Although necrosis also plays a role, β-cell death by apoptosis precipitates type 1 diabetes and also contributes to both type 2 diabetes and islet graft failure after transplantation (1). It is therefore surprising that apoptotic pathways, so well explored in other cell types, have barely been characterized in β-cells. A new study in Diabetes now adds to a growing body of work that addresses how proinflammatory cytokines stimulate the intrinsic apoptotic pathway in β-cells (2).
Apoptotic pathways converge on the activation of cysteine proteases of the caspase family (3). The distal point of this cascade, caspase-3, in turn regulates the morphological and other features that characterize apoptosis. Signaling upstream of caspase-3 involves two major arms known as extrinsic and intrinsic pathways. The extrinsic route is triggered by ligands of so-called death receptors, which include Fas and tumor necrosis factor receptor (TNFR). Activation of these receptors facilitates recruitment and cleavage of initiator caspases (such as caspase-8) that act directly on effector caspases such as caspase-3.
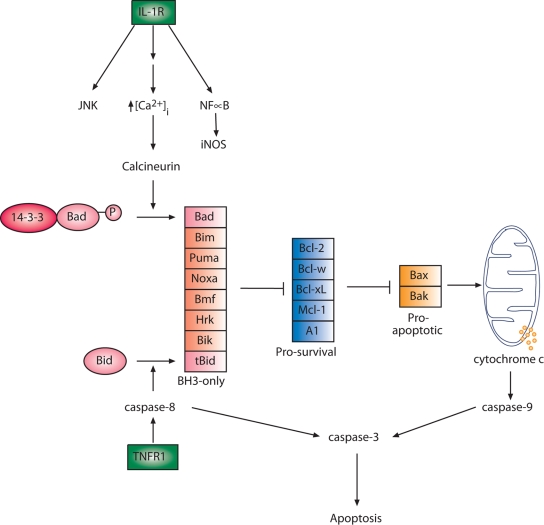
In contrast, the intrinsic pathway (also called the mitochondrial or Bcl-2–regulated pathway) is triggered by a loss of mitochondrial outer membrane potential, which facilitates release of cytochrome c from the mitochondrial membrane to seed a signaling complex that activates a different set of initiator caspases, including caspase-9 (4). This mitochondrial pathway can be initiated by multiple stimuli and is subject to a complex hierarchical regulation by members of the Bcl-2 family (5). Notably, the proapoptotic members, Bax and Bak, directly promote the release of cytochrome c. These are normally held in check by the prosurvival subgroup, including Bcl-2, Bcl-xL, and Mcl-1. Another tier of regulation is provided by the proapoptotic BH3-only proteins, which sequester the prosurvival group and thereby trigger Bax/Bak activation in a cell type– and stimulus-specific manner (Fig. 1).
FIG. 1.
Pathways of extrinsic and intrinsic apoptosis, highlighting roles of Bcl-2 family members. The expression in β-cells of the various Bcl-2 family proteins listed is currently unknown, and their potential contributions are largely uncharacterized. The study of Grunnet et al. highlights a pathway leading from the IL-1 receptor (IL-1R) to the intrinsic apoptotic pathway mediated via dephosphorylation of the BH3-only protein Bad. Sequestering members of the prosurvival group of Bcl-2 proteins relieves inhibition of Bax/Bak leading to downstream caspase activation. Previous work has identified a role for cleavage of Bid downstream of the TNFR, thus defining a cross-talk mechanism between the extrinsic and instrinsic apoptotic pathways (8). How other known signals arising from IL-1R interact with Bcl-2 family members in β-cells is poorly understood. For simplicity, full signaling events downstream of IL-1R and TNFR are omitted, such as their cross-talk with each other, as well as the contribution of other relevant receptors such as that for interferon-γ.
Cytokine signaling in β-cells, especially the pathway leading to nitric oxide (NO) generation, has been studied for many years (6), and more recent studies have begun to characterize the intrinisic pathway of apoptosis (7). However, the links between these two areas have remained unclear, and the precise identity of the key players at each level is poorly understood. Although many gaps remain, the study by Grunnet et al. (2) provides evidence for one route in linking proximal cytokine signaling to the intrinsic pathway. The authors show that Bax activation underlies cytochrome c release and caspase-9 cleavage and place the BH3-only protein Bad as an upstream regulator. This builds on previous findings that the BH3-only protein Bid and the downstream multidomain effector molecules Bax and Bak are required for cytokine-induced β-cell death (8) and that overexpression of the prosurvival molecule Bcl-2 partially protects β-cells from cytokine toxicity (9). Bax and Bak are normally both required for apoptosis, so a role for Bak in the process is also likely (10). Moreover, Bad is a weak binder of the prosurvival proteins and is therefore only a poor inducer of apoptosis (11). However, BH3-only proteins cooperate to induce apoptosis in other cell types, so it is possible that Bad and Bid (and potentially other BH3-only proteins) also interact in β-cells in response to cytokines (Fig. 1).
The new findings are interesting because the phosphorylation status of Bad integrates signals arising on the survival side from the akt pathway and on the proapoptotic side from activation of the stress kinase JNK as well as the calcium-regulated protein phosphatase calcineurin. Of these potential mechanisms, the authors highlight the role of calcineurin-mediated dephosphorylation of Bad in rat β-cells, consistent with earlier studies using MIN6N8 insulinoma cells (12). In human islets no such dephosphorylation was observed, although FK506, a calcineurin inhibitor, did diminish cytokine-stimulated caspase-3 activity and apoptosis under these conditions (2). This suggests that calcineurin might act on additional substrates in human β-cells.
However, the involvement of calcineurin is intriguing because it implicates a rise in cytosolic free–calcium ([Ca2+]i) as a mediator of apoptosis in response to cytokines. How might this come about? One explanation involves activation of low-voltage–activated calcium channels (13). However, there is another possibility whereby cytokines might chronically raise [Ca2+]i by transcriptional downregulation of SERCA2b, a transporter responsible for pumping calcium from the cytosol into the endoplasmic reticulum (ER) (14). This mechanism has been hitherto viewed as a potential trigger of ER stress. However, it might be more directly linked to the intrinsic apoptotic pathway, which might explain why ER stress is present but not always necessary for cytokine-stimulated apoptosis (15). In any event, the study by Grunnert et al. now impels further investigation into the role and source of the increased [Ca2+]i caused by proinflammatory cytokines.
Other interesting questions are raised. c-Jun NH2-terminal kinase (JNK) is a key player in β-cell apoptosis in models of type 1 diabetes (6,16), but activation of this stress kinase was not reduced by inhibition of calcineurin—in contrast to the situation in some other cell types. How JNK interacts with the intrinsic apoptotic pathway in β-cells therefore remains a key unresolved question. Likewise, the current study did not address the impact of calcineurin on nuclear factor-κB (NF-κB). Although the NF-κB–regulated gene iNOS plays less of a role in human versus rodent β-cells (6), the contribution of this transcription factor is likely to be complex and its interaction with calcineurin would be a productive topic for future investigation.
Finally, there is independent evidence of a role for a phosphorylation-dependent interplay of Bad with glucokinase in the maintenance of glucose-stimulated insulin secretion (17,18). Thus, in addition to its potential role in cytokine-mediated death, this molecule might also contribute to secretory defects in the context of type 1 as well as type 2 diabetes. In any event, Bad-deficient mice, such as those employed in the glucokinase studies, could prove useful in designing future experiments to determine the involvement of Bad in apoptosis versus its role in β-cell growth/survival. Elucidation of the roles of other Bcl-2 family proteins in this context also awaits the application of appropriate mouse models. The study by Grunnet et al. now helps refine the best avenues for future investigation.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1807.
REFERENCES
- 1.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL: Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54( Suppl.2): S97– S107 [DOI] [PubMed] [Google Scholar]
- 2.Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Størling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T: Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes 2009; 58: 1807– 1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann KC, Bonzon C, Green DR: The machinery of programmed cell death. Pharmacol Ther 2001; 92: 57– 70 [DOI] [PubMed] [Google Scholar]
- 4.Riedl SJ, Salvesen GS: The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 2007; 8: 405– 413 [DOI] [PubMed] [Google Scholar]
- 5.Youle RJ, Strasser A: The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47– 59 [DOI] [PubMed] [Google Scholar]
- 6.Eizirik DL, Mandrup-Poulsen T: A choice of death: the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001; 44: 2115– 2133 [DOI] [PubMed] [Google Scholar]
- 7.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW: Beta cell apoptosis in diabetes. Apoptosis 26March2009. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.McKenzie MD, Carrington EM, Kaufmann T, Strasser A, Huang DC, Kay TW, Allison J, Thomas HE: Proapoptotic BH3-only protein Bid is essential for death receptor–induced apoptosis of pancreatic β-cells. Diabetes 2008; 57: 1284– 1292 [DOI] [PubMed] [Google Scholar]
- 9.Rabinovitch A, Suarez-Pinzon W, Strynadka K, Ju Q, Edelstein D, Brownlee M, Korbutt GS, Rajotte RV: Transfection of human pancreatic islets with an anti-apoptotic gene (bcl-2) protects β-cells from cytokine-induced destruction. Diabetes 1999; 48: 1223– 1229 [DOI] [PubMed] [Google Scholar]
- 10.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB: The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389– 1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ: Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995; 80: 285– 291 [DOI] [PubMed] [Google Scholar]
- 12.Chang I, Cho N, Kim S, Kim JY, Kim E, Woo JE, Nam JH, Kim SJ, Lee MS: Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol 2004; 172: 7008– 7014 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Bhattacharjee A, Zuo Z, Hu F, Honkanen RE, Berggren PO, Li M: A low voltage-activated Ca2+ current mediates cytokine-induced pancreatic beta-cell death. Endocrinology 1999; 140: 1200– 1204 [DOI] [PubMed] [Google Scholar]
- 14.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL: Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 2005; 54: 452– 461 [DOI] [PubMed] [Google Scholar]
- 15.Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ, Laybutt DR: Cytokine-induced β-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008; 57: 3034– 3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF: Cell-permeable peptide inhibitors of JNK: novel blockers of β-cell death. Diabetes 2001; 50: 77– 82 [DOI] [PubMed] [Google Scholar]
- 17.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ: BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003; 424: 952– 956 [DOI] [PubMed] [Google Scholar]
- 18.Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ: Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 2008; 14: 144– 153 [DOI] [PMC free article] [PubMed] [Google Scholar]