Abstract
OBJECTIVE
The gluco-incretin hormones glucagon-like peptide (GLP)-1 and gastric inhibitory peptide (GIP) protect β-cells against cytokine-induced apoptosis. Their action is initiated by binding to specific receptors that activate the cAMP signaling pathway, but the downstream events are not fully elucidated. Here we searched for mechanisms that may underlie this protective effect.
RESEARCH DESIGN AND METHODS
We performed comparative transcriptomic analysis of islets from control and GipR−/−;Glp-1-R−/− mice, which have increased sensitivity to cytokine-induced apoptosis. We found that IGF-1 receptor expression was markedly reduced in the mutant islets. Because the IGF-1 receptor signaling pathway is known for its antiapoptotic effect, we explored the relationship between gluco-incretin action, IGF-1 receptor expression and signaling, and apoptosis.
RESULTS
We found that GLP-1 robustly stimulated IGF-1 receptor expression and Akt phosphorylation and that increased Akt phosphorylation was dependent on IGF-1 but not insulin receptor expression. We demonstrated that GLP-1–induced Akt phosphorylation required active secretion, indicating the presence of an autocrine activation mechanism; we showed that activation of IGF-1 receptor signaling was dependent on the secretion of IGF-2. We demonstrated, both in MIN6 cell line and primary β-cells, that reducing IGF-1 receptor or IGF-2 expression or neutralizing secreted IGF-2 suppressed GLP-1–induced protection against apoptosis.
CONCLUSIONS
An IGF-2/IGF-1 receptor autocrine loop operates in β-cells. GLP-1 increases its activity by augmenting IGF-1 receptor expression and by stimulating secretion; this mechanism is required for GLP-1–induced protection against apoptosis. These findings may lead to novel ways of preventing β-cell loss in the pathogenesis of diabetes.
The number of pancreatic islet β-cells as well as their capacity to secrete insulin is modulated in normal physiological conditions to respond to the metabolic demand of the organism (1). A failure of the endocrine pancreas to maintain an adequate insulin secretory capacity due to reduced β-cell number and function underlies the pathogenesis of both type 1 and type 2 diabetes. In type 1 diabetes, β-cells are destroyed by an autoimmune mechanism in which cytokine-induced apoptosis is thought to play an important role (2). In the pathogenesis of type 2 diabetes, hyperglycemia appears when β-cell mass and insulin secretory capacity are no longer sufficient to compensate for insulin resistance. The reduction in β-cell mass results from increased apoptosis, most probably caused by the combined action of cytokines and increased plasma glucose and free fatty acid levels (3,4). Therefore, finding means to protect β-cell against apoptosis may be useful for the treatment or prevention of diabetes.
The gluco-incretin hormones GLP-1 and GIP can protect β-cells against apoptosis induced by cytokines or glucose and free fatty acids (5). Both hormones bind to specific stimulatory G-protein (Gs)–coupled receptors, which trigger cAMP formation (6,7). In β-cells, basal cAMP levels control glucose competence (8), i.e., the magnitude of the insulin secretion response to a given increase in extracellular glucose concentration. Increases in cAMP levels, for instance, as stimulated by GLP-1 or GIP action, potentiate glucose-stimulated insulin secretion by both protein kinase A (PKA)-dependent and -independent mechanisms (9); they also stimulate gene transcription through PKA-dependent phosphorylation of the transcription factor CREB (cAMP-response element binding) (10).
In β-cells, increased cAMP levels also activate the mitogen-activated protein (MAP) kinase cascade, leading to rapid phosphorylation of Erk1/2 (11). An activation of the phosphatidylinositol (PI) 3-kinase/Akt pathway is also observed. PI 3-kinase may be directly activated by the βγ subunit of Gs (12), be secondary to transactivation of the EGF receptor by betacellulin (13), or follow transcriptional induction of insulin receptor substrate (IRS)-2 through the PKA/CREB pathway. The IRS-2/PI 3-kinase/Akt pathway is known to have antiapoptotic effects (14–16); however, it is unclear why increased expression of IRS-2 leads to activation of its signaling pathway. IRS-2 may be downstream of the insulin receptor (IR) or IGF-1 receptor (IGF-1R). Studies of mice with β-cell–specific inactivation of either receptor (17–19) have indicated that the insulin receptor was important for compensatory growth of the β-cells in response to insulin resistance, whereas the IGF-1 receptor was involved in the control of glucose competence.
Here we studied islets from mice with inactivation of both the GLP-1 and GIP receptor genes. We showed them to be more sensitive than control islets to cytokine-induced apoptosis. By comparative transcriptomic analysis of islets from control and mutant mice, we found that expression of the IGF-1R was markedly decreased in the mutant islets. In control islets, GLP-1–activated IGF-1R expression and signaling and GLP-1–induced IGF-1R signaling required autocrine secretion of IGF-2. We provided evidence that increasing the activity of this autocrine loop was required for GLP-1 to protect β-cells against apoptosis. These data provide an integrated description of the interaction between the GLP-1 and IGF-1 receptor signaling pathways that operate to protect β-cells against apoptosis.
RESEARCH DESIGN AND METHODS
Mice.
Male C57BL/6 and GipR−/−;Glp-1-R−/− mice backcrossed for seven generations into the C57BL/6 background were used between 8 and 10 weeks of age. All experimental procedures received approval from the Service Vétérinaire du Canton de Vaud.
Antibodies.
Rabbit polyclonal antibody against actin was purchased from Sigma (St. Louis, MO); rabbit polyclonal antibodies against the IGF-1R (C20; sc-713), the insulin receptor (C19; sc-711), and total Akt ([H-136]: sc-8312) were from Santa Cruz Biotechnology (Nunningen, Switzerland); rabbit monoclonal antibody against phosphorylated Akt (pAktThr-308) (no. 4056), rabbit polyclonal antibodies against phosphorylated Akt (pAktSer-473) (no. 9271), phosphorylated Bad (pBadSer-112) (no. 9291), and total Bad (no. 9292) were from Cell Signaling (Danvers, MA); and goat polyclonal antibody against IGF-2 (ab10731) was purchased from Abcam (Cambridge, U.K.).
Pancreatic islet isolation.
Islets were isolated according to published procedures (20) using a Histopaque density gradient. Islets were cultured overnight at 37°C in 5% CO2 in RPMI-1640, 10% FCS, 2 mmol/l glutamax, 100 units/ml penicillin, and 100 μg/ml streptomycin. β-Cells were purified by cell sorter as described (21).
Microarray and quantitative PCR analyses.
Islets were isolated from 8-to 10-week-old mice and kept in culture overnight before RNA extraction. Total islet RNA was extracted using RNeasy Mini kit (Qiagen); purity and quality were assessed by an Agilent bioanalyzer. After linear amplification with T7 polymerase, the RNA was reverse transcribed and labeled with Cy3 or Cy5 and hybridized to cDNA arrays containing 17,664 different probes, prepared by the University of Lausanne DNA Array Facility (www.unil.ch/dafl). Hybridization, data acquisition, and quality control were performed as previously described (22). Differentially expressed genes were identified using linear models implemented in the Limma package from Bioconductor (http://www.bioconductor.org). All statistical analyses were performed using the R software (http://www.r-project.org). Real-time quantitative PCR was performed using Light Cycler Technology (Roche, France). Primers sets were chosen to amplify products of ∼200 bp. cDNAs were obtained from 2.5 μg total RNA with SuperScript II RNAse H- Reverse Transcriptase (Invitrogen, Carlsbad, CA) and 50 pmol random hexamer (Applied Biosystem). cDNA amplification was performed in a 20-μl reaction mixture including 1× QuantiTect SYBR Green PCR Master Mix (Qiagen) and 10 pmol of each primer. Primers for mouse IGF-1 (sense, 5′-GGCATTGTGGATGAGTGTTG-3′; antisense, 5′-AGTTGCCTCCGTTACCTCCT-3′), mouse IGF-2 (sense, 5′-GTCGATGTTGGTGCTTCTCA-3′; antisense, 5′-AAGCAGCACTCTTCCACGAT-3′), and mouse cyclophilin (sense, 5′-TCCATCGTGTCATCAAGGAC-3′; antisense, 5′-CTTGCCATCCAGCCAGGAG-3′) were used. All measurements were normalized to cyclophilin.
MIN6 cell culture and secretion tests.
MIN6 cells were grown in Dulbecco's modified Eagle's medium, 15% FCS, 2 mmol/l glutamine, and 50 μmol/l β-mercaptoethanol and used between passages 19 and 30. For pharmacological treatments, 106 MIN6 cells were plated in 6-cm-diameter tissue culture dishes for 4 days, the medium was replaced, and cells were incubated for 2 h at 37°C before addition of various reagents at the concentrations and for the periods of time indicated in the figure legends.
For gene silencing, MIN6 cells were transfected 1 day after plating with 0.5 μg of a green fluorescent protein (GFP) expression plasmid and siRNAs at a final concentration of 100 nmol/l using lipofectamine (Invitrogen, Carlsbad, CA). After 6 h at 37°C, the growth medium was replaced, and the cells were used 48 h later.
For secretion tests, MIN6 cells were seeded at 106 cells in sixwell plates. Three days later, cells were washed and preincubated for 2 h in Krebs-Ringer bicarbonate HEPES buffer (120 mmol/l NaCl, 4 mmol/l KH2PO4, 20 mmol/l HEPES, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 5 mmol/l NaHCO3, and 0.5% BSA, pH 7.4) supplemented with 2 mmol/l glucose. Then, the medium was replaced with Krebs-Ringer bicarbonate HEPES buffer containing 2 or 20 mmol/l glucose for an additional hour. Secretion and cellular IGF-2 (extracted in PBS-Tween 5%) was assessed by ELISA (no. DY702, R&D; Minneapolis, MN).
Gel electrophoresis and immunoblotting.
MIN6 cells were placed on ice, washed twice with PBS, and lysed for 30 min on ice in 50 mmol/l Tris (pH 7.5) containing 1 mmol/l EDTA, 1 mmol/l EGTA, 1% Triton, 10 mmol/l β-glycerol-phosphate, 1 mmol/l Na3VO4, 50 mmol/l NaF, 5 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, leupeptin, and pepstatin. The lysates were centrifuged for 15 min at 13,000 rpm at 4°C.
Mouse islets were washed twice with PBS and lysed in 160 mmol/l Tris (pH 6.8) containing 5 mmol/l EDTA, 20% glycerol, 10% β-mercaptoethanol, 10% SDS, 1 mmol/l Na3VO4, 50 mmol/l NaF, 5 mmol/l phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin. Lysates were sonicated for 15 s before protein determination. Western blot analysis was performed as described (21) with antibodies diluted in Tris-buffered saline–5% nonfat dry milk (1/500 dilution for IGF-1R, IR and phosphorylated Akt [Thr308 and Ser473], and phosphorylated Bad and 1/1,000 for actin, total Akt, total Bad, and IGF-2) and revealed using horseradish peroxidase–conjugated donkey anti-rabbit IgG or horseradish peroxidase–conjugated sheep anti-mouse IgG as secondary antibodies (Amersham, Buckinghamshire, U.K.). Band intensities were determined using a Bio-Rad densitometer (Strasbourg, France).
Apoptosis assay.
Apoptosis was induced by exposing the MIN6 cells or islets to cytokines at the indicated concentrations for 24 h; GLP-1(7–36)amide (100 nmol/l) was added 4 h before the cytokines and was present for the entire cytokine incubation period (24 h). Apoptosis was assessed by scoring the number of cells expressing GFP and displaying pyknotic nuclei labeled with Hoechst 33342 (10 μg/ml) (23). Islets were first dissociated in 100 μl dissociation buffer (Gibco, product number 13151-014) for 15 min at 37°C before apoptosis was assessed.
Adenovirus.
Recombinant adenoviruses expressing enhanced GFP alone (Ad-GFP) or together with IGF-1R (Ad-IGF-1R) were generated using the AdEasy system (24). Islets were infected with a multiplicity of infection of 40 in 500 μl of medium for 3 h at 37°C and then cultured for 48 h before insulin secretion tests, as described above. Preliminary experiments showed that ∼85% of islet cells were infected and that infection did not affect glucose-induced insulin secretion.
Statistical analysis.
All experiments were performed at least three times. Results are expressed as means ± SD. Comparisons were performed using unpaired Student's t test or one-way or two-way ANOVA for the different groups followed by post hoc pair-wise multiple-comparison procedures (Tukey test or Bonferroni, respectively).
RESULTS
Increased susceptibility to apoptosis of islets from GipR−/−;Glp-1R−/− mice.
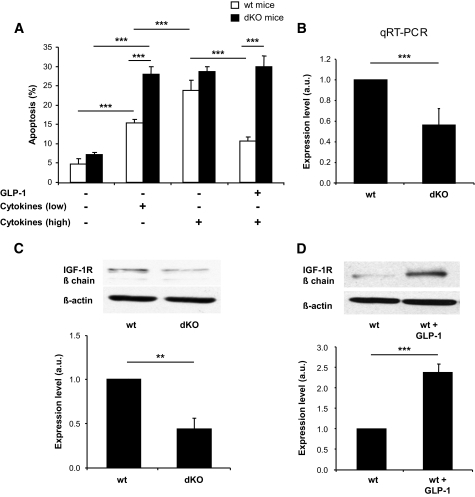
To evaluate whether absence of both gluco-incretin hormone receptor expression led to increased susceptibility to cytokine-induced apoptosis, islets from GipR−/−;Glp-1-R−/− mice were exposed to low or high concentrations of cytokines (interleukin [IL]-1β, tumor necrosis factor [TNF]-α, and interferon [IFN]-γ), and the percent of apoptotic cells was determined. Figure 1A shows that islets from mutant mice were significantly more sensitive than control islets to apoptosis induced by the low dose of cytokine; at the high dose, apoptosis was similar in both types of islets. As expected, GLP-1 protected control but not mutant islets against cytokine-induced apoptosis.
FIG. 1.
Gluco-incretin signaling controls susceptibility to apoptosis and IGF-1R expression. A: Islets from control or GipR−/−;Glp-1R−/− mice were exposed or not to cytokines (IL-1β, TNF-α, and IFN-γ) at low or high concentrations (5, 12.5, and 5 ng/ml or 10, 25, and 10 ng/ml, respectively) and in the presence or absence of GLP-1 (100 nmol/l). B: Quantitative RT-PCR analysis of IGF-1R mRNA expression in islets from control (wt) and GipR−/−;Glp-1R−/− mice (dKO). C: Reduced expression of IGF-1R expression in GipR−/−;Glp-1R−/− mouse islets as detected by Western blot analysis. Lower panel: Quantitation of the expression data. D: GLP-1 (100 nmol/l) treatment for 18 h increased IGF-1R expression as detected by Western blot analysis. Lower panel: Quantitation of the expression data. For all panels, data are means ± SD, n = 3 independent experiments. **P < 0.01; ***P < 0.001.
Gluco-incretins control IGF-1R expression and signaling.
To search for genes differentially expressed in control and mutant islets and that could be involved in the increased susceptibility to cytokine-induced apoptosis, we performed microarray analysis of transcripts expressed in control and mutant islets. We found that the IGF-1R mRNA was downregulated in the mutant islets (Fig. 1B) and that IGF-1R protein expression was also markedly decreased (Fig. 1C). This suggested that expression of the IGF-1R could be regulated by gluco-incretin hormones. Figure 1D shows that GLP-1 indeed induced a marked increase in IGF-1 receptor expression in control islets.
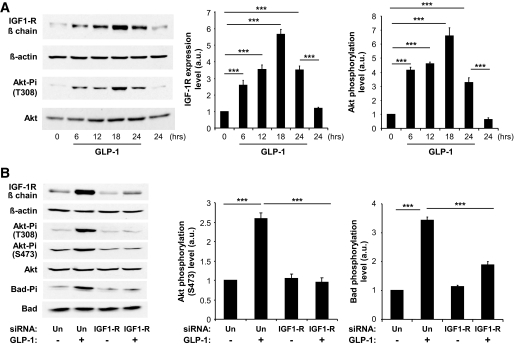
To investigate in more detail the regulation by gluco-incretins of IGF-1R expression and signaling, we used MIN6 cells as a model system and concentrated our study on the effect of GLP-1, since GLP-1 and GIP use the same signaling pathway. We first demonstrated that, as in primary islets, GLP-1 increased IGF-1R expression with maximal induction reached after ∼18 h of treatment (Fig. 2A). In addition, we found that induction of IGF-1R expression was associated with a corresponding increase in Akt phosphorylation on Thr308 (Fig. 2A). We then showed that GLP-1 treatment also induced Akt phosphorylation on Ser473 and the phosphorylation of Bad, a proapoptotic protein inactivated by phosphorylation by IGF-1R signaling (25,26) (Fig. 2B). We also determined that preventing IGF-1R expression by transfecting the cells with an IGF-1R–specific siRNA suppressed GLP-1–induced Akt phosphorylation on both sites and the phosphorylation of Bad (Fig. 2B). GLP-1 treatment of the cells induced an approximately threefold increase in IRS-2 expression (see Fig. S1 in an online-only appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0063/DC1).
FIG. 2.
GLP-1 increased IGF-1R expression and signaling in MIN6 cells. A: MIN6 cells were treated with GLP-1 for the indicated periods of time, and IGF-1R expression and Akt phosphorylation were analyzed by Western blot analysis. B: MIN6 cells were transfected with a control (Un) or an IGF-1R siRNA and treated for 18 h with GLP-1. GLP-1 induced IGF-1 receptor expression, Akt phosphorylation on both T308 and S473, and Bad phosphorylation. Preventing IGF-1R expression suppressed GLP-1–induced Akt and Bad phosphorylation. For all panels, data are means ± SD, n = 3 independent experiments. ***P < 0.001.
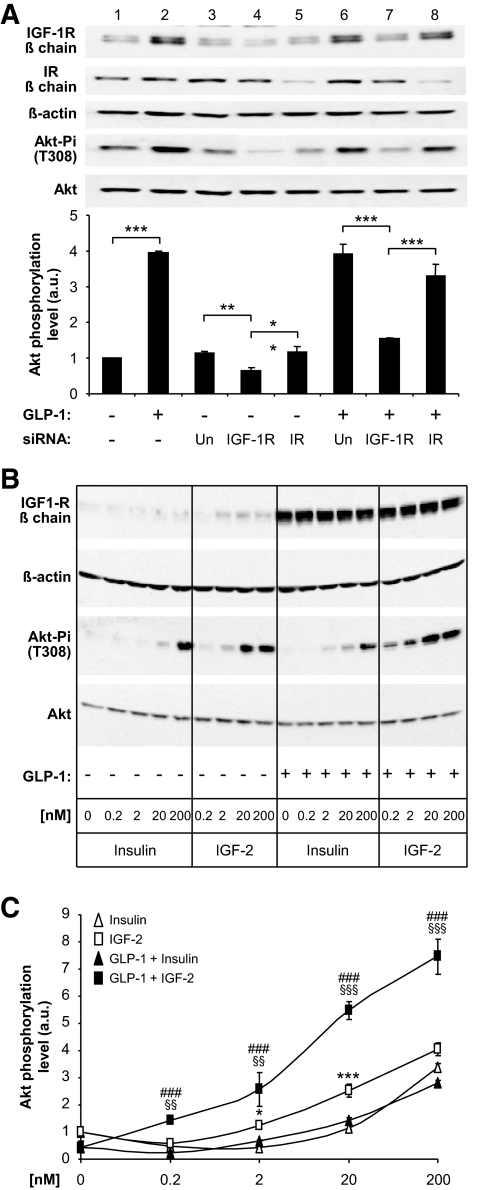
Because β-cells also express the IR and secrete insulin, we searched for direct evidence that the IGF-1R, and not the IR, was responsible for the induction of Akt phosphorylation. First, we transfected MIN6 cells with IGF-1R– or IR-specific siRNAs before treating the cells with GLP-1 for 18 h and analyzing Akt phosphorylation. Figure 3A (lanes 1 and 2) shows that GLP-1 induced expression of the IGF-1R but not the IR. The receptor-specific, but not the unrelated, siRNAs led to a reduction of either IGF-1R or IR expression in the basal state (in the absence of GLP-1 stimulation; Fig. 2A, lanes 3–5) or after GLP-1 treatment (Fig. 2A, lanes 6–8). Importantly, Akt phosphorylation in the basal state or after GLP-1 treatment was reduced only when the IGF-1R, but not the IR expression, was decreased (lanes 4 and 7 vs. lanes 5 and 8).
FIG. 3.
GLP-1–induced Akt phosphorylation depends on IGF-1R but not IR expression. A: MIN6 cells were transfected with control (Un, lanes 3 and 6), IGF-1R (lanes 4 and 7), or IR-specific (lanes 5 and 8) siRNAs and exposed (+) or not (−) to GLP-1 for 18 h before Western analysis. Lanes 1 and 2 show the induction by GLP-1 of IGF-1R expression in nontransfected cells. Lane 3 shows the basal level of IGF-1R and IR expression in transfected cells; reducing IGF-1R expression (lane 4) but not the IR (lane 5) reduced Akt phosphorylation. Lane 6: GLP-1–treated cells showed higher expression of the IGF-1R but not of the IR; reducing IGF-1R expression (lane 7) but not IR (lane 8) reduced Akt phosphorylation. Bottom panel: Quantitation of the data. Data are means ± SD, n = 3 independent experiments. **P < 0.01; ***P < 0.001. B: MIN6 cells were treated or not for 18 h with GLP-1 to increase IGF-1R expression, then incubated with 2 mmol/l glucose for 2 h, and exposed for 15 min to the indicated concentrations of insulin or IGF-2. IGF-1R and total and phosphorylated Akt were determined by Western blot analysis. C: Quantification of the data in B. *P < 0.05, ***P < 0.001 for IGF-2 vs. insulin; §§P < 0.01, §§§P < 0.001 for IGF-2 in control vs. GLP-1–treated cells; ###P < 0.001 for IGF-2 vs. insulin in GLP-1–treated cells. a.u., arbitrary units.
To further evaluate the respective roles of the IGF-1R and IR in activating the Akt pathway, we measured the efficiency of insulin and IGF-2 to activate Akt phosphorylation in MIN6 cells. Indeed, insulin binds to the IR with high affinity (Kd ∼1 nmol/l) and to IGF-1R with an ∼100-fold lower affinity, whereas IGF-2 binds to the IGF-1R with high affinity (∼1 nmol/l) and with much lower affinity to the IR (27). MIN6 cells were thus pretreated or not with GLP-1 for 18 h and kept in the presence of 2 mmol/l glucose for 2 h to prevent insulin secretion. The cells were then exposed to different concentrations of each hormone for 15 min, and IGF-1R expression and Akt phosphorylation were determined (Fig. 3B). The data show that in the absence of GLP-1 pretreatment, IGF-2 was significantly more efficient in inducing Akt phosphorylation than insulin (Fig. 3B and C). After GLP-1 treatment, IGF-1R expression was markedly augmented and IGF-2 induced Akt phosphorylation with a greater efficacy than in nontreated cells; insulin-induced Akt phosphorylation, however, was not increased.
Together, the above data indicated that GLP-1–induced Akt phosphorylation was dependent on IGF-1R upregulation and independent of IR expression.
IGF-1R activation is triggered by autocrine secretion of IGF-2.
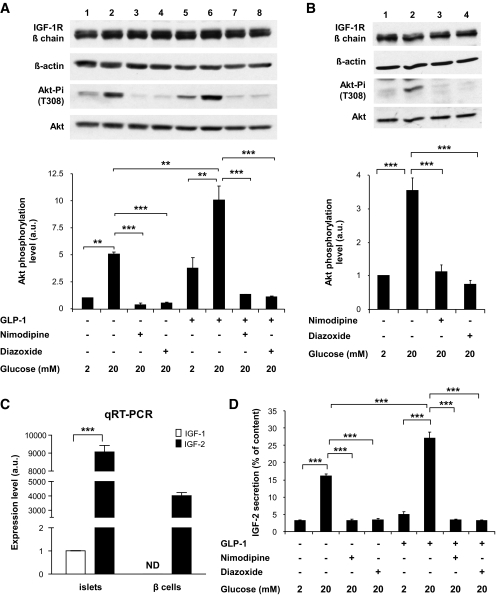
To determine whether activation of the IGF-1R signaling pathway was dependent on secretion, we evaluated the level of Akt phosphorylation in cells, which had previously been treated for 18 h with or without GLP-1 to increase IGF-1R expression, then exposed for 2 h to a low glucose concentration, and then exposed to low or high glucose concentrations in the presence or absence of secretion inhibitors. We first evaluated Akt phosphorylation in cells pretreated or not pretreated with GLP-1. In the absence of pretreatment, high glucose induced an approximately threefold increase in Akt phosphorylation, whereas this increase was ∼12-fold in GLP-1–treated cells (see Fig. S2 in the online appendix and Fig. 4A). The increased phosphorylation at 20 mmol/l glucose was suppressed by the ATP-sensitive K+ (KATP) channel opener diazoxide or the Ca2+ channel inhibitor nimodipine (Fig. 4A). When the cells were exposed to GLP-1 during the last incubation period, Akt phosphorylation was higher than in the absence of GLP-1 but equally suppressed by the secretion inhibitors. To determine if the same regulation of Akt phosphorylation was observed in primary β-cells, mouse islets were similarly treated with GLP-1 for 18 h and then exposed to 2 or 20 mmol/l glucose in the presence or absence of nimodipine and diazoxide. As for MIN6 cells, phosphorylation of Akt was very low at 2 mmol/l glucose, increased at 20 mmol/l glucose, and suppressed by the secretion inhibitors (Fig. 4B). Thus, these observations indicated that activation of Akt phosphorylation was dependent on active secretion.
FIG. 4.
Activation of Akt phosphorylation by GLP-1 depends on glucose-induced secretion. A: MIN6 cells were incubated for 18 h with GLP-1 to induce IGF-1R expression, then with 2 mmol/l glucose for 2 h, and finally for 1 h with 2 or 20 mmol/l glucose with or without GLP-1 and nimodipine (1 μmol/l) or diazoxide (200 μmol/l). Bottom panel: Quantitation of the data. B: Pancreatic islets were treated as the MIN6 cells in A. C: qRT-PCR analysis of IGF-1 and IGF-2 mRNA expression in mouse islets and cell sorter–purified β-cells. D: Secretion of IGF-2 by MIN6 cells incubated to 2 or 20 mmol/l glucose and in the presence of nimodipine, diazoxide, or GLP-1, as indicated. Total IGF-2 content was 10 ng/4 × 106 cells. A–D: Data are means ± SD, n = 3 independent experiments. **P < 0.01; ***P < 0.001. a.u., arbitrary units.
Three ligands can potentially activate the IGF-1R: insulin, IGF-1, and IGF-2. Because the data of Fig. 3 indicated that activation of Akt phosphorylation was through the IGF-1R but not the IR, it was highly unlikely that insulin was the ligand activating Akt phosphorylation. On the other hand, IGF-1 was not detectable by RT-PCR in MIN6 cells (not shown) and was expressed at an extremely low level in islets and not detectable in cell sorter–purified β-cells (Fig. 4C); IGF-1 was therefore not a candidate for activating the IGF-1 receptor signaling pathway in our experiments. Previous reports described the expression of IGF-2 in mature β-cells (28,29), and we confirmed that IGF-2 mRNA was expressed at high level in islets and purified β-cells (Fig. 4C). Furthermore, incubation of MIN6 cells at high glucose triggered IGF-2 secretion in the culture medium; this secretion was blocked by diazoxide and nimodipine and was increased when GLP-1 was present (Fig. 4D).
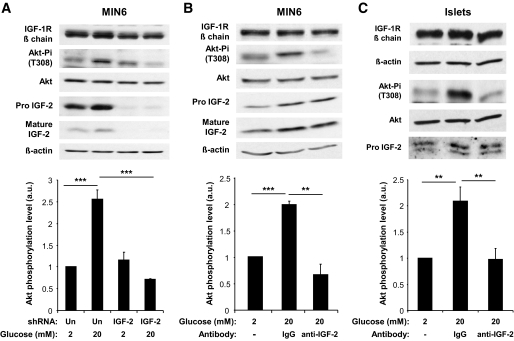
We thus tested whether IGF-2 was involved in activation of Akt phosphorylation in MIN6 cells exposed to GLP-1. MIN6 cells were first transfected with an IGF-2–specific or an unrelated shRNA, then exposed to GLP-1 for 18 h to induce IGF-1R expression, and finally exposed to 2 or 20 mmol/l glucose before analysis of Akt phosphorylation. Figure 5A shows that expression of pro–IGF-2 and mature IGF-2 was strongly decreased by transfection of the specific shRNA, and this suppressed the induction of Akt phosphorylation in cells exposed to high glucose. As a second test for an autocrine role of IGF-2 secretion in Akt phosphorylation, MIN6 cells were treated as in the previous experiment but the final incubation with 20 mmol/l glucose was conducted in the presence of an IGF-2 blocking antibody or an unrelated IgG fraction. Figure 5B shows that the IGF-2 blocking antibody suppressed Akt phosphorylation. The same inhibition of Akt phosphorylation by the IGF-2 neutralizing antibody was made using primary islets (Fig. 5C). Thus, both in MIN6 cells and primary islets, activation of Akt phosphorylation by GLP-1 requires secretion of IGF-2 under these conditions. These data also indicated that an IGF-2/IGF-1R autocrine loop operated in β-cells, and its activity could be increased by GLP-1 by enhancing IGF-1R expression and by stimulating secretion in the presence of high glucose.
FIG. 5.
GLP-1–increased Akt phosphorylation depends on secreted IGF-2. A: MIN6 cells were transfected with an unrelated (Un) or an IGF-2–specific shRNA. Forty-eight hours later, they were stimulated with GLP-1 for 18 h to increase IGF-1R expression and then incubated with 2 mmol/l glucose for 2 h and for 1 h in either 2 or 20 mmol/l glucose. B: MIN6 cells were stimulated with GLP-1 for 18 h and then incubated with 2 mmol/l glucose for 2 h and for 1 h in either 2 or 20 mmol/l glucose and with nonspecific IgGs or an IGF-2 blocking antibody. C: Pancreatic islets were stimulated with GLP-1 for 18 h and then incubated with 2 mmol/l glucose for 2 h and for 1 h in either 2 or 20 mmol/l glucose and with nonspecific IgGs or an IGF-2 blocking antibody. A–C: Data are means ± SD, n = 3 independent experiments. **P < 0.01; ***P < 0.001. a.u., arbitrary units.
GLP-1 signaling protects β-cells against apoptosis by increasing the IGF-2/IGF-1R autocrine loop.
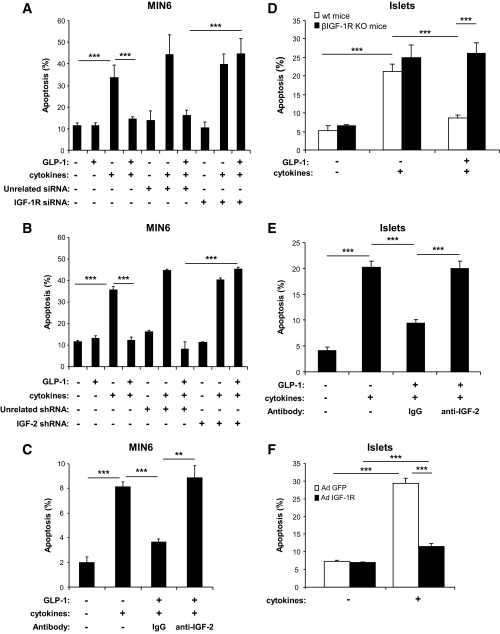
To determine whether the protective effect of GLP-1 against cytokine-induced apoptosis was dependent on activation of the IGF-2/IGF-1R autocrine loop, we measured apoptosis in MIN6 cells exposed to cytokines and treated or not treated with GLP-1. Figure 6A shows that cytokine-induced apoptosis was suppressed by treatment with GLP-1 and that this protective effect was blunted when IGF-1R expression was prevented by transfection of a specific siRNA. The same blunting of the GLP-1 protective effect was obtained when the cells were transfected with an shRNA that specifically suppressed IGF-2 expression (Fig. 6B) or when the cells were incubated in the presence of an IGF-2 blocking antibody (Fig. 6C).
FIG. 6.
GLP-1–induced protection against cytokine-induced apoptosis depends on activation of an IGF-2/IGF-1R autocrine loop. A: MIN6 cells were transfected with an unrelated or an IGF-1R siRNA, and 48 h later they were incubated in the presence or absence of GLP-1 for 4 h before addition of cytokines (IL-1β, 10 ng/ml; TNF-α, 25 ng/ml; IFN-γ, 10 ng/ml); the incubations were continued for 24 h in the continuous presence of GLP-1. B: MIN6 cells were transfected with an unrelated or an IGF-2 shRNA and then treated as described for A. C: MIN6 cells were incubated in the presence or absence of GLP-1 and in the presence of a nonspecific IgG fraction or an IGF-2 blocking antibody for 4 h before addition of cytokines (see above). D: Islets from β-cell–specific Igf-1R−/− mice or from their control littermates were incubated in the presence or absence of GLP-1 for 4 h before addition of cytokines (see above). E: Control islets were incubated for 4 h in the presence or absence of GLP-1 and with a nonspecific or an IGF-2 blocking antibody before cytokines treatment. F: Islets from GipR−/−;Glp-1R−/− mice were infected with a control adenovirus (Ad-GFP) or an IGF-1R–expressing adenovirus (Ad-IGF1-R) and 1 day later exposed to cytokines, as described in A. A–F: Data are means ± SD, n = 3 independent experiments. **P < 0.01; ***P < 0.001.
To determine whether GLP-1 protected primary islets using the same IGF-2/IGF-1R autocrine loop, we performed three sets of experiments. First, we tested whether GLP-1 protected islets from mice with a β-cell–specific IGF-1R gene inactivation against cytokine-induced apoptosis. As shown in Fig. 6D, apoptosis was not suppressed by GLP-1 in these islets—in contrast to the marked protection observed in control islets. Second, we showed that GLP-1 protection against cytokine-induced apoptosis was also suppressed when the islets were incubated in the presence of the neutralizing IGF-2 antibody (Fig. 6E). Third, we overexpressed the IGF-1R in islets from GipR−/−;Glp-1R−/− mice using a recombinant adenovirus (see Fig. S3 in the online appendix). Cytokine-induced apoptosis was markedly reduced in IGF-1R–overexpressing islets compared with control-infected cells (Fig. 6F). Thus, both in MIN6 cells and in primary islets, GLP-1 protected β-cells against cytokine-induced apoptosis by modulating the activity of the IGF-2/IGF-1R autocrine loop.
DISCUSSION
GLP-1 has previously been demonstrated to protect β-cells against apoptosis by increasing IRS-2 expression (14,16), PI 3-kinase activity (30), Akt phosphorylation (31) and Foxo1 phosphorylation, and nuclear exclusion (32). Our data are in agreement with these previous studies but present an integrated view of the operation of the GLP-1 and IGF-1R signaling pathways by providing novel findings on 1) the regulation of IGF-1R expression by GLP-1, 2) the predominant role of the IGF-1R compared with the IR in GLP-1–induced Akt phosphorylation, 3) the role of IGF-2 as an autocrine regulator of this signaling pathway, and 4) the modulation of this autocrine loop by GLP-1 as a basis for this hormone antiapoptotic effect.
Induction of IGF-1R expression by GLP-1 follows relatively slow kinetics, with a peak expression at ∼18 h. This is in contrast with the induction of immediate early genes, whose expression peaks between 30 and 60 min after initiation of GLP-1 treatment (21). Thus, increased expression of the IGF-1 receptor after GLP-1 treatment may be due to transcriptional or posttranscriptional regulations that are secondary to the initial wave of GLP-1–mediated transcriptional events. A detailed understanding of these mechanisms will, however, need further investigation. The slow induction of IGF-1R expression is thus an adaptive process, which, in vivo, may link the amount of GLP-1 secreted daily to the β-cell susceptibility to apoptosis. Because there is a link between feeding and GLP-1 secretion (33), reduced susceptibility to apoptosis may also participate in the increased β-cell mass observed in obesity. This slow induction kinetics should also be considered when using GLP-1 to protect β-cells from apoptosis after islet transplantation (34). To be efficacious, this treatment may need to be started before transplanting the islets to ensure full expression of the IGF-1R and maximal antiapoptotic protection.
A question that had not previously been addressed is the mechanism by which GLP-1–induced IRS-2 expression leads to activation of its downstream signaling pathway. Our data show that GLP-1–induced Akt phosphorylation was suppressed by knocking-down IGF-1R but not IR expression; furthermore, the relative efficacy of insulin and IGF-2 induction of Akt phosphorylation in cells stimulated by GLP-1 supported a primary role of the IGF-1R. Thus, our data indicate that the IGF-1R but not the IR lies upstream of activated Akt in our experimental conditions.
Phosphorylation of Akt in GLP-1–treated cells required secretion of IGF-2, as supported by several data. First, the IR is not involved in GLP-1–induced Akt phosphorylation, and insulin is therefore unlikely to be responsible for the autocrine activation of the IGF-1R; second, IGF-1 is not expressed in MIN6 cells nor in cell sorter–purified β-cells and is present at extremely low levels in isolated islets, which is in agreement with the reported expression of IGF-1 in non–β-cells (35); third, IGF-2 mRNA is detected at relatively high levels in primary islets and in cell sorter–purified β-cells, and its secretion can be stimulated by glucose, which is in agreement with previous data showing the presence of IGF-2 in insulin granules (29,36).
The importance of secreted IGF-2 in activating IGF-1R signaling was further demonstrated by showing that IGF-2 downregulation or neutralization with a specific antibody suppressed GLP-1–induced Akt phosphorylation. Furthermore, we showed that IGF-2 was responsible for GLP-1–induced protection against apoptosis, since this protective effect was suppressed by IGF-2 knockdown, by antibody-mediated blocking of secreted IGF-2, or by suppressing IGF-1R expression. This was observed both in MIN6 cells and in pancreatic islets.
This proposed autocrine role of IGF-2 in protecting β-cells against apoptosis is in agreement with previous data suggesting a role of this hormone in the physiological regulation of β-cells during embryonic development (36) and with the reported link between reduced IGF-2 expression by the pancreas during the fetal life and impaired β-cell function in the adult GK rat (37,38). Also, in the neonatal period, when the endocrine pancreas goes through a wave of β-cell apoptosis, there is a decreased expression of IGF-2 in the islets (39). This wave of apoptosis can, however, be prevented by transgenic expression of IGF-2 in peripheral tissues, which leads to elevated circulating plasma levels of this hormone (40). Also, transgenic expression of IGF-2 in β-cells leads to increased β-cell mass in adult mice (41). However, a role for IGF-2 in the control of adult islet function has not been previously established.
Therefore, several lines of evidence support an autocrine role for secreted IGF-2 in the control of β-cell mass both during embryonic development and after birth. Interestingly, recent genome-wide association studies have identified Igf2bp2 as a gene associated with increased incidence of type 2 diabetes (42). IGF2BP2 is an IGF-2 mRNA binding protein that regulates the translation of this mRNA (43), suggesting that the control of IGF-2 expression may also participate in regulating β-cell physiology.
Together, our studies provide an integrated view of the GLP-1 and IGF-1R signaling pathways. They show that an IGF-2/IGF-1R autocrine loop operates in β-cells and that GLP-1 increases its activity by augmenting IGF-1R expression, a long-term effect, and also by acutely stimulating IGF-2 secretion. These results therefore have important pathophysiological implications, since they can lead to alternative ways of preventing β-cell mass decrease: by modulating IGF-1R expression and/or expression and secretion of IGF-2.
Supplementary Material
Acknowledgments
This work was supported by grants to B.T. from the Swiss National Science Foundation (3100A0-113525), the Juvenile Diabetes Research Foundation (Program Project 7-2005-1158), and the European Union (Integrated Project Eurodia LSHM-CT-2006-518153, Framework Programme 6 [FP6] of the European Community).
No potential conflicts of interest relevant to this article were reported.
We thank the University of Lausanne DNA Array Facility for help with cDNA microarray experiments, D. Accili and A. Efstratiadis for β-IGF1-R knockout mice, and C.B. Wollheim for helpful comments on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bouwens L, Rooman I: Regulation of pancreatic beta-cell mass. Physiol Rev 2005; 85: 1255– 1270 [DOI] [PubMed] [Google Scholar]
- 2.Eizirik DL, Mandrup-Poulsen T: A choice of death: the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001; 44: 2115– 2133 [DOI] [PubMed] [Google Scholar]
- 3.Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802– 1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ: The biology of incretin hormones. Cell Metab 2006; 3: 153– 165 [DOI] [PubMed] [Google Scholar]
- 6.Thorens B: Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide I. Proc Natl Acad Sci U S A 1992; 89: 8641– 8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI: Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993; 133: 2861– 2870 [DOI] [PubMed] [Google Scholar]
- 8.Schuit FC, Pipeleers DG: Regulation of adenosine 3′,5′-monophosphate levels in the pancreatic B cell. Endocrinology 1985; 117: 834– 840 [DOI] [PubMed] [Google Scholar]
- 9.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano M, Matsura Y, Iwanaga T, Takai Y, Seino S: cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2000; 2: 805– 811 [DOI] [PubMed] [Google Scholar]
- 10.Dyachok O, Isakov Y, Sagetorp J, Tengholm A: Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 2006; 439: 349– 352 [DOI] [PubMed] [Google Scholar]
- 11.Gomez E, Pritchard C, Herbert TP: cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic β-cells. J Biol Chem 2002; 277: 48146– 48151 [DOI] [PubMed] [Google Scholar]
- 12.Kerchner KR, Clay RL, McCleery G, Watson N, McIntire WE, Myung CS, Garrison JC: Differential sensitivity of phosphatidylinositol 3-kinase p110gamma to isoforms of G protein betagamma dimers. J Biol Chem 2004; 279: 44554– 44562 [DOI] [PubMed] [Google Scholar]
- 13.Buteau J, Foisy S, Joly E, Prentki M: Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003; 52: 124– 132 [DOI] [PubMed] [Google Scholar]
- 14.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M: cAMP promotes pancreatic beta-cells survival via CREB-mediated induction of IRS2. Genes Dev 2003; 17: 1575– 1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF: Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest 2003; 112: 1521– 1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF: Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 2006; 281: 1159– 1168 [DOI] [PubMed] [Google Scholar]
- 17.Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D, Efstratiadis A: Defective insulin secretion in pancreatic β cells lacking type 1 IGF receptor. J Clin Invest 2002; 110: 1011– 1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR: Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet 2002; 31: 111– 115 [DOI] [PubMed] [Google Scholar]
- 19.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN: Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 2006; 38: 583– 588 [DOI] [PubMed] [Google Scholar]
- 20.Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP: Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation 1987; 43: 725– 730 [DOI] [PubMed] [Google Scholar]
- 21.Klinger S, Poussin C, Debril MB, Dolci W, Halban PA, Thorens B: Increasing GLP-1–induced β-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-α and DUSP14. Diabetes 2008; 57: 584– 593 [DOI] [PubMed] [Google Scholar]
- 22.de Fourmestraux V, Neubauer H, Poussin C, Farmer P, Falquet L, Burcelin R, Delorenzi M, Thorens B: Transcript profiling suggests that differential metabolic adaptation of mice to a high fat diet is associated with changes in liver to muscle lipid fluxes. J Biol Chem 2004; 279: 50743– 50753 [DOI] [PubMed] [Google Scholar]
- 23.Yang JY, Widmann C: Antiapoptotic signaling generated by caspase-induced cleavage of RasGAP. Mol Cell Biol 2001; 21: 5346– 5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B: A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 1998; 95: 2509– 2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Ordas R, Klein JD, Price SR: Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. J Appl Physiol 2008; 105: 1772– 1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Chin-Chance C, Lee EJ, Lowe WL, Jr: Activation of phosphatidylinositol 3-kinase contributes to insulin-like growth factor I-mediated inhibition of pancreatic beta-cell death. Endocrinology 2002; 143: 3802– 3812 [DOI] [PubMed] [Google Scholar]
- 27.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R: Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 1999; 19: 3278– 3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoog A, Hu W, Abdel-Halim SM, Falkmer S, Qing L, Grimelius L: Ultrastructural localization of insulin-like growth factor-2 (IGF-2) to the secretory granules of insulin cells: a study in normal and diabetic (GK) rats. Ultrastruct Pathol 1997; 21: 457– 466 [DOI] [PubMed] [Google Scholar]
- 29.Hoog A, Kjellman M, Nordqvist AC, Hoog CM, Juhlin C, Falkmer S, Schalling M, Grimelius L: Insulin-like growth factor-II in endocrine pancreatic tumours: immunohistochemical, biochemical and in situ hybridization findings. APMIS 2001; 109: 127– 140 [DOI] [PubMed] [Google Scholar]
- 30.Buteau J, Roduit R, Susini S, Prentki M: Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 1999; 42: 856– 864 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL: Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 2004; 47: 478– 487 [DOI] [PubMed] [Google Scholar]
- 32.Buteau J, Spatz ML, Accili D: Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes 2006; 55: 1190– 1196 [DOI] [PubMed] [Google Scholar]
- 33.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ: Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003; 88: 2706– 2713 [DOI] [PubMed] [Google Scholar]
- 34.Crutchlow MF, Yu M, Bae YS, Deng S, Stoffers DA: Exendin-4 does not promote beta-cell proliferation or survival during the early post-islet transplant period in mice. Transplant Proc 2008; 40: 1650– 1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith FE, Rosen KM, Villa-Komaroff L, Weir GC, Bonner-Weir S: Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc Natl Acad Sci U S A 1991; 88: 6152– 6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portela-Gomes GM, Hoog A: Insulin-like growth factor II in human fetal pancreas and its co-localization with the major islet hormones: comparison with adult pancreas. J Endocrinol 2000; 165: 245– 251 [DOI] [PubMed] [Google Scholar]
- 37.Calderari S, Gangnerau MN, Thibault M, Meile MJ, Kassis N, Alvarez C, Portha B, Serradas P: Defective IGF2 and IGF1R protein production in embryonic pancreas precedes beta cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 2007; 50: 1463– 1471 [DOI] [PubMed] [Google Scholar]
- 38.Serradas P, Goya L, Lacorne M, Gangnerau MN, Ramos S, Alvarez C, Pascual-Leone AM, Portha B: Fetal insulin-like growth factor-2 production is impaired in the GK rat model of type 2 diabetes. Diabetes 2002; 51: 392– 397 [DOI] [PubMed] [Google Scholar]
- 39.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ: A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 1999; 140: 4861– 4873 [DOI] [PubMed] [Google Scholar]
- 40.Hill DJ, Strutt B, Arany E, Zaina S, Coukell S, Graham CF: Increased and persistent circulating insulin-like growth factor II in neonatal transgenic mice suppresses developmental apoptosis in the pancreatic islets. Endocrinology 2000; 141: 1151– 1157 [DOI] [PubMed] [Google Scholar]
- 41.Devedjian JC, George M, Casellas A, Pujol A, Visa J, Pelegrin M, Gros L, Bosch F: Transgenic mice overexpressing insulin-like growth factor-II in beta cells develop type 2 diabetes. J Clin Invest 2000; 105: 731– 740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC: A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999; 19: 1262– 1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.