Abstract
OBJECTIVE
To examine the prospective association between objectively measured time spent sedentary and insulin resistance and whether this association is independent of moderate- and vigorous-intensity physical activity (MVPA) and other relevant confounders.
RESEARCH DESIGN AND METHODS
This was a population-based study (Medical Research Council Ely study) in 376 middle-aged adults (166 men; 210 women) over 5.6 years of follow-up. Physical activity and sedentary time were measured objectively by individually calibrated minute-by-minute heart rate monitoring at both baseline and follow-up. Sedentary time was calculated as the heart rate observations (in minutes) below an individually predetermined threshold (flex heart rate) and expressed as a percentage of total monitored time during waking hours over 4 days. The percentage of time spent above 1.75 × resting heart rate represented MVPA. Fasting plasma insulin was used as a surrogate measure of insulin resistance.
RESULTS
Time spent sedentary at baseline was significantly and positively associated with log fasting insulin at follow-up (β = 0.003, 95% CI 0.0006–0.006, P = 0.015) independent of baseline age, sex, fat mass, fasting insulin, smoking status, and follow-up time. After further adjustment for MVPA, this association was somewhat strengthened (β = 0.004, 95% CI 0.0009–0.006, P = 0.009).
CONCLUSIONS
Time spent sedentary predicts higher levels of fasting insulin independent of the amount of time spent at moderate- and vigorous-intensity activity levels. This highlights the importance of reducing sedentary time in order to improve metabolic health, possibly in addition to the benefits associated with a physically active lifestyle.
Insulin resistance is a precursor of type 2 diabetes and a major characteristic of the metabolic syndrome (1). Hyperinsulinemia and impaired insulin sensitivity are common clinical findings yielding independent health risks, including metabolic, cardiovascular, and neoplastic disorders (2–4).
Several etiological factors have been identified for impaired insulin sensitivity, including genotype, body composition, inflammation, and lifestyle factors (5–7). Low levels of physical activity and lack of moderate- and vigorous-intensity physical activity (MVPA) are associated with insulin resistance (8,9).
Sedentary time has been linked to various cardiometabolic health outcomes, sometimes independent of overall physical activity in cross-sectional analyses (10–12). Because the cardiometabolic consequences are suggested to be a unique feature in hazardous physical activity behavior, sedentary behavior should be considered distinctively from physical activity when examining associations with these health outcomes (13).
A recent prospective analysis suggested that MVPA but not sedentary time was associated with insulin resistance in high-risk individuals over a 1-year follow-up period (14). Further prospective research is needed to examine these associations and the direction of causality in normal-risk populations with longer duration of follow-up. Therefore, the purpose of the present study was to 1) examine the prospective association between objectively measured sedentary time and fasting insulin, a marker of insulin resistance, in healthy middle-aged Caucasian subjects and 2) examine whether this association is independent of MVPA and other confounding variables.
RESEARCH DESIGN AND METHODS
This study is part of the Medical Research Council Ely study, a prospective population-based cohort study of the etiology and pathogenesis of type 2 diabetes and related metabolic disorders. Data were collected in 1994–1996 (baseline) and again in 2001–2003 (median follow-up time 5.6 years). A total of 393 participants with complete data on anthropometry, body composition, and physical activity energy expenditure (PAEE) at both baseline and follow-up were initially selected (15). Participants with missing data on fasting plasma insulin, fat mass, smoking status, or MVPA were excluded. Therefore, the present report comprises 376 (166 male) healthy middle-aged Caucasian subjects. Missing data were random with respect to body composition and physical activity at baseline. Ethics permission for the study was granted by the Cambridge Local Research Committee, and all participants provided written informed consent.
Plasma insulin, glucose, and confounding variables.
Data collection procedures have been described in detail previously (9,16). In brief, blood samples were taken after an overnight fast. Plasma insulin and glucose levels were determined using standardized protocols. Levels of fasting plasma insulin were used as a surrogate measure for insulin resistance. The homeostasis model assessment of insulin resistance (HOMA-IR) score was calculated by dividing the product of fasting plasma insulin (microunits per milliliter) and fasting plasma glucose (millimoles per liter) by 22.5. Anthropometry data were obtained using standardized procedures (9,16). Body composition was measured by bio-impedance (Bodystat 1500 analyzer; Bodystat, Isle of Man, U.K.). Information on smoking status was self-reported.
Sedentary behavior, physical activity, and aerobic fitness.
A standard valid protocol for measuring energy expenditure was used at baseline and follow-up (17). The individual relationship between energy expenditure and heart rate was assessed during rest and exercise according to the flex heart rate (FHR) method (15–17). At baseline, individual calibration was completed through a graded cycle ergometer test using indirect calorimetry (P.K. Morgan oxygen analyzer). At follow-up, a submaximal graded walking treadmill test was used. Oxygen consumption and carbon dioxide production were measured by indirect calorimetry (Vista XT metabolic system; Vacumed, Ventura, CA).
Heart rate was monitored on a minute-by-minute basis during the waking hours over 4 days of free living (Polar Electro, Kemple, Finland). In the analysis of the free-living heart rate data, sedentary time was assessed as all heart rate observations (in minutes) below the FHR and expressed as a percentage of total monitored time. MVPA was defined as the percentage of all heart rate points above 1.75 × resting heart rate (RHR), reported previously (9). Aerobic fitness (Vo2max) was estimated as the oxygen uptake at the age-predicted maximal heart rate of the extrapolated regression line for each individual's oxygen consumption and heart rate relationship. Vo2max was expressed per kilogram of fat-free mass.
Statistical methods.
Fasting insulin and HOMA-IR were logarithmically transformed (ln) in order to normalize skewed distributions. Because these variables were highly correlated (Pearson's r = 0.99) at both baseline and follow-up and the results for HOMA-IR and fasting insulin were almost identical, results are only reported for fasting insulin.
Univariate and multivariate linear regression analysis was used to examine the associations between men and women, between baseline and follow-up, and between the percentage of time spent sedentary at baseline and fasting insulin at follow-up. Confounders included in the multivariate models were baseline fasting insulin, age, sex, fat mass, smoking status, and duration of follow-up. To examine whether the association was independent of baseline MVPA, this variable was added to the model additionally. Collinearity was controlled for by means of the variance inflation factor. Statistical analyses were conducted using Stata/SE version 10.0 (StataCorp, College Station, TX).
RESULTS
Table 1 shows the descriptive characteristics of the participants. Sedentary time was inversely correlated with MVPA (r = −0.34; P < 0.001). Table 2 shows the associations between sedentary time and fasting insulin. No significant interaction effect (sex by sedentary time) on follow-up fasting insulin was found (P = 0.80). Therefore, results are presented for men and women combined, adjusting for sex. Percentage of time spent sedentary at baseline was significantly and positively associated with fasting insulin at follow-up. The unadjusted association between time spent in MVPA with fasting insulin approached statistical significance. In model 1 (adjusting for confounders except MVPA), the association between sedentary time and fasting insulin at follow-up was attenuated but remained statistically significant. When this model was reanalyzed with substitution of fat mass for body fat percentage or waist circumference, the results were essentially unchanged (data not shown). Model 2 (additionally adjusting for time spent in MVPA) showed virtually no change in the magnitude or direction of the association for sedentary time. To examine whether the effect of sedentary time was also independent of change in fat mass over the follow-up time, we added change in fat mass to model 2. The association pertained in magnitude and direction after further adjustment for change in fat mass (β = 0.004, 95% CI 0.001–0.006, P = 0.005).
TABLE 1.
Descriptive characteristics of participants (n = 376) at baseline and follow-up: the Medical Research Council Ely Study, 1994–2003
| Total (n = 376) |
Men (n = 166) |
Women (n = 210) |
||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Age (years) | 49.4 ± 7.7 | 55.0 ± 7.9* | 49.6 ± 8.1 | 55.2 ± 8.3* | 49.2 ± 7.4 | 54.8 ± 7.5* |
| Height (cm) | 168.7 ± 8.7 | 168.5 ± 8.6† | 175.7 ± 6.3 | 175.4 ± 6.2† | 163.2 ± 6.0‡ | 163.1 ± 6.0‡ |
| Weight (kg) | 75.0 ± 13.7 | 76.3 ± 15.4* | 82.8 ± 10.6 | 83.7 ± 12.0† | 68.8 ± 12.7‡ | 70.3 ± 15.2*‡ |
| BMI (kg/m2) | 26.3 ± 3.9 | 26.7 ± 4.6* | 26.8 ± 2.9 | 27.2 ± 3.4* | 25.8 ± 4.5 | 26.4 ± 5.3* |
| Fat mass (kg) | 22.2 ± 7.8 | 24.4 ± 9.2* | 19.1 ± 5.4 | 21.3 ± 6.4* | 24.7 ± 8.5‡ | 26.9 ± 10.4*‡ |
| FFM (kg) | 52.8 ± 11.5 | 51.8 ± 11.5* | 63.7 ± 6.8 | 62.4 ± 7.4* | 44.1 ± 5.5‡ | 43.5 ± 6.0*‡ |
| Waist circumference (cm) | 85.9 ± 12.0 | 90.5 ± 13.0* | 94.3 ± 8.5 | 98.2 ± 9.7* | 79.3 ± 10.2‡ | 84.5 ± 12.1*‡ |
| Current smokers (%) | 16.8 | — | 15.1 | — | 18.1 | — |
| Vo2max (ml · kg FFM−1 · min−1) | 44.9 ± 11.6 | 56.5 ± 13.4* | 46.3 ± 10.9 | 58.0 ± 12.2* | 43.8 ± 12.0 | 55.4 ± 14.2* |
| Flex–heart rate | 76.1 ± 10.1 | 81.5 ± 11.1* | 73.1 ± 9.5 | 77.7 ± 9.8* | 78.4 ± 9.9‡ | 84.6 ± 11.0*‡ |
| Time spent sedentary (% of total time) | 32.9 ± 20.6 | 51.7 ± 22.8* | 30.7 ± 19.9 | 47.2 ± 21.5* | 34.7 ± 20.9 | 55.2 ± 23.2*‡ |
| MVPA (% of total time) | 1.9 ± 3.4 | 1.9 ± 2.2 | 2.2 ± 3.1 | 2.2 ± 2.5 | 1.7 ± 3.6 | 1.7 ± 2.8 |
| Fasting plasma insulin (pmol/l) | 38.1 (35.9–40.4) | 45.6 (42.6–48.7)* | 41.7 (38.2–45.6) | 52.6 (48.1–57.5)* | 35.4 (32.8–38.3) | 40.7 (37.0–44.7)*‡ |
| Fasting plasma glucose (mmol/l) | 4.9 (4.8–4.9) | 5.4 (5.3–5.4)* | 5.0 (4.9–5.1) | 5.6 (5.5–5.7)* | 4.7 (4.7–4.8)‡ | 5.2 (5.1–5.3)*‡ |
| HOMA-IR | 1.37 (1.28–1.46) | 1.80 (1.67–1.94)* | 1.54 (1.40–1.70) | 2.19 (1.98–2.41)* | 1.24 (1.15–1.35)‡ | 1.54 (1.39–1.71)*‡ |
Data are means ± SD or geometric means (95% CI).
*P < 0.001,
†P < 0.05 for baseline vs. follow-up,
P < 0.001 for men vs. women. FFM, fat-free mass.
TABLE 2.
Regression coefficients (95% CI) for the association between objectively measured sedentary time and MVPA time at baseline with fasting insulin (log pmol/l) at follow-up in healthy, middle-aged, Caucasian subjects (n = 376)
| β (95% CI) | P | R2 | |
|---|---|---|---|
| Unadjusted | |||
| Sedentary (%) | 0.005 (0.001 to 0.008) | 0.005 | 0.021 |
| MVPA (%) | −0.018 (−0.04 to 0.002) | 0.080 | 0.008 |
| Model 1 | |||
| Sedentary (%) | 0.003 (0.0006 to 0.006) | 0.015 | 0.439 |
| MVPA (%) | 0.002 (−0.01 to 0.02) | 0.818 | 0.430 |
| Model 2 | |||
| Sedentary (%)* | 0.004 (0.0009 to 0.006) | 0.009 | 0.441 |
| MVPA (%)† | 0.009 (−0.007 to 0.02) | 0.290 | 0.441 |
Models 1 and 2 adjusted for baseline age, sex, fat mass, fasting insulin, smoking status, and duration of follow-up.
*Additionally adjusted for MVPA.
†Additionally adjusted for sedentary time.
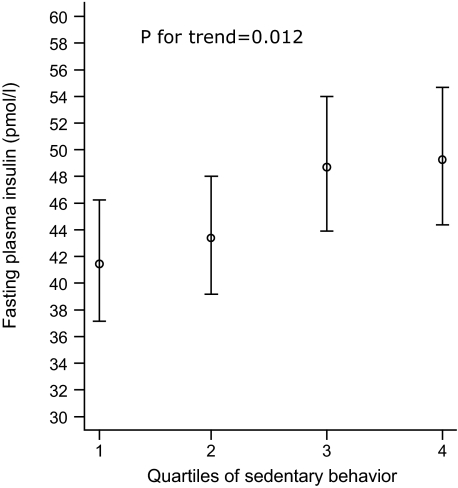
Baseline aerobic fitness was not associated with follow-up fasting insulin (P = 0.32) after adjusting for baseline age, sex, fat mass, fasting insulin, smoking status, and follow-up time. Adjusted geometric means of fasting insulin, stratified by quartiles of time spent sedentary, are presented in Fig. 1. This model accounted for 44% of the variance in fasting insulin. Sedentary time and time spent in MVPA accounted for 2.1 and 0.8% of the variance, respectively. Excluding individuals with impaired glucose tolerance (n = 22) at baseline did not materially change the associations (data not shown).
FIG. 1.
Geometric means ± SE of fasting insulin (follow-up) stratified by quartiles of time spent sedentary (baseline) in healthy middle-aged individuals (n = 376). Data are adjusted for baseline age, sex, fat mass, fasting insulin, smoking status, follow-up time, and time spent at MVPA.
DISCUSSION
The results from the present study showed that physiologically measured sedentary time using individual calibration was associated with hyperinsulinemia measured 5.6 years later in healthy middle-aged Caucasian subjects. This association was independent of baseline confounders including age, sex, fat mass, fasting insulin, smoking status, follow-up time, and MVPA. Similar results were found when we modeled sedentary time as quartiles. Moreover, reliability estimates from a repeated-measures substudy of this cohort suggest that the true association between behavior and health is likely to be twice as strong as those observed (18).
Our observations are consistent with previous cross-sectional findings using objective measurements of sedentary time suggesting that this behavior is associated with 2-h blood glucose (19) and metabolic syndrome features (11) in adults. Our results extend these previous cross-sectional observations by suggesting that sedentary time predicts higher levels of fasting insulin independent of time spent in MVPA and other confounding factors. This observation is, however, in contrast to recent prospective observations in high-risk individuals, where accelerometry-measured MVPA and not sedentary time predicted insulin resistance at follow-up (14). Differences between studies are likely explained by differences in methodology for assessing sedentary time, duration of follow-up, and population (i.e., age, diabetes risk, and obesity status). Future studies are needed to determine whether objectively measured subcomponents of physical activity differentially predict insulin resistance and other metabolic outcomes caused by baseline age, obesity status, and diabetes risk.
The following study strengths and limitations should be considered. First, because the present study is observational, the extent to which causality can be inferred is less than that for a randomized controlled trial. However, our prospective study design, the relatively precise measure of our main exposure variable, and the possibility to control for many confounding factors support a causal independent positive association between sedentary time and the development of insulin resistance. Second, the use of individually calibrated heart rate monitoring for measurement of sedentary time is likely to be more accurate compared with self-report methods. Although this method is somewhat susceptible to heart rate fluctuations due to external factors (environmental and emotional influences), the individually defined FHR will greatly reduce misclassification of time spent sedentary. The thresholds used for defining sedentary time and MVPA are somewhat arbitrary. However, these intensity thresholds are feasible when using heart rate monitoring in large population-based studies (9,20). Our estimate of time spent sedentary and at MVPA, based on the FHR and RHR, is adjusted relative to the fitness level of each individual because RHR is lower in more fit individuals. This means that our results may not be directly comparable with results obtained with other objective methods such as accelerometry. The confounding effect of aerobic fitness on our results is likely to be minimal. Indeed, additional adjustments for aerobic fitness did not change results for any of the models (data not shown). Third, the associations observed in this group of healthy middle-aged Caucasian subjects may not be generalizable to other populations that differ in age and physical activity. Volunteers more than 65 years of age at follow-up were not included due to safety precautions for the exercise test. However, our results were unchanged after excluding those with impaired fasting glucose, suggesting that this condition did not explain our findings. Fourth, other potential confounders not accounted for in the analyses, such as genotype, dietary habits, birth weight, early growth patterns, and pharmaceutical factors, may explain some of the observed associations. Also, although we adjusted for MVPA, we could not adjust for total PAEE because of collinearity. Finally, fasting insulin is not a direct measure of insulin resistance, but it has been shown consistently that fasting insulin is a robust surrogate for insulin resistance and is associated with the metabolic syndrome, diabetes, and their etiology (21).
Using data from this cohort, we recently showed that sedentary time at baseline was not significantly associated with a wide range of obesity indicators at follow-up; interestingly, greater fat mass, waist circumference, and BMI at baseline significantly predicted sedentary time at follow-up (22). This suggests that sedentary time may be differentially associated with various health outcomes and also urges the need for more prospective studies using objective methods for assessing the dose-response relationships between various subdimensions of physical activity with obesity, insulin resistance, and other chronic disease risk factors.
The results from this study may have important clinical implications. In a meta-analysis, Ruige et al. (23) reported an increased risk of 18% (8–29%) for cardiovascular diseases per increment of 50 pmol/l in fasting insulin. Further, a recent meta-analysis demonstrated a linear association between fasting insulin levels and cardiovascular mortality independent of other risk factors (2).
Pathways through which inactivity can lead to insulin resistance and its comorbidities include vascular changes. Vascular and endothelial dysfunction may contribute to reduced blood flow, decreased peripheral insulin-stimulated glucose uptake, and reduced glucose-stimulated insulin secretion (24). A sedentary lifestyle also has a direct effect on inactivity-induced factors including deep venous thrombosis and poor lipid metabolism (25).
In summary, this is the first study suggesting that increased time spent sedentary is prospectively associated with elevated fasting insulin levels regardless of the amount of time spent in MVPA in healthy middle-aged adults. From a public health perspective, these findings urge the need for recommendations aimed at reducing sedentary time in addition to those aimed at increasing MVPA.
Acknowledgments
The Medical Research Council (MRC) U.K. provided funding for the MRC Ely study.
No potential conflicts of interest relevant to this article were reported.
We are grateful to the volunteers, who gave their time; to Susie Hennings, Sue Emms, and Ema Lucia De Rolfe, who coordinated the study and collected data; to Kate Westgate, who assisted with the analysis of physical activity data; and to Stephen Sharp, who provided statistical advice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R, Diet M, Nilsson P, Hedblad B: Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab 2002; 28: 364– 376 [PubMed] [Google Scholar]
- 2.Hu G, Qiao Q, Tuomilehto J, Eliasson M, Feskens EJ, Pyorala K: Plasma insulin and cardiovascular mortality in non-diabetic European men and women: a meta-analysis of data from eleven prospective studies. Diabetologia 2004; 47: 1245– 1256 [DOI] [PubMed] [Google Scholar]
- 3.Li C, Ford ES, McGuire LC, Mokdad AH, Little RR, Reaven GM: Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care 2006; 29: 2396– 2402 [DOI] [PubMed] [Google Scholar]
- 4.Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Lanza E, Schatzkin A: Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology 2007; 133: 1423– 1429 [DOI] [PubMed] [Google Scholar]
- 5.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Bostrom KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jorgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjogren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D: Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008; 40: 638– 645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzali G, Di Francesco V, Zoico E, Fantin F, Zamboni G, Benati C, Bambara V, Negri M, Bosello O, Zamboni M: Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr 2006; 84: 1193– 1199 [DOI] [PubMed] [Google Scholar]
- 7.Festa A, D'Agostino R, Jr, Tracy RP, Haffner SM: Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes 2002; 51: 1131– 1137 [DOI] [PubMed] [Google Scholar]
- 8.Mayer-Davis EJ, D'Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN: Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA 1998; 279: 669– 674 [DOI] [PubMed] [Google Scholar]
- 9.Assah FK, Brage S, Ekelund U, Wareham NJ: The association of intensity and overall level of physical activity energy expenditure with a marker of insulin resistance. Diabetologia 2008; 51: 1399– 1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, Cameron AJ, Dwyer T, Jolley D, Shaw JE: Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia 2005; 48: 2254– 2261 [DOI] [PubMed] [Google Scholar]
- 11.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N: Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008; 31: 369– 371 [DOI] [PubMed] [Google Scholar]
- 12.Wijndaele K, Duvigneaud N, Matton L, Duquet W, Delecluse C, Thomis M, Beunen G, Lefevre J, Philippaerts RM: Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults. Eur J Clin Nutr 2009; 63: 421– 429 [DOI] [PubMed] [Google Scholar]
- 13.Owen N, Leslie E, Salmon J, Fotheringham MJ: Environmental determinants of physical activity and sedentary behavior. Exerc Sport Sci Rev 2000; 28: 153– 158 [PubMed] [Google Scholar]
- 14.Ekelund U, Brage S, Griffin S, Wareham N: Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predict insulin resistance in high-risk individuals. Diabetes Care 2009; 32: 1081– 1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ: Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care 2007; 30: 2101– 2106 [DOI] [PubMed] [Google Scholar]
- 16.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ: Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 2005; 28: 1195– 1200 [DOI] [PubMed] [Google Scholar]
- 17.Livingstone MB, Prentice AM, Coward WA, Ceesay SM, Strain JJ, McKenna PG, Nevin GB, Barker ME, Hickey RJ: Simultaneous measurement of free-living energy expenditure by the doubly labeled water method and heart-rate monitoring. Am J Clin Nutr 1990; 52: 59– 65 [DOI] [PubMed] [Google Scholar]
- 18.Wareham NJ, Wong MY, Day N: Glucose intolerance and physical inactivity: the relative importance of low habitual energy expenditure and cardiorespiratory fitness. Am J Epidemiol 2000; 152: 132– 139 [DOI] [PubMed] [Google Scholar]
- 19.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N: Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007; 30: 1384– 1389 [DOI] [PubMed] [Google Scholar]
- 20.Wareham NJ, Hennings SJ, Prentice AM, Day NE: Feasibility of heart-rate monitoring to estimate total level and pattern of energy expenditure in a population-based epidemiological study: the Ely Young Cohort Feasibility Study 1994–5. Br J Nutr 1997; 78: 889– 900 [DOI] [PubMed] [Google Scholar]
- 21.Vaccaro O, Masulli M, Cuomo V, Rivellese AA, Uusitupa M, Vessby B, Hermansen K, Tapsell L, Riccardi G: Comparative evaluation of simple indices of insulin resistance. Metabolism 2004; 53: 1522– 1526 [DOI] [PubMed] [Google Scholar]
- 22.Ekelund U, Brage S, Besson H, Sharp S, Wareham NJ: Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality? Am J Clin Nutr 2008; 88: 612– 617 [DOI] [PubMed] [Google Scholar]
- 23.Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM: Insulin and risk of cardiovascular disease: a meta-analysis. Circulation 1998; 97: 996– 1001 [DOI] [PubMed] [Google Scholar]
- 24.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE: Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 2004; 96: 101– 106 [DOI] [PubMed] [Google Scholar]
- 25.Hamilton MT, Hamilton DG, Zderic TW: Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007; 56: 2655– 2667 [DOI] [PubMed] [Google Scholar]