Abstract
OBJECTIVE
Using the Hawaii component of the Multiethnic Cohort (MEC), we estimated diabetes incidence among Caucasians, Japanese Americans, and Native Hawaiians.
RESEARCH DESIGN AND METHODS
After excluding subjects who reported diabetes at baseline or had missing values, 93,860 cohort members were part of this analysis. New case subjects were identified through a follow-up questionnaire (1999–2000), a medication questionnaire (2003–2006), and linkage with two major health plans (2007). We computed age-standardized incidence rates and estimated hazard ratios (HRs) for ethnicity, BMI, education, and combined effects of these variables using Cox regression analysis.
RESULTS
After a total follow-up time of 1,119,224 person-years, 11,838 incident diabetic case subjects were identified with an annual incidence rate of 10.4 per 1,000 person-years. Native Hawaiians had the highest rate with 15.5, followed by Japanese Americans with 12.5, and Caucasians with 5.8 per 1,000 person-years; the adjusted HRs were 2.65 for Japanese Americans and 1.93 for Native Hawaiians. BMI was positively related to incidence in all ethnic groups. Compared with the lowest category, the respective HRs for BMIs of 22.0–24.9, 25.0–29.9, and ≥30.0 kg/m2 were 2.10, 4.12, and 9.48. However, the risk was highest for Japanese Americans and intermediate for Native Hawaiians in each BMI category. Educational achievement showed an inverse association with diabetes risk, but the protective effect was limited to Caucasians.
CONCLUSIONS
Within this multiethnic population, diabetes incidence was twofold higher in Japanese Americans and Native Hawaiians than in Caucasians. The significant interaction of ethnicity with BMI and education suggests ethnic differences in diabetes etiology.
Based on prevalence studies, type 2 diabetes is considerably more common among individuals with ethnic backgrounds other than Caucasian (1–3). Both diabetes and obesity are highly prevalent among Native Hawaiians (4,5), but Japanese Americans also suffer a disproportionate rate of the disease despite their relatively low body weight (6,7). This might be due to the higher proportion of body fat and the larger amount of visceral adipose tissue in Asians compared with Caucasians (8–10). In particular, the visceral fat component appears to be associated with impaired glucose tolerance (11,12) and development of type 2 diabetes (13). Incidence data on type 2 diabetes are limited because of the lack of population-based registries, but health plans store information for large parts of the population (14,15). The Hawaii component of the Multiethnic Cohort (MEC) study (16) offers the opportunity to study diabetes incidence by ethnicity. The cohort, with more than 44,000 Japanese Americans, 14,000 Native Hawaiians, and 35,000 Caucasians in Hawaii, has been followed for more than 10 years. To estimate annual incidence rates for type 2 diabetes since cohort entry, we combined information from MEC follow-up questionnaires with data from diabetes care registries maintained by the two major health plans in Hawaii that capture at least 90% of the population: the Blue Cross and Blue Shield (BCBS) association and Kaiser Permanente Hawaii (KP) (17). Our goal was to estimate incidence rates by sex, ethnicity, age at cohort entry, BMI, and education.
RESEARCH DESIGN AND METHODS
The MEC was established from 1993 through 1996 to study diet and cancer among different ethnic groups in Hawaii and California (16). Subjects entered the cohort by completing a 26-page, self-administered mailed survey that asked about demographic background, medical conditions, anthropometric measures, diet, and lifestyle factors. The Hawaii component of the MEC consists of 103,898 members (48,936 men and 54,962 women) of different ethnicities (43% Japanese Americans, 34% Caucasians, 14% Native Hawaiians, and 9% other ancestries including 6% Filipino). Individuals who reported more than one ancestry were assigned to one of the categories according to the following priority ranking: Native Hawaiian, Japanese American, and Caucasian. The percentages of mixed ethnic backgrounds were 82, 3, and 5% for Native Hawaiians, Japanese Americans, and Caucasians, respectively. The following response rates to the baseline questionnaire mailings were achieved: 28 and 35% for Native-Hawaiian men and women, respectively; 39 and 47% for Caucasian men and women; and 46 and 51% for Japanese-American men and women. A comparison of the cohort with census data indicated that the MEC represents all levels of education, although cohort members were somewhat better educated than the general population (16). The overrepresentation of college-educated subjects was greater for men than for women and for Native Hawaiians and Caucasians than for Japanese Americans (16).
Follow-up and vital status.
Between February 1999 and September 2003, a short follow-up questionnaire (FuQx) was sent to all MEC members to update information on medical conditions, including diabetes (Table 1). Information from the FuQx was available for 84% of the Hawaii part of the cohort. In addition, a biorepository of blood and urine specimens was created for the MEC between 2003 and 2006. A medication questionnaire (MedQx), including diabetes drugs, was administered as part of the procedure and was available for 38% of the 103,898 subjects. Since the MEC was established, annual linkages with state death certificate files have been performed to update vital status information.
TABLE 1.
Diabetes status of subjects in the Hawaii component of the MEC study at different follow-up times
| N | Diabetes cases | Prevalence (%) | |
|---|---|---|---|
| Baseline questionnaire (1993–1996) | 103,898 | 10,028 | 9.7 |
| Follow-up questionnaire (1999–2003) | 86,732 | 9,964 | 11.5 |
| Medication use questionnaire (2003–2006) | 39,787 | 4,425 | 11.1 |
| Linkage with BCBS (2007)* | 67,465 | 11,375 | 16.9 |
| Linkage with KP (2007) | 20,539 | 4,003 | 19.5 |
*Number of MEC subjects provided to BCBS minus KP members; health plan membership is not established for noncase subjects.
Linking with health insurance plans.
After excluding 15,884 subjects who were known to be deceased, had refused further participation in the cohort, or had missing information on diabetes at baseline or follow-up time after baseline, the MEC data for 88,004 members were linked with the diabetes care registries of the two major insurers that also covered low-income and elderly individuals through government-sponsored health plans in July 2007 (17). The registries evaluate quality of diabetes care and monitor health outcomes according to the National Committee for Quality Assurance/Health Plan Employer Data and Information Set Reporting Guidelines (18). After approval by the Committee on Human Studies at the University of Hawaii and the institutional review board (IRB) at KP, a memorandum of agreement with both health plans was signed. The KP IRB approved a Health Insurance Portability and Accountability Act (HIPAA) waiver.
The BCBS plan uses an algorithm based on multiple claims for diabetes-related treatment services over a 2-year time span (including pharmacy) to identify diabetic individuals for the care registry established in 2000. This approach reduces the number of false positives that would arise from considering single diagnostic claims. Physicians can also make referrals into the program. All identified patients receive mailings and may ask to be removed from the registry unless a physician counters their claim. Based on the terms negotiated in the memorandum of agreement, MEC subjects were not linked with the entire BCBS membership file but only with the diabetes registry database. Thus, MEC subjects who were BCBS members without diabetes were not ascertained, and a denominator of BCBS members within the MEC was not established. Linkage of the MEC database with the health plan was performed through probability matching of last and first name, middle initial, birth month and year, and sex. Over 2,000 possible matches that agreed on birth year, birth month, and last name but not perfectly on first name were reviewed manually. Using the current address as ancillary information, a decision was made regarding whether an error in the name or birth date was responsible for the lack of a perfect match.
The comprehensive data systems at KP provide longitudinal data on health care utilization, laboratory results, coverage, pharmaceutical use, and other data elements. Selection of KP members into the diabetes care registry is also based on multiple pieces of evidence and records from several databases including clinical information (A1C testing), pharmacy records (insulin, sulfonylurea drugs, metformin, and blood glucose testing supplies), hospital discharge diagnoses reflecting the presence of diabetes, and outpatient encounters. Linkage with the KP membership file was performed for all MEC members using social security numbers, sex, and birthdates. As a result, KP members with and without diabetes were identified. Data on linked subjects were then examined in three databases: Problem List, Diagnoses, and Diabetes Registry. Subjects detected in any of these were considered diabetic case subjects.
Available data and categorization of case subjects.
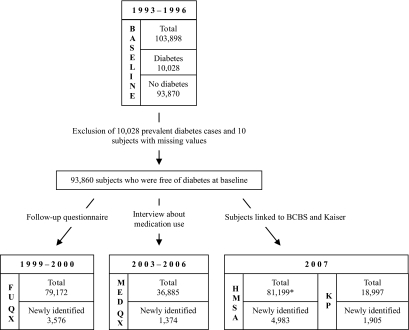
Of the original 103,898 members within the Hawaii subset of the MEC, 10,028 (9.7%) subjects who reported a diagnosis of diabetes at baseline (Table 1) and 10 subjects who had missing information were excluded from the incidence analysis (Fig. 1). Subjects who indicated having diabetes at any point after baseline or who were classified as case subjects by one of the health plans were considered incident at the time of the first report. Individuals who never reported diabetes and who were not identified as diabetic patients by the health plans were categorized as noncase subjects. As described above, data on diabetes status were available at three subsequent time points from four different sources: the FuQx, the MedQx, and the linkage with the BCBS and KP health plans. Of the 86,732 participants who completed the FuQx, 9,964 (11.5%) indicated diabetes. At the time of the MedQx, 4,425 (11.1%) of the 39,787 subjects reported use of diabetes medications. Finally, of the 88,004 MEC subjects linked with the BCBS plan, 11,375 were identified as diabetic case subjects (16.9% of estimated BCBS members, i.e., 88,004 minus 20,539 KP members), while 20,539 (23.3%) MEC subjects were identified as KP members of whom 4,003 (19.5%) were diabetic case subjects.
FIG. 1.
Incident diabetic case subjects (N = 11,838) identified at different follow-up periods within the Hawaii component of the MEC. *Number of subjects provided to BCBS; health plan membership not established for noncase subjects.
Follow-up time.
For noncase subjects, the follow-up time was calculated as the time between the date of the baseline questionnaire and either of the following events, if applicable: the date of death or the last date when data on diabetes status were available (i.e., the date of the FuQx or MedQx or the date of the health plan linkage). For incident case subjects, the follow-up time was calculated as the time between the baseline questionnaire and an estimated diagnosis date according to the following rules. For incident case subjects lost to follow-up before linkage with the health plan, the estimated date of diagnosis was the midpoint in time between the last report of not having diabetes and the first indication of diabetes. For case subjects linked and insured with KP, the date of diagnosis was assumed as 1 July of the diagnosis year and as 1 July 2007 for 159 case subjects with missing dates. For incident case subjects from BCBS, the date of diagnosis was known for all except 14 case subjects diagnosed before 1 December 1999 for whom the midpoint between the last date without diabetes and 1 December 1999 was computed.
Statistical analysis.
The average annual incidence rates were computed by sex and ethnic group as the sum of the number of newly diagnosed diabetic case subjects divided by the sum of person-years of follow-up. The incidence rates were age standardized by the direct method to the truncated U.S. 2000 population standard given that the three ethnic groups have different age distributions. Annual incidence rates were estimated for subgroups defined by age at cohort entry, BMI, and education. Confidence intervals (CIs) were computed for the incidence rates assuming normal distribution because of the large sample size using the following equation:
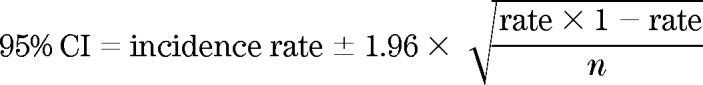 |
Cox proportional hazards regression models using PROC PHREG in the SAS software (version 9.1; SAS Institute, Cary, NC) were applied to estimate diabetes risk by ethnicity. We calculated hazard ratios (HRs) and 95% CIs using models stratified by age at cohort entry (continuous) and adjusted for ethnicity (Caucasian, Japanese American, and Native Hawaiian), education (less than or at least high school degree, some college education, and college graduate), and BMI (<22, 22–24.9, 25–29.9, and ≥30 kg/m2). For BMI and education, we created indicator variables that reflected ethnicity in combination with the four BMI or three education levels, respectively. When included in the Cox regression analysis, Caucasians in the lowest category were the reference category. For the BMI analyses, 1,037 subjects with missing and 318 with invalid BMI information were excluded.
RESULTS
The ethnic groups within the Hawaii component of the MEC differed by age; Japanese Americans were older and Native Hawaiians relatively younger than Caucasians (Table 2). Native Hawaiians were more likely and Japanese Americans less likely to be overweight than Caucasians. Diabetes prevalence differed by time of assessment and increased over time (Table 1). The estimated prevalence of diabetes was 16.9% for MEC members linked with the BCBS plan and 19.5% for subjects linked with KP. The ethnic distribution for KP was 45% Caucasians, 26% Japanese Americans, and 18% Native Hawaiians, whereas among non-KP members who may not all be BCBS plan members 38% were Caucasians, 42% Japanese Americans, and 12% Native Hawaiians.
TABLE 2.
Composition of the Hawaii component of the MEC study at baseline
| Caucasian (n = 35,042) |
Japanese American (n = 44,513) |
Native Hawaiian (n = 14,346) |
Other (n = 9,997) |
All (n = 103,898) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| n | 17,299 | 17,743 | 21,011 | 23,502 | 6,140 | 8,206 | 4,486 | 5,511 | |
| Age at cohort entry (years) | |||||||||
| <55 | 42.6 | 44.4 | 29.6 | 29.9 | 44.5 | 49.8 | 43.2 | 43.5 | 38.2 |
| 55–64 | 27.6 | 26.9 | 27.5 | 29.6 | 30.9 | 29.6 | 31.5 | 32.3 | 28.7 |
| ≥65 | 29.8 | 28.7 | 42.9 | 40.5 | 24.6 | 20.6 | 25.3 | 24.2 | 33.2 |
| BMI (kg/m2) | |||||||||
| <22.0 | 13.8 | 33.2 | 18.5 | 41.5 | 9.7 | 20.9 | 16.6 | 30.6 | 25.5 |
| 22.0–24.9 | 31.6 | 27.4 | 37.2 | 29.7 | 22.8 | 22.6 | 29.5 | 27.5 | 30.0 |
| 25.0–29.9 | 40.7 | 25.2 | 37.2 | 22.6 | 41.0 | 30.7 | 39.6 | 26.6 | 31.7 |
| ≥30.0 | 13.9 | 14.1 | 7.2 | 6.2 | 26.4 | 25.9 | 17.4 | 15.3 | 12.8 |
| Education (years)* | |||||||||
| ≤12 | 22.6 | 27.5 | 42.6 | 46.2 | 48.4 | 51.6 | 44.2 | 47.4 | 38.9 |
| 13–15 | 29.2 | 32.8 | 28.1 | 26.5 | 29.5 | 28.0 | 28.0 | 26.8 | 28.7 |
| >15 | 48.2 | 39.7 | 29.2 | 27.3 | 22.1 | 20.4 | 27.8 | 25.8 | 32.4 |
| Smoking status* | |||||||||
| Never | 31.7 | 44.9 | 29.3 | 68.0 | 31.1 | 47.6 | 30.4 | 56.6 | 44.2 |
| Past | 50.8 | 37.9 | 54.8 | 21.5 | 47.5 | 31.1 | 49.0 | 26.6 | 39.7 |
| Current | 16.9 | 16.5 | 15.3 | 9.2 | 20.7 | 20.0 | 19.6 | 15.6 | 15.3 |
| Family history | |||||||||
| Parents | 12.2 | 15.2 | 16.8 | 19.0 | 19.9 | 24.8 | 14.2 | 19.7 | 17.1 |
| Siblings | 5.4 | 7.0 | 9.6 | 12.8 | 8.7 | 12.6 | 7.3 | 10.8 | 9.3 |
| Hypertension | 27.1 | 26.9 | 42.9 | 38.4 | 45.4 | 44.8 | 37.7 | 36.5 | 36.2 |
| Mean follow-up time (years) | 12.0 ± 3.5 | 12.5 ± 2.9 | 11.4 ± 3.7 | 12.2 ± 3.1 | 10.9 ± 4.0 | 11.4 ± 3.7 | 11.6 ± 3.7 | 12.2 ± 3.2 | 11.9 ± 3.4 |
Data are % unless otherwise indicated.
*Totals may not add up to 100% because of missing values.
After combining the information from questionnaires and linkages, a total of 11,838 incident diabetic case subjects were identified (Fig. 1). Of the 93,860 participants not reporting diabetes at baseline, 3,576 individuals (30% of incident case subjects) reported diabetes in the FuQx and 1,374 individuals (12% of incident case subjects) were newly identified through the MedQx. Of the 88,004 participants linked with the health plans, 81,199 (92%) were free of diabetes at baseline. KP identified 1,905 (16% of incident) new case subjects, and the BCBS plan identified another 4,983 (42% of incident) new case subjects. The total follow-up time for all 93,860 subjects who were free of diabetes at baseline was 1,119,224 person-years.
Based on the subjects with self-reported diabetes at baseline who were part of any follow-up (n = 8,327), 64% were correctly identified as diabetic case subjects by all questionnaires and linkages in which they were included. Another 24% were identified by at least one follow-up method, whereas 12% were not considered diabetic case subjects at any follow-up. Of the 20,539 KP members who reported diabetes in a questionnaire (n = 2,524), 83% were identified as case subjects by KP. The missing denominator does not allow similar computations for BCBS. Of all MEC members who reported diabetes medication use in the MedQx and were part of the health plan linkage, 83% were identified as diabetic case subjects.
The annual age-adjusted incidence rate was estimated as 10.4 cases per 1,000 person-years (95% CI 9.8–11.1) (Table 3). Men had a higher incidence rate than women with 11.7 vs. 9.4 per 1,000 person-years, both for KP (14.8 and 12.4) and for non-KP members (11.1 and 8.7). Native Hawaiians had the highest incidence rate at 15.5, followed by Japanese Americans at 12.5 and others at 12.2, while Caucasians were lowest at 5.8 cases per 1,000 person-years. The 95% CIs for Native Hawaiians and Japanese Americans overlapped with each other but not with Caucasians. BMI was directly related to incidence; overweight and obese subjects had rates of 13.8 and 25.8 per 1,000 person-years, respectively, compared with 3.6 and 7.4 for the two lowest categories. Education was inversely related to incidence; subjects with a college degree had an incidence rate of 8.0, and those with some college education and equal to or less than a high school diploma had rates of 10.3 and 12.9 per 1,000 person-years, respectively. The same trends, but somewhat higher incidence rates, were observed for KP members.
TABLE 3.
Annual age-adjusted incidence rates of diabetes (per 1,000 person-years) within the Hawaii component of the MEC study, 1993–1996
| N | Incident cases | Incidence rate (95% CI) | Incidence rate for KP members (95% CI) | |
|---|---|---|---|---|
| Overall | 93,860 | 11,838 | 10.4 (9.8–11.1) | 13.5 (11.8–15.1) |
| Sex | ||||
| Male | 43,801 | 6,033 | 11.7 (10.7–12.7) | 14.8 (12.3–17.3) |
| Female | 50,059 | 5,805 | 9.4 (8.5–10.2) | 12.4 (10.3–14.6) |
| Ethnicity | ||||
| Caucasian | 33,229 | 2,386 | 5.8 (5.0–6.6) | 8.7 (6.7–10.7) |
| Japanese American | 39,675 | 5,957 | 12.5 (11.4–13.5) | 15.8 (12.4–19.2) |
| Native Hawaiian | 12,159 | 2,182 | 15.5 (13.3–17.6) | 20.5 (15.7–25.3) |
| Other | 8,797 | 1,313 | 12.2 (9.9–14.4) | 15.8 (10.6–21.0) |
| Age at cohort entry (years) | ||||
| 45–49 | 21,017 | 2,278 | 8.5 (7.7–10.2) | 9.8 (7.0–12.5) |
| 50–54 | 16,111 | 2,152 | 10.7 (9.0–12.2) | 12.0 (8.5–15.5) |
| 55–59 | 12,832 | 1,887 | 12.2 (9.8–13.5) | 15.0 (10.5–19.5) |
| 60–64 | 13,815 | 1,955 | 11.9 (10.1–13.7) | 14.8 (10.2–19.3) |
| 65–69 | 14,903 | 1,969 | 11.5 (9.6–13.0) | 16.8 (11.9–21.7) |
| 70–74 | 12,502 | 1,364 | 10.1 (8.7–12.2) | 14.6 (9.3–19.9) |
| 75–79 | 2,683 | 233 | 8.5 (4.9–11.7) | 16.7 (3.9–29.5) |
| BMI (kg/m2)* | ||||
| <22.0 | 23,906 | 1,102 | 3.6 (1.5–5.6) | 4.6 (0.0–10.0) |
| 22.0–24.9 | 28,889 | 2,703 | 7.4 (6.6–8.1) | 9.1 (7.2–11.1) |
| 25.0–29.9 | 29,289 | 4,816 | 13.8 (12.4–15.1) | 16.6 (13.4–19.8) |
| ≥30.0 | 10,576 | 3,068 | 25.8 (22.9–28.7) | 32.4 (25.9–39.0) |
| Education (years)* | ||||
| ≤12 | 34,486 | 5,087 | 12.9 (10.6–15.2) | 16.9 (10.7–23.1) |
| 13–15 | 27,119 | 3,428 | 10.3 (9.1–11.5) | 14.0 (10.7–17.4) |
| >15 | 31,486 | 3,196 | 8.0 (7.0–9.0) | 10.0 (7.6–12.3) |
Total follow-up time is 1,119,224 person-years.
*Totals may not add up to 100% because of missing values.
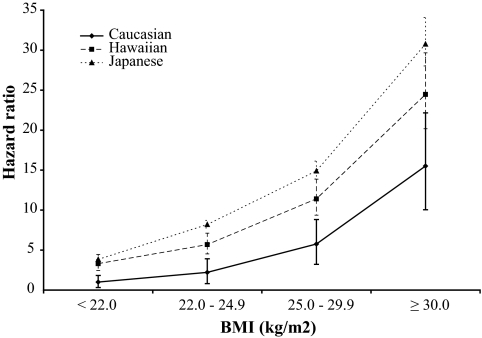
Cox regression models stratified by age at cohort entry and adjusted for sex, BMI, and education showed results similar to unadjusted incidence rates. Japanese Americans and Native Hawaiians were twice as likely to be diagnosed with diabetes as Caucasians with HRs 2.65 (95% CI 2.52–2.78) and 1.93 (1.82–2.06), respectively. The respective HRs for BMI were 2.10 (1.94–2.26), 4.12 (3.83–4.43), and 9.48 (8.77–10.25). When we examined the combined effect of BMI and ethnicity (Fig. 2), diabetes risk for Japanese Americans and Native Hawaiians was higher than for Caucasians at all BMI levels. In comparison with the reference category of Caucasians with a BMI <22 kg/m2, the HR for Caucasian obese subjects was 15.51 (12.78–18.82), whereas for obese Native Hawaiians and Japanese Americans the respective HRs were 24.47 (20.17–29.67) and 30.76 (25.30–37.40). Even for individuals with a BMI of 22.0–24.9 kg/m2, the risk was significantly elevated for all ethnic groups.
FIG. 2.
Diabetes risk by ethnicity and BMI. Data are HRs and 95% CIs from the Cox regression analysis (adjusted for age, sex, and education). Caucasians with a BMI <22 kg/m2 are the reference category.
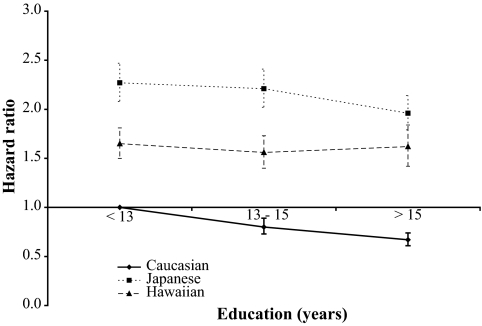
With regard to education, subjects with >12 years of education experienced a significantly lower diabetes risk; the HRs were 0.94 (95% CI 0.89–0.98) for subjects with 13–15 years of education and 0.83 (0.79–0.87) for college graduates. In the interaction model (Fig. 3), the respective HRs were 0.67 (0.61–0.74), 1.96 (1.79–2.14), and 1.62 (1.42–1.84) for Caucasians, Japanese Americans, and Native Hawaiians when the highest level was compared with the lowest category in Caucasians. As indicated by the CIs, risk in Japanese Americans and Native Hawaiians did not differ significantly by education.
FIG. 3.
Influence of education on diabetes incidence by ethnicity. Data are HRs and 95% CIs from the Cox regression analysis (adjusted for age, sex, and BMI). Caucasians with <13 years of education are the reference category.
DISCUSSION
This analysis of the MEC showed that the annual diabetes incidence rate was twice as high in Native Hawaiians as in Caucasians and even higher in Japanese Americans. In agreement with findings for the prevalence of diabetes at baseline (19), this difference was not explained by age, BMI, or education. BMI showed a significant association with diabetes risk among all ethnic groups, but the incidence was higher for Japanese Americans and Native Hawaiians than for Caucasians at all BMI levels, even at the lowest level. The high incidence rate in Japanese Americans despite their lower prevalence of overweight and obesity suggests greater sensitivity to body fat in this population (7,20,21). Also noteworthy was the significant interaction with education. Educational achievement was only protective among Caucasians but not in the two other ethnic groups. These observations must be considered in light of the advanced age of the cohort; the mean age was ∼71 years in 2007.
Our results are consistent with findings of previous studies reporting higher incidence rates in Japanese Americans than in Caucasians (6,7,13,22,23). Although the strong relation of BMI with diabetes prevalence (7,24,25) and incidence (6) in Japanese Americans at relatively low BMI levels and the high diabetes prevalence among Native Hawaiians (1,5) have previously been described (19), the disparate influence of BMI on incidence is noteworthy (Fig. 2). In agreement with our findings, a study in China described a higher diabetes risk in Chinese than in Caucasians with a BMI >25 kg/m2; however, at lower levels there was little difference in risk (26). Mechanistic studies indicate that insulin secretion decreases during early stages of diabetes development among Japanese but not among Caucasian subjects (7,20,21). This impaired β-cell function may not allow Japanese subjects to compensate for insulin resistance through an increase in insulin production to the same degree as Caucasian and Native Hawaiian subjects (27). Therefore, Japanese are more susceptible to changes in BMI, extra body fat, and, in particular, central obesity and an excess of metabolically active visceral fat (8–10). As shown in Japanese studies, visceral fat is a risk factor for insulin resistance (11,12) and central obesity confers a higher diabetes risk than overall adiposity (28).
The weak relation of education with diabetes incidence among Japanese Americans and Native Hawaiians agrees with a report from the Honolulu Heart Program (29) but disagrees with studies showing lower diabetes rates in better educated individuals (30–32). Education, in combination with income and lifestyle, often explains ethnic differences in diabetes. However, one report described less protection against diabetes from education for African American than Caucasian women (33). Possible explanations include the fact that education is an incomplete description of socioeconomic status and that quality of education and its relation to income and health behavior may differ by ethnic group (33).
This analysis has a number of limitations. Foremost, the validity of the diagnosis obtained through different methods varied. The questionnaires relied on self-reports, and the health plan linkages were dependent on health insurance, contact with the health care system, and an accurate algorithm to identify cases. The estimation of follow-up time as the midpoint between follow-up events may have introduced some error; however, there is no obvious reason why the diagnoses of diabetes should not have been distributed evenly over time. Missing case subjects in the diabetes registry or failure of the probability linking procedure, in particular within BCBS, was possible. Even within KP, a minimal number of MEC subjects matched based on social security numbers but did not match on exact birthdates. A number of self-reported diabetic case subjects in the FuQx or MedQx were not identified by BCBS or KP, but exclusion of these 1,545 subjects would have lowered the incidence rate only to 9.1 per 1,000 person-years. Although no data documenting the quality of the diabetes care registries for Hawaii are available, validation studies of similar registries in other locations have shown that care registries are highly specific and adequately sensitive (34,35). In terms of validity of self-reported diabetes, a study from the Netherlands (36) showed very high specificity for self-reported diabetes (99.4%) but relatively low sensitivity (58.9%) in comparison with measured glucose levels. Because the comparison in our study is not self-report versus glucose levels but rather self-report versus health care system, the sensitivity should be considerably higher (37).
The fact that we do not know the number of MEC subjects who were insured with BCBS may have lowered incidence rates. Given the large market shares of the two insurers both for private and for government-sponsored plans and the fact that only 4% of subjects who reported use of diabetes medication in the MedQx were missed, we are confident that <10% of MEC subjects received medical coverage through other health plans or through the military (17). Still, the prevalence within BCBS is most likely an underestimate because the true denominator is lower than the difference between 88,004 and the KP members. The lower incidence for BCBS members is also surprising in light of the lower proportion of Caucasians within BCBS than within KP. Internal BCBS numbers suggest that the diabetes prevalence in the BCBS members is only 12.5% compared with our estimate of 16.9%; possibly, the MEC members are more likely to be diagnosed than the general BCBS members. Among MEC members who were KP members in 2007, the incidence should be closer to a true rate because of the clearly established denominator. In comparison with BCBS, the linkage probably captured a higher proportion of case subjects because KP used several databases to identify cases. It is also possible that diabetic patients are more likely to enroll in a KP health plan because of lower-cost diabetes care or that KP members are more likely to be diagnosed as diabetic as a result of enhanced preventive care services.
The large multiethnic study population with a great variation in diabetes risk is a strength of this report. During >10 years of follow-up, we detected a more than twofold higher diabetes incidence in Japanese Americans and a close to twofold higher risk in Native Hawaiians than in Caucasians. In this population of elderly cohort members, a strong association of BMI with diabetes was observed for all ethnic groups; however, given the higher risk in Japanese Americans and Native Hawaiians, their incidence rates were higher at each BMI level than those in Caucasians. In combination with the differential effect of education on diabetes incidence, our findings suggest ethnic differences in diabetes etiology and support variable risk classifications for Asians and Pacific Islanders (10,38,39). Although previous research with insurance data has been performed (14,15), our linkage is novel in that it demonstrates the usefulness of this approach for large cohorts. The success of this approach offers excellent opportunities for future research, such as identification of diabetes risk factors and investigations into other chronic conditions for which no population-based registries exist.
Acknowledgments
The MEC has been supported by the National Cancer Institute Grant R37CA54281 (principal investigator [PI]: L.N.K.). The recruitment of Native Hawaiians was funded by the Department of Defense Breast Cancer Research Program Grant DAMD 17-94-T-4184 (PI: Dr. A. Nomura). The diabetes project is funded by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK073816 (PI: G.M.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sloan NR: Ethnic distribution of diabetes mellitus in Hawaii. JAMA 1963; 183: 419– 424 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Detailed data for prevalence of diabetes [article online], 2007. Available from http://www.cdc.gov/diabetes/statistics/prev/national/menuraceethsexage.htm Accessed 24 October 2008
- 3.McNeely MJ, Boyko EJ: Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 2004; 27: 66– 69 [DOI] [PubMed] [Google Scholar]
- 4.Maskarinec G, Takata Y, Pagano I, Carlin L, Goodman MT, Le ML, Nomura AM, Wilkens LR, Kolonel LN: Trends and dietary determinants of overweight and obesity in a multiethnic population. Obesity (Silver Spring) 2006; 14: 717– 726 [DOI] [PubMed] [Google Scholar]
- 5.Grandinetti A, Kaholokula JK, Theriault AG, Mor JM, Chang HK, Waslien C: Prevalence of diabetes and glucose intolerance in an ethnically diverse rural community of Hawaii. Ethn Dis 2007; 17: 250– 255 [PubMed] [Google Scholar]
- 6.Burchfiel CM, Curb JD, Rodriguez BL, Yano K, Hwang LJ, Fong KO, Marcus EB: Incidence and predictors of diabetes in Japanese-American men. The Honolulu Heart Program. Ann Epidemiol 1995; 5: 33– 43 [DOI] [PubMed] [Google Scholar]
- 7.Hara H, Egusa G, Yamakido M: Incidence of non-insulin-dependent diabetes mellitus and its risk factors in Japanese-Americans living in Hawaii and Los Angeles. Diabet Med 1996; 13: S133– S142 [PubMed] [Google Scholar]
- 8.Deurenberg P, Deurenberg-Yap M, Guricci S: Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002; 3: 141– 146 [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Allison DB, Heymsfield SB, Gallagher D: Larger amounts of visceral adipose tissue in Asian Americans. Obes Res 2001; 9: 381– 387 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization/International Association for the Study of Obesity /International Obesity Taskforce The Asia-Pacific Perspective: Redefining Obesity and its Treatment Geneva, World Health Org, 2000 [Google Scholar]
- 11.Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Maruyama N, Morioka K, Nakatani K, Yano Y, Adachi Y: Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care 2003; 26: 2341– 2344 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY: Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 2008; 57: 1269– 1275 [DOI] [PubMed] [Google Scholar]
- 13.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L: Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000; 23: 465– 471 [DOI] [PubMed] [Google Scholar]
- 14.Young TK, Roos NP, Hammerstrand KM: Estimated burden of diabetes mellitus in Manitoba according to health insurance claims: a pilot study. Can Med Assoc J 1991; 144: 318– 324 [PMC free article] [PubMed] [Google Scholar]
- 15.Maskarinec G: Diabetes in Hawaii: estimating prevalence from insurance claims data. Am J Public Health 1997; 87: 1717– 1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS: A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000; 151: 346– 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawaii Health Information Corporation Health trends in Hawaii: a profile of the health care system [article online], 2004. Available from www.healthtrends.org Accessed 3 September 2008
- 18.Smith JJ: NCQA/HEDIS guidelines for diabetes. Manag Care 2001; 10( Suppl. 2): 3– 5 [PubMed] [Google Scholar]
- 19.Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, Kolonel LN: Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis 2009; 19: 49– 55 [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE: β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002; 51: 2170– 2178 [DOI] [PubMed] [Google Scholar]
- 21.Fukushima M, Suzuki H, Seino Y: Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66( Suppl. 1): S37– S43 [DOI] [PubMed] [Google Scholar]
- 22.Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB: Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006; 29: 1585– 1590 [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH: A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992; 268: 63– 67 [PubMed] [Google Scholar]
- 24.Rodriguez BL, Curb JD, Burchfiel CM, Huang B, Sharp DS, Lu GY, Fujimoto W, Yano K: Impaired glucose tolerance, diabetes, and cardiovascular disease risk factor profiles in the elderly: the Honolulu Heart Program. Diabetes Care 1996; 19: 587– 590 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez BL, D'Agostino R, Abbott RD, Kagan A, Burchfiel CM, Yano K, Ross GW, Silbershatz H, Higgins MW, Popper J, Wolf PA, Curb JD: Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke 2002; 33: 230– 236 [DOI] [PubMed] [Google Scholar]
- 26.Stevens J, Truesdale KP, Katz EG, Cai J: Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People's Republic of China Study and the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2008; 167: 1365– 1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandinetti A, Keawe'aimoku KJ, Chang HK, Chen R, Rodriguez BL, Melish JS, Curb JD: Relationship between plasma glucose concentrations and Native Hawaiian Ancestry: The Native Hawaiian Health Research Project. Int J Obes Relat Metab Disord 2002; 26: 778– 782 [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi H, Saitoh S, Takagi S, Katoh N, Chiba Y, Akasaka H, Nakamura Y, Shimamoto K: Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: the Tanno and Sobetsu Study. Diabetes Care 2006; 29: 1128– 1129 [DOI] [PubMed] [Google Scholar]
- 29.Huang B, Rodriguez BL, Burchfiel CM, Chyou PH, Curb JD, Yano K: Acculturation and prevalence of diabetes among Japanese-American men in Hawaii. Am J Epidemiol 1996; 144: 674– 681 [DOI] [PubMed] [Google Scholar]
- 30.Smith JP: Nature and causes of trends in male diabetes prevalence, undiagnosed diabetes, and the socioeconomic status health gradient. Proc Natl Acad Sci U S A 2007; 104: 13225– 13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins JM, Vaccarino V, Zhang H, Kasl SV: Socioeconomic status and diagnosed diabetes incidence. Diabetes Res Clin Pract 2005; 68: 230– 236 [DOI] [PubMed] [Google Scholar]
- 32.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, Hargreaves MK, Blot WJ: Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health 2007; 97: 2260– 2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins JM, Vaccarino V, Zhang H, Kasl SV: Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health 2001; 91: 76– 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F: Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol 2004; 14: 507– 516 [DOI] [PubMed] [Google Scholar]
- 35.Brown JB, Nichols GA, Glauber HS: Case-control study of 10 years of comprehensive diabetes care. West J Med 2000; 172: 85– 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molenaar EA, Van Ameijden EJ, Grobbee DE, Numans ME: Comparison of routine care self-reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health 2007; 17: 199– 205 [DOI] [PubMed] [Google Scholar]
- 37.Bowlin SJ, Morrill BD, Nafziger AN, Jenkins PL, Lewis C, Pearson TA: Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol 1993; 46: 561– 571 [DOI] [PubMed] [Google Scholar]
- 38.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, Teo KK, McQueen M, Yusuf S: Defining obesity cut points in a multiethnic population. Circulation 2007; 115: 2111– 2118 [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez BL, Abbott RD, Fujimoto W, Waitzfelder B, Chen R, Masaki K, Schatz I, Petrovitch H, Ross W, Yano K, Blanchette PL, Curb JD: The American Diabetes Association and World Health Organization classifications for diabetes: their impact on diabetes prevalence and total and cardiovascular disease mortality in elderly Japanese-American men. Diabetes Care 2002; 25: 951– 955 [DOI] [PubMed] [Google Scholar]