Type 1 diabetes is a disorder caused by the autoimmune-mediated destruction of insulin-producing β-cells in the pancreas (1). Many promising intervention trials are currently underway or in development to prevent β-cell loss in patients with recent-onset type 1 diabetes and in individuals at increased risk for type 1 diabetes due to the presence of autoantibodies or elevated genetic risk (2). Despite this hope for future interventions, islet transplantation remains the only therapeutic option currently available for individuals with established type 1 diabetes when insulin therapy fails to maintain adequate metabolic control (3). Islet allograft transplantation has demonstrated great success in terms of restoring normoglycemia (4). Unfortunately, the long-term efficacy following transplantation has been limited due to chronic graft rejection (5). Thus, an optimal approach would involve an islet transplantation protocol that would prevent graft rejection without the need for potentially dangerous long-term and nonspecific immune suppression.
Mesenchymal stem cells (MSCs) have been proposed to be one possible means to enhance current islet transplantation protocols (6). MSCs represent a population of nonhematopoetic precursor cells that have generated marked interest and attention for their capacity to elicit tissue regeneration (7–9). For the treatment of type 1 diabetes, the therapeutic potential of MSCs is potentially twofold. First, these cells are reported to provide critical growth factors for tissue regeneration (8). Second, these cells possess potent immunoregulatory properties that could be exploited to suppress allograph rejection following transplantation (6).
In this issue of Diabetes, Ding et al. (10) provide evidence that MSCs possess the capacity to impede T-cell activation both in the context of a delayed-type hypersensitivity reaction and following an islet allotransplantation protocol. The authors essentially accomplished this through a series of in vitro and in vivo studies suggesting that MSCs exert potent immunosuppressive properties by blocking the interleukin (IL)-2 cytokine signaling pathway required for T-lymphocyte activation, expansion, and differentiation (11,12).
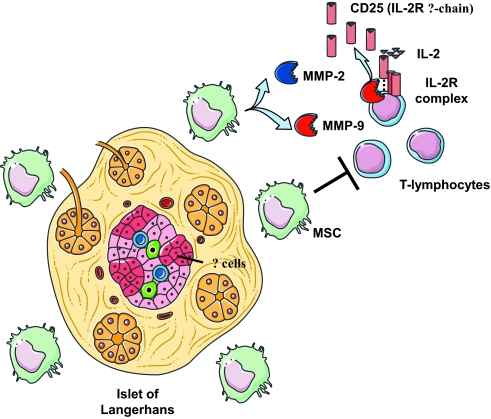
Given the potent immunosuppressive properties of MSCs, the authors of this article sought to interrogate the potential mechanism(s) of suppression. A prior study had implicated various immunomodulatory pathways in the ability of MSCs to suppress T-cell responses. These include production of the immunomodulatory cytokines TGF-β and IL-10 (13) as well as enzymatic pathways involving indolamine 2,3-dioxygenase (12), nitric oxide synthase (14), and heme oxygenase-1 (15). Adding to the existing literature, Ding et al. demonstrate the potent immunosuppressive properties of MSCs in vitro either when cocultured with polyclonal T-cells that are activated with microbeads coated with anti-CD3 and anti-CD28 or during allogeneic antigen stimulation. The authors noted little influence on T-cell activation when the pathways described previously were specifically targeted. On the other hand, these authors did observe that production of the enzymes matrix metalloproteinase (MMP)-2 and -9 by MSCs had a potent immunosuppressive effect on T-cell activation. This finding is analogous to that described previously by Sheu et al. (16), who showed that MMP-2 and -9 production by tumors is capable of creating an immunosuppressive microenvironment. The authors also showed that the mechanism of suppression of T-cell activation involved the specific cleavage of CD25 (the α-chain of the IL-2 receptor) from the surface of responding CD4+ T-cells (see Fig. 1).
FIG. 1.
MSCs protect transplanted allogeneic islets by creating an immunosuppressive microenvironment. MMP-2 and -9 produced by MSCs suppress T-cell responses by cleaving the high-affinity growth factor receptor CD25 (the α-chain of the IL-2 receptor) from the surface of infiltrating T-lymphocytes.
How can the findings by Ding et al. be translated into the clinic to benefit patients with type 1 diabetes? Whereas the authors demonstrated the efficacy of MSCs in suppressing a delayed-type hypersensitivity response and in preventing rejection of an islet allograft, additional studies are clearly warranted in animal models that more closely approximate clinical treatments in humans. Specifically, it will be helpful for future studies to test the capacity of MSCs to prevent allograft rejection in nonimmunodeficient recipient hosts (i.e., the authors reconstituted immunodeficient BALB/c Rag−/−γ−/− mice with naïve BALB/c CD4+CD25− T-cells and transplanted B6 islets in the presence or absence of BALB/c MSCs). Moreover, it will be informative to assess the site of action for MSCs. Are MSCs simply guarding the border of the transplanted tissue to create a local immunosuppressive milieu, or is there a long-term tolerogenic effect of MSCs in the draining lymph nodes of the target organ, presumably the site where the majority of antigen priming occurs? Finally, it is of interest whether MSCs will show efficacy in blocking recurrent autoimmunity and preventing allograft rejection, with the former presumably a more difficult response to impede because memory T-cell responses may be less dependent on IL-2 signaling for effector function (16). One could also speculate that the cleavage of CD25 from the surface of regulatory T-cells (a population of cells characterized as IL-2 dependent and constitutively CD25+) might be detrimental in some settings (17,18). These questions are critical for understanding the mechanism(s) of action of MSCs and in the future design of translational therapies.
From a broader immune standpoint, questions remain regarding the immunomodulatory properties of MMP-2 and -9. These proteolytic enzymes are produced not only by MSCs (as highlighted in this issue) but also by innate antigen-presenting cells as well as in an autocrine fashion by T-cells following activation (reviewed in (19)). Their role in modulating the normal immune response remains poorly defined and requires additional consideration.
This study brings to light a novel mechanism by which MSCs can protect transplanted tissues and raises the possibility that these cells could be added to current protocols to improve the long-term engraftment of transplanted islets. Clearly, more studies in animal models are required to validate these results and further describe the therapeutic mechanisms of MSCs.
Acknowledgments
The author is supported by funding from the Juvenile Diabetes Research Foundation as well as funding from the Dee and William Brehm Foundation.
No potential conflicts of interest relevant to this article were reported.
The author thanks the members of the Bluestone Laboratory for comments and helpful discussion.
Footnotes
See accompanying original article, p. 1797.
REFERENCES
- 1.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358: 221– 229 [DOI] [PubMed] [Google Scholar]
- 2.St Clair EW, Turka LA, Saxon A, et al. : New reagents on the horizon for immune tolerance. Annu Rev Med 2007; 58: 329– 346 [DOI] [PubMed] [Google Scholar]
- 3.Hogan A, Pileggi A, Ricordi C: Transplantation: current developments and future directions: the future of clinical islet transplantation as a cure for diabetes. Front Biosci 2008; 13: 1192– 1205 [DOI] [PubMed] [Google Scholar]
- 4.Ichii H, Ricordi C: Current status of islet cell transplantation. J Hepatobiliary Pancreat Surg 2009; 16: 101– 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorina P, Shapiro AM, Ricordi C, et al. : The clinical impact of islet transplantation. Am J Transplant 2008; 8: 1990– 1997 [DOI] [PubMed] [Google Scholar]
- 6.Vija L, Farge D, Gautier JF, et al. : Mesenchymal stem cells: stem cell therapy perspectives for type 1 diabetes. Diabete Metab 2009; 35: 85– 93 [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. : Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143– 147 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Li C, Jiang X, et al. : Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol 2004; 32: 657– 664 [DOI] [PubMed] [Google Scholar]
- 9.Majumdar MK, Thiede MA, Haynesworth SE, et al. : Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841– 848 [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Xu D, Feng G, et al. : Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 2009; 58: 1797– 1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antony PA, Paulos CM, Ahmadzadeh M, et al. : Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol 2006; 176: 5255– 5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meisel R, Zibert A, Laryea M, et al. : Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619– 4621 [DOI] [PubMed] [Google Scholar]
- 13.Nauta AJ, Fibbe WE: Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499– 3506 [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Ozaki K, Oh I, et al. : Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007; 109: 228– 234 [DOI] [PubMed] [Google Scholar]
- 15.Chabannes D, Hill M, Merieau E, et al. : A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007; 110: 3691– 3694 [DOI] [PubMed] [Google Scholar]
- 16.Sheu BC, Hsu SM, Ho HN, et al. : A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 2001; 61: 237– 242 [PubMed] [Google Scholar]
- 17.Bayer AL, Yu A, Adeegbe D, et al. : Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med 2005; 201: 769– 777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloy KJ, Powrie F: Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol 2005; 6: 1071– 1072 [DOI] [PubMed] [Google Scholar]
- 19.McCawley LJ, Matrisian LM: Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol 2001; 13: 534– 540 [DOI] [PubMed] [Google Scholar]