Abstract
OBJECTIVE
Ca2+-regulated K+ channels are involved in numerous Ca2+-dependent signaling pathways. In this study, we investigated whether the Ca2+-activated K+ channel of intermediate conductance SK4 (KCa3.1, IK1) plays a physiological role in pancreatic β-cell function.
RESEARCH DESIGN AND METHODS
Glucose tolerance and insulin sensitivity were determined in wild-type (WT) or SK4 knockout (SK4-KO) mice. Electrophysiological experiments were performed with the patch-clamp technique. The cytosolic Ca2+ concentration ([Ca2+]c) was determined by fura-2 fluorescence. Insulin release was assessed by radioimmunoassay, and SK4 protein was detected by Western blot analysis.
RESULTS
SK4-KO mice showed improved glucose tolerance, whereas insulin sensitivity was not altered. The animals were not hypoglycemic. Isolated SK4-KO β-cells stimulated with 15 mmol/l glucose had an increased Ca2+ action potential frequency, and single-action potentials were broadened. These alterations were coupled to increased [Ca2+]c. In addition, glucose responsiveness of membrane potential, [Ca2+]c, and insulin secretion were shifted to lower glucose concentrations. SK4 protein was expressed in WT islets. An increase in K+ currents and concomitant membrane hyperpolarization could be evoked in WT β-cells by the SK4 channel opener DCEBIO (100 μmol/l). Accordingly, the SK4 channel blocker TRAM-34 (1 μmol/l) partly inhibited KCa currents and induced electrical activity at a threshold glucose concentration. In stimulated WT β-cells, TRAM-34 further increased [Ca2+]c and broadened action potentials similar to those seen in SK4-KO β-cells. SK4 channels were found to substantially contribute to Kslow (slowly activating K+ current).
CONCLUSIONS
SK4 channels are involved in β-cell stimulus-secretion coupling. Deficiency of SK4 current induces elevated β-cell responsiveness and coincides with improved glucose tolerance in vivo. Therefore, pharmacologic modulation of these channels might provide an interesting approach for the development of novel insulinotropic drugs.
SK4 channels are Ca2+-activated K+ channels of intermediate conductance (synonymous with IK1 and KCa3.1) encoded by the KCNN4 gene. They are primarily expressed in cells of the hematopoietic system, where they represent the Gardos channel (1). Channel activation requires Ca2+ increase and determines the cell volume of T-cells and erythrocytes by elevating K+ efflux. In organs regulating salt and fluid transport (e.g., colon, salivary glands, and lung), SK4 current provides the driving force for secondary electrogenic ion transport (2–4). SK4 channels are suggested to be involved in mast cell stimulation (5), and channel upregulation is important for lymphocyte activation and cell proliferation (6,7). For enteric neurons, SK4 channels seem to mediate the late after-hyperpolarization (8). In 1997, SK4 channels were cloned from human pancreatic tissue (9). A detailed investigation of mRNA and protein expression of KCa channels of intermediate (SK4) and small conductance (SK1–3) was performed by Tamarina et al. (10) showing mRNA expression of these channels in murine islets.
In the past, ATP-sensitive K+ (KATP) channels were considered to be essential for glucose homeostasis. Consequently, KATP channel inhibitors are important drugs to augment insulin secretion in type 2 diabetic subjects. However, with the generation of two KATP channel-deficient mouse models (SUR1 and Kir6.2 knockout), it was shown that KATP channels are not indispensable for glycemic control (11–14). Neither SUR1 nor Kir6.2 knockout mice show severe hypoglycemia or any symptoms of insulin hypersecretion. Several reports provide evidence that efficient blood glucose regulation and even glucose-dependent insulin secretion (15–17) is possible despite KATP channel ablation. In the search for compensatory mechanisms, modulation of insulin release by other K+ channels gains particular interest.
Besides KCa channels, pancreatic β-cells express K+ channels exclusively regulated by voltage (Kv channels) (10,18,19). Several studies indicate that Kv channel activation plays a role in action potential (AP) repolarization (20–22). Blocking these channels broadens APs and increases insulin secretion (23–25). Recently, it was shown that Kv2.1 ablation drastically reduces Kv currents of isolated β-cells (26). Interestingly, this coincides with improved glucose tolerance pointing to a specific role for Kv2.1 in the regulation of insulin secretion.
For decades, it was discussed whether KCa channels participate in the regulation of β-cell activity (27). An early report (28) described KCa currents that were periodically activated by inositol-trisphosphate–dependent Ca2+ mobilization. The existence of large conductance KCa channels (BK channels) in pancreatic β-cells and insulin-secreting cell lines has been verified by several groups (29–31). However, since blockage of BK channels does not alter membrane potential oscillations (31,32), these channels are not considered to play a major role in glucose-stimulated insulin release. In 1999, a K+ current activating with increasing Ca2+ influx during burst phases of glucose-stimulated β-cells was detected (33). The current, termed Kslow because of its delayed and slow onset, strongly depends on [Ca2+]c. Further analysis suggested that ∼50% could be ascribed to KATP current (34). However, the remaining sulfonylurea-insensitive component of Kslow does not resemble the characteristics of any known KCa channel (33), and its precise nature remains to be identified. It has been suggested that KCa channels of small conductance (SK1–3) play a functional role in β-cells (10,35), but at present, there is only limited information about their contribution to glucose handling of the whole organism.
Because up to now nothing is known about the significance of SK4 channels in pancreatic β-cells, this study was performed to elucidate whether SK4 channels are suitable candidates for modulation of β-cell function. We demonstrate that SK4 channels are expressed in murine islets and investigated the influence of constitutive SK4 channel knockout (SK4-KO) and of pharmacological SK4 channel inhibition on glucose homeostasis, insulin sensitivity, and the stimulus-secretion cascade of murine pancreatic β-cells.
RESEARCH DESIGN AND METHODS
Animals and cell and islet preparation.
Experiments were performed with SK4-KO and wild-type (Sv129/C57Bl6 or C57Bl/6) mice. The principles of laboratory animal care were followed (NIH publication number 85-23, revised 1985), and experiments were carried out according to German laws (Regierungspräsidium Stuttgart, Germany, approval number PZ 1/08). SK4-KO mice were generated as previously described (5). In brief, the targeting vector was constructed by flanking the pore exon by a single loxP site and a floxed neo/tk cassette. Correctly targeted L1/+ clones were injected into C57Bl6 blastocysts. Resulting chimeras were mated with Sv129 mice to obtain germ-line transmission. Heterozygous offspring were intercrossed with C57Bl6 mice, yielding a Sv129/C57Bl6 hybrid background. For in vitro experiments, mice were killed with CO2, and islets were isolated by collagenase digestion. Islets were dispersed in Ca2+-free medium and cultured for up to 4 days in RPMI-1640 medium (11.1 mmol/l glucose) supplemented with 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Solutions and chemicals.
The bath solution for [Ca2+]c and membrane potential (Vm) was as follows (in mmol/l): 140 NaCl, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, 15 glucose, and 10 HEPES, pH 7.4. The pipette solution for Vm recordings (in mmol/l) was as follows: 10 KCl, 10 NaCl, 70 K2SO4, 4 MgCl2, 2 CaCl2, 10 EGTA, 5 HEPES, pH 7.15, and amphotericin B (250 μg/ml). The pipette solution for inside-out recordings was as follows (in mmol/l): 130 KCl, 1.2 MgCl2, 2 CaCl2, 10 EGTA, and 20 HEPES, pH 7.4. Bath solution included the following (in mmol/l): 130 KCl, 10 EDTA, and 20 HEPES, pH 7.2; free Ca2+ was adjusted to 10 μmol/l by CaCl2. Incubation medium for insulin secretion was as follows (in mmol/l): 122 NaCl, 4.8 KCl, 2.5 CaCl2, 1.1 MgCl2, 10 HEPES, and 0.5% BSA, pH 7.4. Lysis buffer for Western blot included the following (in mmol/l): 125 NaCl, 1% Triton X-100, 0.5% Na-deoxycholate, 0.1% SDS, 10 EDTA, 25 HEPES, 10 NaPP, 10 NaF, 1 Na-vanadate, and protease inhibitor cocktail (Roche), pH 7.3.
Fura-2AM was obtained from Molecular Probes (Eugene, OR). RPMI-1640 medium was from PromoCell (Heidelberg, Germany) and penicillin/streptomycin from GIBCO/BRL (Karlsruhe, Germany). All other chemicals were purchased from Sigma (Deisenhofen, Germany) and Merck (Darmstadt, Germany).
Glucose tolerance and insulin sensitivity.
In vivo experiments were performed with male SK4-KO mice and WT littermates aged 12, 24, and 36 weeks. Glucose (2 g/kg body wt) or insulin (0.7 IU/kg body wt) was injected intraperitoneally. Changes in plasma glucose concentration were monitored for 120 or 60 min, respectively. Mice were fasted for 16 h before glucose tolerance testing.
Measurement of [Ca2+]c.
[Ca2+]c was measured in single cells or small clusters by the fura-2 method (36) using equipment and software from TILL photonics (Gräfelfing, Germany). Cells were identified as β-cells when [Ca2+]c was not decreased by 15 mmol/l glucose as described for α-cells (37). Cells were loaded with fura-2AM (5 μmol/l) for 30 min at 37°C. Fura-2 was excited alternately at 340 or 380 nm. The emitted light was filtered (LP515 nm) and measured by a digital camera. [Ca2+]c was calculated after an in vitro calibration with fura-2 K+ salt (36).
Electrophysiology.
Patch pipettes were pulled from borosilicate glass capillaries (Clark, Pangbourne, U.K.). Vm was recorded at 32°C with an EPC-9 patch-clamp amplifier (HEKA, Lambrecht, Germany). K+ currents were elicited by 10 mV voltage steps (300 ms) from a holding potential of −70 mV. Kslow currents were determined according to the methodology of Göpel et al. (33): after a 30-mV depolarizing step, a train of 26 voltage ramps (−40 to 0 to −40 mV within 200 ms) was applied and followed by at least 10 s at −40 mV before the voltage step back to −70 mV. Data were analyzed with “Chart” software (ADInstruments, Spechbach, Germany). Inside-out recordings were performed at a holding potential of −50 mV.
Insulin secretion.
Batches of five islets were incubated for 60 min at 37°C. Insulin was determined by radioimmunoassay using rat insulin (Linco Research, St. Charles, MO) as the standard.
Western blot analysis.
For determination of SK4 channel protein, ∼300 islets per genotype were collected, rinsed with PBS, and homogenized in lysis buffer (see above). Protein amount was determined by a Bradford assay. The homogenates (100 μg per lane) were separated on a 12.5% SDS-PAGE. Peptides were blotted on a polyvinylidene difluoride membrane. The primary antibody was directed against the COOH-terminus of SK4 (1:200; Santa Cruz Technology, Santa Cruz, CA). PKB was used as loading control (1:1,000; Cell Signaling, Beverly, MA).
Presentation of results.
[Ca2+]c and electrophysiological experiments are illustrated by representative recordings. At least three different cell preparations were used for each series. Means ± SE are given in the text for the indicated number of experiments. Western blots were performed in duplicate. Statistical significance of differences was assessed by a one-sample or Student's t test for paired values; multiple comparisons were made by ANOVA followed by a Student-Newman-Keuls test. For AP characteristics, five APs of each experiment were averaged. Peak values were set to t = 0 ms, and data were analyzed every 50 ms within the preceding and following 200 ms (Fig. 1B and D). Dose-response curves of [Ca2+]c were fitted with the Hill equation. Curves were defined by the following parameters: P(D) = 1/[1 + (D50/D)c], where P(D) is the probability of glucose-induced stimulation, D50 is the dose level with 50% response probability, D is the glucose concentration (in mmol/l), and c reflects the slope of the concentration-response curve. The equation was adjusted by a maximum-likelihood procedure. A P value of <0.05 was considered significant.
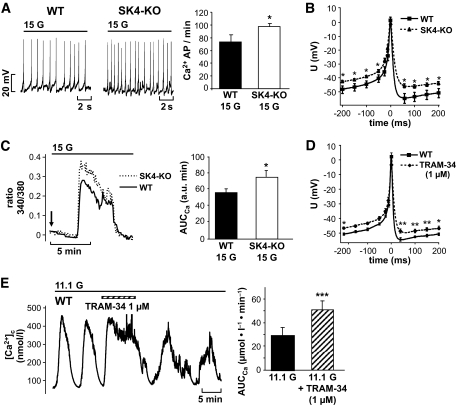
FIG. 1.
Genetic ablation or pharmacologic inhibition of SK4 channels influences electrical activity and [Ca2+]c of pancreatic β-cells. A: In the presence of 15 mmol/l glucose, action potential frequency was increased in SK4-KO β-cells compared with WT controls. Data are given as means ± SEM of 18 WT and 53 SK4-KO β-cells tested. B and D: Analysis of single Ca2+ action potentials in SK4-KO and WT β-cells. SK4 deficiency or blockage with the SK4 channel inhibitor TRAM-34 (1 μmol/l) resulted in action potential broadening and depolarized the plateau potential from which action potentials started. In the series with TRAM-34, the shape of action potentials before drug application was compared with action potentials 3–4 min after addition of TRAM-34. The traces were compiled by averaging action potentials of 11 experiments with SK4-KO and 12 experiments with WT β-cells. The series with TRAM-34 results from five independent experiments. C: SK4-KO β-cells stimulated with 15 mmol/l glucose display an augmented Ca2+ response compared with WT β-cells. The figure shows an overlay of two representative traces of the first increase in [Ca2+]c induced by switching glucose from 0.5 to 15 mmol/l (arrow). A total of 31 SK4-KO and 26 WT β-cells were analyzed. The values for AUCCa ± SEM of this series of experiments are summarized in the diagram. E: Blocking SK4 channels elevates [Ca2+]c in WT β-cells. β-Cells exposed to 11.1 mmol/l glucose show regular oscillations of [Ca2+]c. Addition of TRAM-34 (1 μmol/l) increased [Ca2+]c and altered the pattern of oscillations. The experiment is representative of five with similar results. The diagram summarizes the increase in the AUCCa analyzed for a time period of 4 min in the presence of TRAM-34 compared with control conditions. *P ≤ 0.05, **P < 0.001, ***P ≤ 0.001.
RESULTS
Role of SK4 channels in glucose-induced stimulus-secretion coupling.
To elucidate whether SK4 channels interact with β-cell function, we tested whether knockout of SK4 channels influences β-cell activity in response to glucose. SK4-KO was accompanied by several alterations in glucose responsiveness (Fig. 1). SK4-KO β-cells stimulated with 15 mmol/l glucose showed an increased frequency of Ca2+ APs (74 ± 11 AP/min in WT cells, n = 18, vs. 97 ± 5 AP/min in SK4-KO cells, n = 53, P ≤ 0.05; Fig. 1A) and the plateau potential at which APs started was more depolarized (WT: −50 ± 2 mV, n = 12, vs. SK4-KO: −43 ± 1 mV, n = 11, P ≤ 0.001). Further analysis demonstrated that single APs were broadened (Fig. 1B): the width at half-maximum amplitude averaged 23 ± 3 ms in WT (n = 12) and 37 ± 3 ms (n = 11) in SK4-KO β-cells (P ≤ 0.01). Membrane depolarization represents the link between glucose metabolism and Ca2+ influx. Consequently, the loss of SK4 channels should alter [Ca2+]c. The increased electrical activity of SK4-KO β-cells was reflected by an augmented [Ca2+]c response (Fig. 1C). In SK4-KO β-cells, the area under the curve (AUCCa) for the first rise of [Ca2+]c after elevating glucose from 0.5 to 15 mmol/l increased by ∼34% (AUCWT: 56 ± 4 arbitrary units [a.u.] min, n = 26, vs. AUCKO: 75 ± 8 a.u. min, n = 31, P ≤ 0.05, Fig. 1C). These data show that in SK4-KO β-cells, the elevated electrical activity is paralleled by Ca2+ influx.
Influence of pharmacological modulation of SK4 channels on β-cell function.
To test whether drug-induced alterations of SK4 channel activity influences β-cell stimulus-secretion coupling, the SK4 channel blocker TRAM-34 (38) was investigated for effects on electrical activity and [Ca2+]c. In stimulated β-cells, the SK4 channel inhibitor induced similar changes in the shape of Ca2+ APs, as observed in SK4-KO β-cells (compare Fig. 1B and D). TRAM-34 (1 μmol/l) elevated the width at half-maximum amplitude from 19 ± 4 to 29 ± 3 ms (n = 5, P ≤ 0.05), and the plateau potential was shifted from −51 ± 1 to −47 ± 1 mV (n = 5, P ≤ 0.05). Importantly, [Ca2+]c was also modulated by blocking SK4 channels (Fig. 1E). In this series of experiments, 1 μmol/l TRAM-34 was added to β-cells stimulated with 11.1 mmol/l glucose. Acute application of the SK4 channel blocker abrogated the oscillatory pattern of [Ca2+]c that characterizes glucose-stimulated β-cells (39) and clearly augmented [Ca2+]c. Quantification of the AUCCa for 4 min before changes in the bath solution showed that TRAM-34 increased the AUCCa 1.8-fold vs. control conditions (i.e., Δ of 22 ± 3 μmol/l min, n = 5, P ≤ 0.001). For specificity testing, SK4-KO β-cells were also treated with TRAM-34, yielding a slight change in the pattern of oscillations but no increase in [Ca2+]c. On average, the AUCCa in G11.1 was 21 ± 4 μmol/l min without and 25 ± 5 μmol/l min with 1 μmol/l TRAM-34, respectively (i.e., Δ of 5 ± 2 μmol/l min, n = 5, NS vs. control, not shown).
Next, the effect of DCEBIO, a potent SK4 channel activator (40), was investigated. In agreement with activation of a K+ current, DCEBIO (100 μmol/l) rapidly hyperpolarized Vm (Fig. 2A). This series of experiments was performed in the presence of high glucose (15 mmol/l), tolbutamide (1 mmol/l), and nifedipine (5 μmol/l) to exclude any influence of KATP and Ca2+ currents. [Ca2+]c was elevated by 1 μmol/l ionomycin. On average, Vm was altered from −37 ± 2 to −56 ± 4 mV after addition of DCEBIO (n = 7, P ≤ 0.001). The K+ current elicited by a 10-mV depolarizing voltage step (from −70 to −60 mV) amounted to 3.61 ± 0.51 pA in the presence of DCEBIO and was reduced to 1.60 ± 0.17 pA after washout (n = 5, P ≤ 0.01). DCEBIO is not entirely specific for SK4 channels and has been reported to interact with Ca2+ and Cl− channels (40–42). Therefore, we performed analogical experiments with SK4-KO β-cells (Fig. 2B). Vm was −38 ± 3 mV under control conditions and −33 ± 3 mV after addition of DCEBIO (n = 13), strongly suggesting that the hyperpolarization in WT β-cells was in fact due to SK4 channel activation. To directly show that SK4 channels are present in β-cells, we performed inside-out single-channel measurements (Fig. 2C). Besides BK and SK channels, we identified a KCa channel with a single channel conductance of 39 ± 1 pS (n = 5), fitting with the properties of SK4 channels in other tissues (43,44). The expression of SK4 protein was confirmed in isolated WT islets by Western blot analysis (Fig. 2D, left). Specificity of the antibody was confirmed by the absence of immunostaining in SK4-KO islets (Fig. 2D, right).
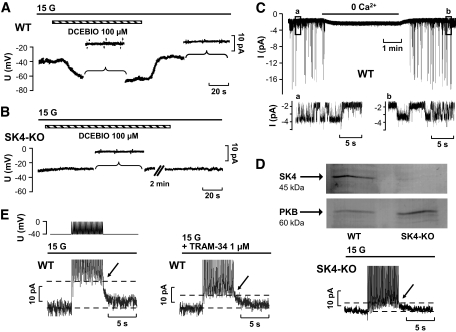
FIG. 2.
Activation of SK4 currents in WT β-cells. A and B: Membrane potential was determined in the perforated-patch configuration in WT (A) and SK4-KO (B) β-cells. Bath solution contained 15 mmol/l glucose (15 G). To eliminate any effect of KATP channels and to clamp the intracellular Ca2+ concentration, tolbutamide (1 mmol/l), nifedipine (5 μmol/l), and ionomycin (1 μmol/l) were present in the perifusion solution throughout the experiment. DCEBIO (100 μmol/l) was added, as indicated by the horizontal bars. To measure the currents elicited by 10 mV de- and hyperpolarizing voltage steps, Vm recordings were interrupted at the time periods marked by the brackets. The recordings are representative of 5 experiments with WT and 13 experiments with SK4-KO cells yielding similar results. C: Single-channel currents were determined in the inside-out configuration in WT β-cells (130 mmol/l K+ in bath and pipette solution, −50 mV). For the time indicated by the horizontal bar, patches were perifused with Ca2+-free bath solution. The insets (a, b) show at an extended scaling the openings of Ca2+-activated K+ channels of ∼40 pS, which is attributed to SK4. The experiment is representative of five with similar results. D: Western blots of protein extracts from freshly isolated islets of WT and SK4-KO mice, respectively (100 μg protein per lane), with an SK4-specific antibody (1:200, Santa Cruz). Immunoblot of PKB protein was used as the loading control. E: Activation of Ca2+-dependent ion currents in isolated WT (left and middle trace) and SK4-KO β-cells (right trace). The pulse protocol used for these experiments is illustrated above the current traces. Glucose (15 mmol/l) was present throughout the experiments. The simulated burst of action potentials induced an outward current that was partly suppressed by 1 μmol/l TRAM-34 (the peak of the Kslow current is marked by the arrows) within 5–10 min after application of the drug. In SK4-KO β-cells, the Kslow currents elicited by the pulse protocol were markedly decreased compared with WT β-cells. The experiments are representative of seven (WT) and five (SK4-KO) with similar results.
These data demonstrate that SK4 channels are operative in β-cells and that pharmacological modulation influences glucose-induced stimulus-secretion cascade.
Contribution of SK4 channels to Kslow currents.
To test whether SK4 channels contribute to the Ca2+-regulated component of Kslow, β-cells were stimulated with 15 mmol/l glucose, and a pulse protocol similar to that described by Göpel et al. (33,34) was used to imitate a burst of Ca2+ APs (Fig. 2E, upper trace). The increase in current amplitude induced by a train of 26 voltage ramps was quantified in the absence and presence of TRAM-34 (Fig. 2E, lower trace, left and middle). The current elicited by this protocol was significantly reduced by TRAM-34 (compare arrows and areas marked by the dashed lines). Kslow was 13.3 ± 2.1 pA under control conditions and 8.2 ± 1.2 pA with 1 μmol/l TRAM-34 (n = 7, P ≤ 0.01). After washout, the current increased to 13.5 ± 2.3 pA (n = 7, NS vs. control). As SK4 channels have been reported to be sensitive to charybdotoxin, a scorpion toxine widely used to block BK channels (45), we tested whether Kslow was affected by this drug. Up to 100 nmol/l charybdotoxin had no inhibitory effect on Kslow (n = 4, not shown). To further elucidate the involvement of SK4 channels in generation of Kslow, the above-mentioned protocol was applied to SK4-KO β-cells (Fig. 2E, right). SK4 ablation significantly reduced Kslow. In this series of experiments, the current averaged 10.3 ± 1.8 pA in WT (n = 11) and 3.6 ± 0.8 pA in SK4-KO β-cells (n = 5, P ≤ 0.05). Tolbutamide (1 mmol/l) did not completely abolish but further reduced Kslow (2.1 ± 0.3 pA, n = 5, NS compared with control conditions without sulfonylurea) in SK4-KO β-cells. These data clearly show that a significant component of Kslow is carried by SK4 channels.
Lack of SK4 channels leads to a left shift in glucose responsiveness.
Neither SK4-KO nor TRAM-34 influenced the resting membrane potential, which was −77 ± 1 mV in 0.5 mmol/l glucose and −76 ± 1 mV with TRAM-34 (1 μmol/l, n = 3) compared with −75 ± 1 mV in SK4-KO β-cells (n = 7, not shown). To find out whether ablation of SK4 channels affects glucose responsiveness, we investigated whether stimulation of SK4-KO β-cells was shifted to lower glucose concentrations. Cells were perifused with bath solution containing 6 or 8 mmol/l glucose. In WT β-cells, no electrical activity was observed with 6 mmol/l glucose (n = 7), whereas 37.5% of the cells were depolarized and Ca2+ APs occurred with 8 mmol/l glucose (n = 8). By contrast, in SK4-KO mice, 63.6% of the β-cells were already stimulated by 6 mmol/l glucose (n = 11) and all cells (100%) by 8 mmol/l glucose (n = 5) (Fig. 3A). Consistent with the higher fraction of electrically active β-cells, we observed a significant left shift of the glucose concentration–response curve of [Ca2+]c in SK4-KO versus WT β-cells (Fig. 3B). In these experiments, isolated β-cells were perifused with bath solutions containing 0.5–15 mmol/l glucose. Cells were considered to be glucose responsive if they displayed an increase in [Ca2+]c and/or Ca2+ oscillations. The D50 value (50% probability for glucose responsiveness) was 6.37 mmol/l (95% CI 6.09–6.68) for WT β-cells and was reduced to 5.67 mmol/l (5.29–6.05) for SK4-KO β-cells. SK4-KO also affected insulin secretion. Islets were incubated in 3, 6, or 8 mmol/l glucose for 60 min. WT and SK4-KO islets had similar insulin content (WT: 29 ± 3 ng/islet; SK4-KO: 29 ± 1 ng/islet, n = 8 different preparations for both genotypes), and there was no significant change in insulin release under basal conditions (3 mmol/l glucose) (WT: 33 ± 7 pg/[islet h], SK4-KO: 33 ± 10 pg/[islet h], n = 8 for both genotypes). Compared with basal secretion in 3 mmol/l, glucose stimulation of secretion occurred in all experiments when glucose was elevated to 8 mmol/l irrespective of the genotype (n = 8). However, in agreement with a left shift in glucose responsiveness of Vm and [Ca2+]c, only 38% of the WT but 75% of the SK4-KO islet preparations displayed an increase in secretion with 6 mmol/l glucose (Fig. 3C). These data clearly demonstrate that genetic ablation of SK4 channels sensitizes the β-cells to glucose stimulation.
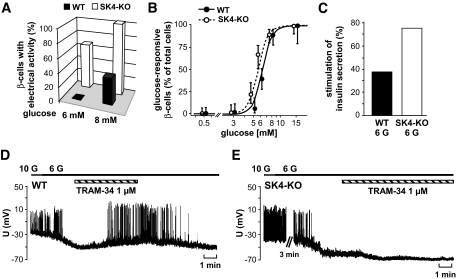
FIG. 3.
Glucose responsiveness of SK4-KO β-cells is shifted to lower glucose concentrations. A: β-Cells were stimulated with either 6 or 8 mmol/l glucose, respectively. The diagram illustrates the fraction of cells showing Ca2+ action potentials in response to the indicated glucose concentration. In this series of experiments, 12 WT and 15 SK4-KO β-cells were tested. B: The concentration-response curve was determined by perifusing isolated β-cells with different glucose concentrations. Cells in which [Ca2+]c increased or displayed oscillations within 15 min of perifusion were regarded as glucose responsive. The number of cells tested with each glucose concentration was as follows (WT/SK4-KO): 0.5 mmol/l glucose, 49 WT/55 SK4-KO cells; 3 mmol/l glucose, 46/51; 5 mmol/l glucose, 49/54; 6 mmol/l glucose, 74/73; 8 mmol/l glucose, 56/68; 15 mmol/l glucose, 16/36. The cells were obtained from preparations of three to nine animals per condition. To avoid overlapping, the data points for WT and SK4-KO β-cells are shifted to left and right within the graph. C: Insulin secretion was compared in islets incubated with 3 or 6 mmol/l glucose (6 G) for 1 h. The diagram shows the percentage of islet preparations with significant increase in insulin release by 6 mmol/l glucose (eight independent preparations for each genotype). D and E: TRAM-34 induces electrical activity in WT β-cells treated with substimulatory glucose concentrations but not in SK4-KO β-cells. In this series of experiments, glucose concentration was lowered from 10 to 6 mmol/l or 5 mmol/l glucose. After termination of electrical activity, TRAM-34 (1 μmol/l) was added and action potentials reoccurred in four of five WT cells tested. In SK4-KO β-cells, TRAM-34 was without depolarizing effect. The experiment is representative of three.
Importantly, SK4 channel inhibition induced similar changes in WT β-cells (Fig. 3D). In this series of experiments, WT and SK4-KO β-cells, respectively, were perifused with 10 mmol/l glucose before lowering glucose below the threshold for Ca2+ APs (5–6 mmol/l glucose). After addition of TRAM-34 (1 μmol/l) to WT β-cells, electrical activity occurred in four of five cells. On average, Vm was −67 ± 2 mV at the subthreshold glucose concentration. With TRAM-34, the plateau potential at which Ca2+ APs started was −51 ± 1 mV (n = 5, P ≤ 0.001). In SK4-KO β-cells, 1 μmol/l TRAM-34 had no depolarizing effect on Vm (Fig. 3E). In this series of experiments, Vm was −67 ± 2 mV after lowering glucose to a concentration terminating electrical activity and −67 ± 1 mV in the presence of TRAM-34 (n = 3).
Knockout of SK4 channels affects glucose tolerance in vivo.
Because the experiments described thus far suggest that SK4 channels participate in regulation of glycemic control, we investigated whether ablation of SK4 channels affects glucose homeostasis in vivo. Therefore, an intraperitoneal glucose tolerance test was performed on 12-week-old male WT and SK4-KO mice. After injection of 2 g glucose/kg body wt, blood glucose was monitored during 120 min. SK4-KO mice had significantly lower blood glucose concentrations than WT mice (Fig. 4A and Table 1). By contrast, blood glucose concentrations in the fasted and fed state were similar in WT and SK4-KO mice, respectively (Table 1).
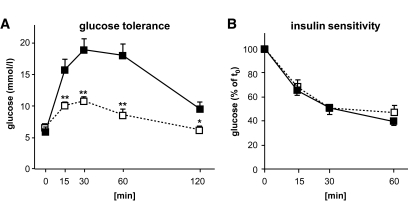
FIG. 4.
SK4 channel KO mice display improved glucose tolerance without alterations in insulin sensitivity. A: Blood glucose concentration of 12-week-old animals was monitored for 2 h after intraperitoneal injection of 2 g/kg body wt glucose (n = 5). B: The decrease in blood glucose concentration was monitored for 1 h after intraperitoneal injection of 0.7 IU/kg body wt insulin in 12-week-old mice. Experiments were performed with five to six male SK4-KO (□) mice and their WT littermates (■), respectively. *P ≤ 0.05, **P ≤ 0.01.
TABLE 1.
Influence of SK4-KO on glucose tolerance and insulin sensitivity
| Plasma glucose (mmol/l) |
||||
|---|---|---|---|---|
| 12 weeks |
24 weeks |
|||
| WT | SK4-KO | WT | SK4-KO | |
| Time after injection of: | ||||
| Glucose | ||||
| 0 min (fasted) | 5.8 ± 0.6 | 6.6 ± 0.5 | 5.6 ± 0.4 | 6.4 ± 0.3 |
| 15 min | 15.7 ± 1.6 | 10.0 ± 0.6† | 21.9 ± 0.9 | 18.6 ± 0.7* |
| 30 min | 18.9 ± 1.7 | 10.7 ± 0.6† | 27.1 ± 1.0 | 22.7 ± 0.5† |
| 60 min | 18.0 ± 1.8 | 8.6 ± 1.0† | 22.1 ± 1.9 | 15.4 ± 0.9† |
| 120 min | 9.5 ± 1.2 | 6.2 ± 0.5* | 10.2 ± 1.0 | 6.4 ± 0.1† |
| Insulin | ||||
| 0 min (fed) | 11.1 ± 1.1 | 10.4 ± 0.8 | 11.2 ± 0.6 | 10.6 ± 0.5 |
| 15 min | 7.0 ± 0.4 | 7.2 ± 1.0 | 7.3 ± 0.4 | 6.8 ± 0.3 |
| 30 min | 5.4 ± 0.3 | 5.3 ± 0.4 | 6.0 ± 0.2 | 5.7 ± 0.3 |
| 60 min | 4.2 ± 0.2 | 4.7 ± 0.5 | 5.7 ± 0.5 | 5.7 ± 0.8 |
Summary of glucose and insulin tolerance tests obtained from 12- and 24-week-old SK4-KO and WT mice. Plasma glucose concentration was monitored for 2 and 1 h after intraperitoneal injection of 2 g/kg body wt glucose or 0.7 IU/kg body wt insulin, respectively. (Five to six SK4-KO and WT littermates were tested for each condition.) Glucose tolerance was tested subsequent to a 16-h fasting period.
*P ≤ 0.05,
†P ≤ 0.01.
The improved glucose tolerance of SK4-KO mice might not exclusively represent a better secretory response of pancreatic β-cells but could also result from improved insulin sensitivity. To address this question, 0.7 IU insulin/kg body wt was injected intraperitoneally, and the decrease of blood glucose concentration was followed for 60 min (Fig. 4B and Table 1). Insulin sensitivity of SK4-KO mice was not different from their WT littermates (n = 5–6). To test whether these results were influenced by age, glucose and insulin tolerance tests were repeated with 24-week-old animals (n = 5–6; data are summarized in Table 1). Even in older animals (∼36 weeks), the beneficial effects of SK4-KO on glucose homeostasis still persisted (blood glucose concentration 2 h after glucose injection in WT animals: 8.7 ± 0.7 mmol/l, n = 8; in SK4-KO mice: 6.5 ± 0.2 mmol/l, n = 8, P ≤ 0.01). These experiments demonstrate that SK4-KO ameliorates glycemic control independent of age. To make sure that the improved secretory response of SK4-KO mice was not accompanied by β-cell exhaustion, we determined the insulin content in islets from animals at different ages (up to 9 months). These experiments confirmed that insulin content did not change with age (4.5–5.5 months: WT 25 ± 6 ng/islet vs. KO 25 ± 5 ng/islet, n = 3 different preparations per genotype; 6–7 months: WT 26 ± 3 ng/islet, n = 5, vs. KO 27 ± 2 ng/islet, n = 3; 8–9 months: WT 34 ± 5 ng/islet, n = 2, vs. KO 28 ± 2 ng/islet, n = 4; NS vs. WT, NS vs. ∼5-month-old mice).
DISCUSSION
Our experiments show for the first time that SK4 channels participate in the regulation of β-cell function and glucose homeostasis in vivo.
Glucose-induced insulin secretion involves tight coupling of glucose metabolism, electrical activity, [Ca2+]c, and exocytosis. The key event linking glucose metabolism to membrane depolarization is the closure of KATP channels. Subsequent opening of L-type Ca2+ channels increases [Ca2+]c, representing the triggering signal for insulin release (46,47). Our data show that SK4 channel protein is expressed in murine pancreatic islets. SK4 channels are operative in β-cells and constitute an important regulator of stimulus-secretion coupling. In WT β-cells, pharmacological opening or closure of SK4 channels crucially alters Vm (Figs. 2 and 3D). Importantly, the SK4 channel blocker TRAM-34 depolarizes Vm and induces electrical activity at a subthreshold glucose concentration, thus enhancing the glucose effect on stimulus-secretion coupling (Fig. 3D). This is of considerable significance, since it demonstrates that SK4 channels contribute to regulation of insulin release in the narrow range around the threshold blood glucose concentration physiologically relevant for glycemic control. In addition, TRAM-34 and SK4-KO significantly reduce Kslow currents that are thought to participate in the characteristic burst pattern of pancreatic β-cells. Our observation that SK4 is one component of Kslow (Fig. 2E) emphasizes the importance of the SK4 channel for β-cell electrical activity. Although the involvement of SK4 in Kslow generation is evidenced by the reduced current in SK4-KO β-cells and in TRAM-34–treated WT cells, charybdotoxin failed to affect Kslow. This observation requires further investigation but is in agreement with reports of others (33) describing inefficiency of the scorpion toxin on Kslow or on whole-cell currents with SK4 characteristics (48). Importantly, the typical oscillations of glucose-stimulated β-cells are not prevented by SK4-KO, and Kslow is not completely absent in SK4-KO β-cells, even in the presence of 1 mmol/l tolbutamide. This suggests, in agreement with what has previously been proposed by Kanno et al. (34), that KATP channels, SK4, and other KCa channels act in concert to regulate the bursting activity of pancreatic β-cells.
SK4-KO did not affect blood glucose concentration of fed or fasted mice, demonstrating that glucose homeostasis can be maintained by other factors, e.g., adaptation in central regulation of energy balance or activation of peripheral counterregulatory mechanisms. However, SK4-KO improved the glucose tolerance after glucose challenge, whereas insulin sensitivity remained unchanged (Fig. 4). This strongly suggests that the β-cell is the major target of SK4-KO with regard to glycemic control. Indeed, SK4-KO β-cells displayed alterations in agreement with improved glucose tolerance: in glucose-stimulated SK4-KO β-cells, the plateau potential was more depolarized compared with WT cells. Consequently, the frequency of Ca2+ APs was increased by ∼30%. In addition, loss or blockade of SK4 channels resulted in AP broadening and elevated Ca2+ influx. These effects are suited to enhance exocytosis and finally to improve glucose tolerance.
For control of insulin secretion, the concentration-response correlation of glucose and electrical activity is very important (49). SK4-KO induced a clear left shift in glucose responsiveness with respect to Vm, [Ca2+]c, and insulin secretion (Fig. 3A–C). Furthermore, electrical activity could be induced by TRAM-34 applied to subthreshold glucose concentrations, i.e., when Vm is already depolarized but has not reached the threshold for Ca2+ APs (Fig. 3D). It is well-known that the resting membrane potential of β-cells is predominantly carried by KATP current (50,51). In agreement, SK4-KO did not affect the responsiveness of β-cells at low glucose concentrations (Fig. 3B) and TRAM-34 did not depolarize WT β-cells under resting conditions. Regulation of insulin secretion occurs via a gradual decrease in the open probability of KATP channels in response to a stepwise elevation of glucose (46), thereby increasing membrane depolarization. SK4 channel opening is largely independent of Vm (2,52) but strictly regulated by [Ca2+]c. Half-maximal activation occurs at Ca2+ concentrations ranging from 300 to 500 nmol/l (53). For myocytes, it has been shown that SK4 channels are already open when Ca2+ is reduced below 100 nmol/l (52). This is in agreement with our observation that SK4 channel inhibition influences Vm under conditions where [Ca2+]c is in the low nanomoles per liter range. For pancreatic β-cells, it was suggested that Ca2+ influx via L-type Ca2+ channels does not increase at a Vm below −40 mV (54). This might raise the question why SK4 channel inhibition does not affect Vm at 0.5 mmol/l glucose but initiates APs at 6 mmol/l glucose. However, because Larsson-Nyrén et al. (54) induced Ca2+ influx by short depolarizing voltage steps starting at −70 mV, they cannot elucidate whether a gradual increase of Vm elevates Ca2+ channel activity, thereby promoting Ca2+ influx even below the threshold for Ca2+ APs. In this context, it is noteworthy that Nelson et al. (55) demonstrated in cell-attached membrane patches of basilar arteries that the open probability of L-type Ca2+ channels already starts to increase at −65 mV, which is ∼20 mV more negative than the threshold potential for APs. Consequently, glucose-regulated membrane depolarization might enhance SK4 channel activity dose dependently even before the threshold for induction of Ca2+ APs.
Our data suggest that membrane depolarization induced by closure of KATP channels leads to Ca2+ influx and subsequent activation of SK4 channels. This mechanism counteracts the depolarization and promotes closure of L-type Ca2+ channels. We hypothesize that modulation of β-cell activity via SK4 channels contributes to the precise adjustment of insulin secretion according to the current metabolic demands. An important regulatory function of SK4 channels concerning intracellular Ca2+ homeostasis has also been described for other cellular systems. In mast cells or in the endothelium, receptor-mediated Ca2+ influx activates SK4 channels, thereby inducing membrane hyperpolarization. However, in contrast to pancreatic β-cells, the increased K+ conductance enforces Ca2+ influx in these cells through transient receptor potential or store-operated Ca2+ channels. The final result is elevation of [Ca2+]c, which triggers mast cell degranulation or endothelium-mediated vasodilation, respectively (5,56). Depending on the pathway of Ca2+ influx, SK4 channel activation could either enhance or limit Ca2+-regulated signaling cascades in different tissues or organs.
As the Ca2+ dependence of SK4 channels is expected to couple channel activity to the metabolic status of pancreatic β-cells, SK4 channels may modulate cell function without bearing the risk for unwanted hypoglycemic episodes, which complicates the use of insulinotropic drugs acting on KATP channels (57,58). Importantly, SK4-KO mice displayed no signs of hypoglycemia after overnight fasting or when they were fed ad libitum, which shows that the genetic manipulation did not result in excessive insulin secretion per se but improved β-cell response when challenged with high blood glucose concentrations. Because SK4-KO markedly elevated the proportion of active β-cells, it is suggested that a reduction of the SK4 current is a suitable tool to recruit more β-cells for nutrient-stimulated insulin release. Thus, targeting SK4 channels pharmacologically might be a useful approach to augment insulin release in β-cells with impaired secretory response.
Acknowledgments
This work was supported by grants from the Deutsche Forschungs Gemeinschaft (DFG) Dr225/6-3 (G.D.) and DU425/1-2 (M.D.).
No potential conflicts of interest relevant to this article were reported.
We thank K. Dietz (Department of Medical Biometry, University of Tübingen) for help with statistical analysis of the Ca2+ concentration response correlation, O. Werz and F. Behnke (Department of Pharmaceutical Chemistry, University of Tübingen) for assistance with the immunoblots, I. Breuning for determination of insulin release, and M. Sausbier (Department of Pharmacology, University of Tübingen) for providing SK4-KO mice.
Parts of this study were recently published in abstract form [Diabetologia 2008;51(Suppl. 1):504].
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hoffman JF, Joiner W, Nehrke K, Potapova O, Foye K, Wickrema A: The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc Natl Acad Sci U S A 2003; 100: 7366– 7371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK: Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol 1998; 275: C848– C856 [DOI] [PubMed] [Google Scholar]
- 3.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE: Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem 2004; 279: 47681– 47687 [DOI] [PubMed] [Google Scholar]
- 4.Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J: Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl-secretion. Acta Physiol 2007; 189: 251– 258 [DOI] [PubMed] [Google Scholar]
- 5.Shumilina E, Lam RS, Wölbing F, Matzner N, Zemtsova IM, Sobiesiak M, Mahmud H, Sausbier U, Biedermann T, Ruth P, Sausbier M, Lang F: Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+-activated K+ channel KCa3.1. J Immunol 2008; 180: 8040– 804718523267 [Google Scholar]
- 6.Chang MC, Khanna R, Schlichter LC: Regulation of Kv1.3 channels in activated human T lymphocytes by Ca2+-dependent pathways. Cell Physiol Biochem 2001; 11: 123– 134 [DOI] [PubMed] [Google Scholar]
- 7.Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR: Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif 2007; 40: 656– 670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TV, Matsuyama H, Baell J, Hunne B, Fowler CJ, Smith JE, Nurgali K, Furness JB: Effects of compounds that influence IK (KCNN4) channels on afterhyperpolarizing potentials, and determination of IK channel sequence, in guinea pig enteric neurons. J Neurophysiol 2007; 97: 2024– 2031 [DOI] [PubMed] [Google Scholar]
- 9.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J: A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A 1997; 94: 11651– 11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamarina NA, Wang Y, Mariotto L, Kuznetsov A, Bond C, Adelman J, Philipson LH: Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes 2003; 52: 2000– 2006 [DOI] [PubMed] [Google Scholar]
- 11.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J: Sur1 knockout mice: a model for KATP channel-independent regulation of insulin secretion. J Biol Chem 2000; 275: 9270– 9277 [DOI] [PubMed] [Google Scholar]
- 12.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA: Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem 2002; 277: 37176– 37183 [DOI] [PubMed] [Google Scholar]
- 13.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S: Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A 1998; 95: 10402– 10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Düfer M, Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G: Oscillations of membrane potential and cytosolic Ca2+ concentration in SUR1-/- beta cells. Diabetologia 2004; 47: 488– 498 [DOI] [PubMed] [Google Scholar]
- 15.Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P: SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 2003; 121: 181– 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G, Düfer M: Crosstalk between membrane potential and cytosolic Ca2+ concentration in beta cells from Sur1-/- mice. Diabetologia 2005; 48: 913– 921 [DOI] [PubMed] [Google Scholar]
- 17.Szollosi A, Nenquin M, Henquin JC: Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J Biol Chem 2007; 282: 14768– 14776 [DOI] [PubMed] [Google Scholar]
- 18.Ferrer J, Wasson J, Salkoff L, Permutt MA: Cloning of human pancreatic islet large conductance Ca2+-activated K+ channel (hSlo) cDNAs: evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia 1996; 39: 891– 898 [DOI] [PubMed] [Google Scholar]
- 19.Yan L, Figueroa DJ, Austin CP, Liu Y, Bugianesi RM, Slaughter RS, Kaczorowski GJ, Kohler MG: Expression of voltage-gated potassium channels in human and rhesus pancreatic islets. Diabetes 2004; 53: 597– 607 [DOI] [PubMed] [Google Scholar]
- 20.Smith PA, Bokvist K, Arkhammar P, Berggren PO, Rorsman P: Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol 1990; 95: 1041– 1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald PE, Wheeler MB: Voltage-dependent K+ channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia 2003; 46: 1046– 1062 [DOI] [PubMed] [Google Scholar]
- 22.Jacobson DA, Philipson LH: Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes Obes Metab 2007; 2 ( Suppl. 9): 89– 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roe MW, Worley JF, 3rd, Mittal AA, Kuznetsov A, DasGupta S, Mertz RJ, Witherspoon SM, 3rd, Blair N, Lancaster ME, McIntyre MS, Shehee WR, Dukes ID, Philipson LH: Expression and function of pancreatic beta-cell delayed rectifier K+ channels: role in stimulus-secretion coupling. J Biol Chem 1996; 271: 32241– 32246 [DOI] [PubMed] [Google Scholar]
- 24.Su J, Yu H, Lenka N, Hescheler J, Ullrich S: The expression and regulation of depolarization-activated K+ channels in the insulin-secreting cell line INS-1. Pflügers Arch 2001; 442: 49– 56 [DOI] [PubMed] [Google Scholar]
- 25.MacDonald PE, Sewing S, Wang J, Joseph JW, Smukler SR, Sakellaropoulos G, Wang J, Saleh MC, Chan CB, Tsushima RG, Salapatek AM, Wheeler MB: Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic beta-cells enhances glucose-dependent insulin secretion. J Biol Chem 2002; 277: 44938– 44945 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ämmälä CE, Philipson LH: Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab 2007; 6: 229– 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribalet B, Beigelman PM: Calcium action potentials and potassium permeability activation in pancreatic beta-cells. Am J Physiol 1980; 239: C124– C133 [DOI] [PubMed] [Google Scholar]
- 28.Ämmälä C, Larsson O, Berggren PO, Bokvist K, Juntti-Berggren L, Kindmark H, Rorsman P: Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature 1991; 353: 849– 852 [DOI] [PubMed] [Google Scholar]
- 29.Ribalet B, Eddlestone GT, Ciani S: Metabolic regulation of the KATP and a maxi-KV channel in the insulin-secreting RINm5F cell. J Gen Physiol 1988; 92: 219– 237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satin LS, Hopkins WF, Fatherazi S, Cook DL: Expression of a rapid, low-voltage threshold K current in insulin-secreting cells is dependent on intracellular calcium buffering. J Membr Biol 1989; 112: 213– 222 [DOI] [PubMed] [Google Scholar]
- 31.Kukuljan M, Goncalves AA, Atwater I: Charybdotoxin-sensitive KCa channel is not involved in glucose-induced electrical activity in pancreatic beta-cells. J Membr Biol 1991; 119: 187– 195 [DOI] [PubMed] [Google Scholar]
- 32.Henquin JC: Role of voltage- and Ca2+-dependent K+ channels in the control of glucose-induced electrical activity in pancreatic B-cells. Pflügers Arch 1990; 416: 568– 572 [DOI] [PubMed] [Google Scholar]
- 33.Göpel SO, Kanno T, Barg S, Eliasson L, Galvanovskis J, Renström E, Rorsman P: Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol 1999; 114: 759– 770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno T, Rorsman P, Göpel SO: Glucose-dependent regulation of rhythmic action potential firing in pancreatic beta-cells by KATP-channel modulation. J Physiol 2002; 545: 501– 507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Houamed K, Kupershmidt S, Roden D, Satin LS: Pharmacological properties and functional role of Kslow current in mouse pancreatic beta-cells: SK channels contribute to Kslow tail current and modulate insulin secretion. J Gen Physiol 2005; 126: 353– 363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440– 3450 [PubMed] [Google Scholar]
- 37.Barg S, Galvanovskis J, Göpel SO, Rorsman P, Eliasson L: Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 2000; 49: 1500– 1510 [DOI] [PubMed] [Google Scholar]
- 38.Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG: Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A 2000; 97: 8151– 8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M: Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Arch 1991; 418: 417– 422 [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ: Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 2001; 296: 600– 611 [PubMed] [Google Scholar]
- 41.Hamilton KL, Kiessling M: DCEBIO stimulates Cl− secretion in the mouse jejunum. Am J Physiol Cell Physiol 2006; 290: C152– C164 [DOI] [PubMed] [Google Scholar]
- 42.Morimura K, Yamamura H, Ohya S, Imaizumi Y: Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J Pharmacol Sci 2006; 100: 237– 241 [DOI] [PubMed] [Google Scholar]
- 43.Hirukawa K, Muraki K, Ohya S, Imaizumi Y, Togari A: Electrophysiological properties of a novel Ca2+-activated K+ channel expressed in human osteoblasts. Calcif Tissue Int 2008; 83: 222– 229 [DOI] [PubMed] [Google Scholar]
- 44.Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y: SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol 2000; 84: 97– 100 [DOI] [PubMed] [Google Scholar]
- 45.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H: International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 2005; 57: 463– 472 [DOI] [PubMed] [Google Scholar]
- 46.Ashcroft FM, Rorsman P: Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 1989; 54: 87– 143 [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, Misler S: Amperometric detection of quantal secretion from patch-clamped rat pancreatic beta-cells. J Biol Chem 1996; 271: 270– 277 [DOI] [PubMed] [Google Scholar]
- 48.Kozak JA, Misler S, Logothetis DE: Characterization of a Ca2+-activated K+ current in insulin-secreting murine betaTC-3 cells. J Physiol 1998; 509: 355– 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner HP, Schmelz H: Membrane potential of beta-cells in pancreatic islets. Pflügers Arch 1974; 351: 195– 206 [DOI] [PubMed] [Google Scholar]
- 50.Rorsman P, Trube G: Biophysics and physiology of ATP-regulated K+ channels (KATP). In Potassium Channels: Structure, Classification, Function and Therapeutic Potential. Cook NS: Ed. Chichester, U.K., Ellis Horwood, 1990, p. 96– 116 [Google Scholar]
- 51.Smith PA, Ashcroft FM, Rorsman P: Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K+-currents in isolated mouse pancreatic beta-cells. FEBS Lett 1990; 261: 187– 190 [DOI] [PubMed] [Google Scholar]
- 52.Vogalis F, Zhang Y, Goyal RK: An intermediate conductance K+ channel in the cell membrane of mouse intestinal smooth muscle. Biochim Biophys Acta 1998; 1371: 309– 316 [DOI] [PubMed] [Google Scholar]
- 53.Ledoux J, Werner ME, Brayden JE, Nelson MT: Calcium-activated potassium channels and the regulation of vascular tone. Physiology 2006; 21: 69– 78 [DOI] [PubMed] [Google Scholar]
- 54.Larsson-Nyrén G, Sehlin J, Rorsman P, Renström E: Perchlorate stimulates insulin secretion by shifting the gating of L-type Ca2+ currents in mouse pancreatic B-cells towards negative potentials. Pflügers Arch 2001; 441: 587– 595 [DOI] [PubMed] [Google Scholar]
- 55.Nelson MT, Patlak JB, Worley JF, Standen NB: Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 1990; 259: C3– C18 [DOI] [PubMed] [Google Scholar]
- 56.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R: Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 2006; 99: 537– 544 [DOI] [PubMed] [Google Scholar]
- 57.Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y: Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159: 281– 284 [DOI] [PubMed] [Google Scholar]
- 58.Schernthaner G, Grimaldi A, Di Mario U, Drzewoski J, Kempler P, Kvapil M, Novials A, Rottiers R, Rutten GE, Shaw KM: GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest 2004; 34: 535– 542 [DOI] [PubMed] [Google Scholar]