PubMed] [Google Scholar]
- 46.Onengut-Gumuscu S, Concannon P: Mapping genes for autoimmunity in humans: type 1 diabetes as a model. Immunol Rev 2002; 190: 182– 194 [DOI] [PubMed] [Google Scholar]
- 47.Margaritte-Jeannin P, Babron MC, Bourgey M, Louka AS, Clot F, Percopo S, Coto I, Hugot JP, Ascher H, Sollid LM, Greco L, Clerget-Darpoux F: HLA-DQ relative risks for coeliac disease in European populations: a study of the European Genetics Cluster on Coeliac Disease. Tissue Antigens 2004; 63: 562– 567 [DOI] [PubMed] [Google Scholar]
- 48.Melanitou E, Fain P, Eisenbarth GS: Genetics of type 1A (immune mediated) diabetes. J Autoimmun 2003; 21: 93– 98 [DOI] [PubMed] [Google Scholar]
- 49.Tiittanen M, Westerholm-Ormio M, Verkasalo M, Savilahti E, Vaarala O: Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clin Exp Immunol 2008; 152: 498– 507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.