Abstract
OBJECTIVE
Mesenchymal stem cells (MSCs) are known to be capable of suppressing immune responses, but the molecular mechanisms involved and the therapeutic potential of MSCs remain to be clarified.
RESEARCH DESIGN AND METHODS
We investigated the molecular mechanisms underlying the immunosuppressive effects of MSCs in vitro and in vivo.
RESULTS
Our results demonstrate that matrix metalloproteinases (MMPs) secreted by MSCs, in particular MMP-2 and MMP-9, play an important role in the suppressive activity of MSCs by reducing surface expression of CD25 on responding T-cells. Blocking the activity of MMP-2 and MMP-9 in vitro completely abolished the suppression of T-cell proliferation by MSCs and restored T-cell expression of CD25 as well as responsiveness to interleukin-2. In vivo, administration of MSCs significantly reduced delayed-type hypersensitivity responses to allogeneic antigen and profoundly prolonged the survival of fully allogeneic islet grafts in transplant recipients. Significantly, these MSC-mediated protective effects were completely reversed by in vivo inhibition of MMP-2 and MMP-9.
CONCLUSIONS
We demonstrate that MSCs can prevent islet allograft rejection leading to stable, long-term normoglycemia. In addition, we provide a novel insight into the mechanism underlying the suppressive effects of MSCs on T-cell responses to alloantigen.
Mesenchymal stem cells (MSCs) were first isolated from bone marrow as stromal cells with characteristics of clonal expansion and were later identified as multipotent stem cells with the capability to differentiate into various kinds of mesodermal tissues (1–3). Aside from their roles in contributing to local microenvironments and supporting hematopoiesis in bone marrow (4–6), MSCs can suppress T-cell proliferation in response to nominal antigens or alloantigens (7). MSC-mediated inhibition is also associated with a reduction in inflammatory cytokine production (8).
Several soluble factors have been implicated in the mechanism of MSC-mediated immunosuppression, including transforming growth factor-β, indoleamine 2,3-dioxygnase (IDO) (9), nitric oxide (NO) (10), and heme oxygenase (HO)-1 (11). However, because of subtle differences in culture conditions, passage numbers, and the input populations used in different studies, direct comparisons are difficult and the mechanisms of MSC-mediated suppression remain elusive. The precise role played by specific molecules and whether this function is an exclusive property of MSCs remain to be clarified.
The use of MSCs for the treatment of graft versus host disease (GvHD) after allogeneic bone marrow transplantation has produced encouraging clinical results. Coinfusion of MSCs with allogeneic hematopoietic stem cells can accelerate hematopoietic recovery and prevent severe GvHD refractory to conventional immunosuppressive therapy (12,13). MSCs may also have significant therapeutic potential for the treatment of autoimmune disease, such as experimental autoimmune encephalomyelitis (14), collagen-induced arthritis (15), autoimmune enteropathy (16), and experimental colitis (17), and in experimental transplantation MSCs are capable of prolonging the survival of heart and skin allografts (18,19).
Islet transplantation offers an attractive and relatively noninvasive therapy for patients with type 1 diabetes, but although the Edmonton protocol and its derivatives have shown great promise in terms of restoration of normoglycemia, they do not appear to provide long-term insulin independence since most patients require exogenous insulin within 2 years posttransplant (20,21). However, the immunosuppressive effects of MSCs may have a particular appeal as an adjunct therapy in islet transplantation because both clinical and experimental procedures lend themselves to islet/MSC codelivery. In this study, we demonstrate that MSCs can attenuate T-cell–mediated rejection responses in a life-sustaining allogeneic islet transplant model and shed new light on the mechanisms involved.
RESEARCH DESIGN AND METHODS
Mice.
CBA.Ca (CBA, H2k), C57BL/6 (B6, H2b), BALB/c (H2d), and BALB/c Rag−.−γ−.− mice were bred and housed in the Biomedical Services Unit of John Radcliffe Hospital and were aged 6–8 weeks at the time of the first procedure.
MSCs.
BALB/c (H2d) bone marrow cells were flushed from femurs and tibias and cultured at 37°C with 5% CO2 in DMEM supplemented with 15% FCS, 2 mmol/l l-glutamine plus penicillin (6 μg/ml) and streptomycin (100 units/ml), and 0.5 mmol/l 2-β-mercaptoethanol. Primary MSCs were identified by morphology, phenotypic analysis, and the capability to differentiate into adipocytes and osteoblasts under appropriate culture conditions as described previously (1,22).
Flow cytometry.
Cell surface markers were stained using established techniques. For analysis of intracellular plus surface CD25, cells were stained for surface CD25 in the conventional manner then fixed and permeabilized for 30 min at 4°C (eBioscience) and incubated with CD25 antibody for intracellular staining at 4°C for 30 min before acquisition.
Delayed-type hypersensitivity assay.
For this assay, 7 × 106 C57BL/6 splenocytes as responders or 7 × 106 irradiated (20 Gy) CBA splenocytes as stimulators were injected into the ear pinnae of immunodeficient BALB/c Rag−.−γ−.− mice either alone or together in the presence or absence of 1 ×105 MSCs using 30-gauge insulin syringes. Ear thickness was measured using a spring-loaded micrometer before and 48 h after injection. Relative delayed-type hypersensitivity (DTH) responses were calculated after 48 h, as previously described (23).
Islet isolation.
Donor CB57L/B6 mice were killed by cervical dislocation. Collagenase (0.25 mg/ml, Liberase RI; Roche Diagnostics) was infused via the common bile duct. Pancreata were retrieved and digested for 10 min at 37°C followed by vigorous agitation. Islet purification was performed by Ficoll gradient centrifugation at 1,230g for 22 min. Islets were visualized by dithizone staining.
Adoptive transfer of cells and islet transplantation.
BALB/c Rag−.−γ−.− mice were reconstituted intravenously with 1 × 105 naïve BALB/c CD4+CD25− cells followed by transplantation of 500 B6 islets mixed with or without 4 × 105 BALB/c MSCs beneath the kidney capsule. Blood glucose levels were determined using a blood glucose meter (Roche Diagnostics). Rejection was defined as a blood glucose of >14.5 mmol/l for at least 2 consecutive days. In some experiments, graft dependence was determined by removing the kidney containing the transplanted islets.
Visualization of transplanted islets.
Kidneys were frozen in Tissue-Tek OCT compound (Sakura) and 8-μm sections stained with a guinea pig anti-insulin antibody followed by a fluorescein isothiocyanate (FITC)–conjugated anti–guinea pig second stage (both DakoCytomation). Nuclear counterstaining was performed using Hoechst dye, and sections were viewed with a Leica confocal microscope.
Statistics.
Data, given as means ± SE, were obtained from at least three individual experiments, and statistical comparisons were performed using a Student's t test. Allograft survival between groups was compared using the log-rank test (Graphpad Prism).
RESULTS
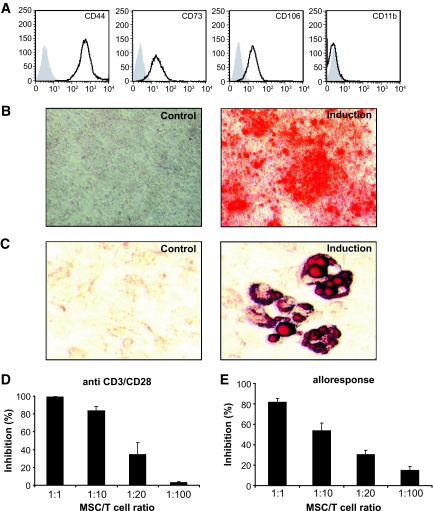
Primary BALB/c MSCs cultured from adherent bone marrow cells had a spindle morphology and exhibit a classical MSC phenotype in that they are positive for CD44, CD73, and CD106 but negative for CD11b (Fig. 1A) and positive for Alizarin red and Oil Red O staining consistent with osteogenic and adipogenic precursors, respectively (Fig. 1B and C).
FIG. 1.
MSCs suppress T-cell proliferation in response to anti-CD3/CD28 cross linking or allogeneic antigen stimulation. A: MSCs obtained from bone marrow cells of BALB/c (H2d) mice show spindle-shaped morphology and exhibit a typical MSC phenotype (solid line), in which they are positive for CD44, CD73, and CD106 but negative for CD11b. Isotype controls are represented as the shaded curve. B: MSCs were able to differentiate into osteogenic and adipogenic lineages when subjected to inductive stimuli (B and C, induction) but not in control medium (B and C, control). Osteogenic differentiation of MSCs was verified by Alizarin red staining (B). The presence of intracellular fat droplets detected by Oil Red O staining indicates induced adipogenesis (C). D: CD4+CD25− T-cells were purified from C57BL/6 (H2b) mice using CD25 MicroBeads. Standard 3-day cultures were set up in 96-well round-bottomed plates using 2 × 105 CFSE-labeled responder T-cells stimulated by CD3/CD28 Dynalbeads at a 1:1 bead:cell ratio in the presence of graded numbers of MSCs. CFSE division history was analyzed by flow cytometry. The percentage of proliferated cells was calculated by dividing the number of cells undergoing divisions with the total T-cells in the cultures. E: For mixed leukocyte cultures, 2 × 105 CFSE-labeled CD4+CD25− T-cells were cocultured with 5 × 105 irradiated (3,600 rad) CBA.Ca (H2k) splenocytes for 5 days. All the data above were obtained from three independent experiments. A high-quality digital representation of this figure is available in the online issue.
MSCs induce interleukin-2 hyporesponsiveness in T-cells.
To examine the effect of MSCs on T-cell responses in vitro, T-cells were stimulated with anti-CD3/CD28 beads with or without graded numbers of MSCs. As shown in Fig. 1D, MSCs mediated suppression of T-cell proliferation in a dose-dependent manner, resulting in a 83.5 and 34.3% inhibition at a ratio of 1:10 or 1:20 (MSC:T-cell), respectively. More importantly, MSCs were capable of inhibiting T-cell responses driven by alloantigen. As shown in Fig. 1E, the addition of MSCs at a ratio of 1:10 reduced the allogeneic T-cell response by 53.7% and at a ratio of 1:1 abolished the proliferative response by 81.8%. In a comparison with conventional CD4+CD25+ naturally occurring Treg, preliminary experiments indicate that on a cell-per-cell basis, MSCs appear to be approximately two to four times more potent in terms of inhibition of in vitro allogeneic T-cell responses (data not shown).
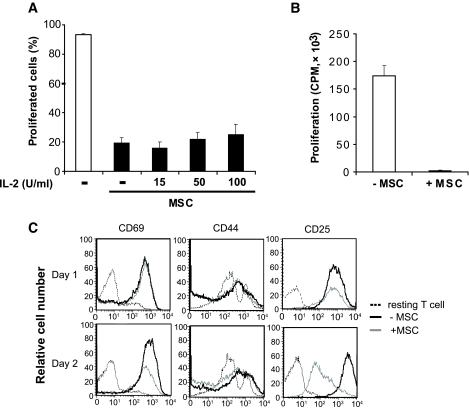
In many situations of in vitro T-cell hyporesponsiveness, T-cell proliferation can be restored by the addition of exogenous interleukin (IL)-2 (24,25). Therefore, we asked whether the MSC-mediated suppressive activity on T-cell proliferation could be rescued by the addition of IL-2. Carboxyfluorescein succinimidyl ester (CFSE)-labeled T-cells were stimulated in the presence or absence of MSCs as in Fig. 1D and proliferation determined by CFSE dilution. As shown in Fig. 2A, in the absence of MSCs, virtually 90% of the T-cells proliferated, but this was reduced to ∼10% by the addition of MSCs. Surprisingly, proliferation was not restored by the addition of exogenous IL-2. We then evaluated whether stimulated T-cells might regain their responsiveness to IL-2 after MSCs were removed. Following stimulation with anti-CD3/CD28 beads in the presence or absence of MSCs, T-cells were reisolated by positive selection then incubated with exogenous IL-2. As shown in Fig. 2B, while cells stimulated in the primary culture without MSCs proliferated vigorously in response to IL-2 in the secondary cultures, cells stimulated initially in the presence of MSCs were unable to respond to exogenous IL-2. Thus, MSC-mediated IL-2 unresponsiveness appears to be a relatively stable phenomenon.
FIG. 2.
T-cell blasts cocultured with MSCs exhibited hyporesponsiveness to exogenous IL-2 and lost surface expression of CD25. A: CFSE-labeled CD4+CD25− T-cells from naïve C57BL/6 mice (H2b) were cultured at a density of 2 × 105 per well in 96-well plates and stimulated with anti-CD3/CD28 beads alone or in the presence of MSCs (2 × 104). Exogenous IL-2 was added at concentrations of 15, 50, and 100 IU/ml. After 72 h, cell proliferation was analyzed by flow cytometry. Data are represented as means ± SD. Results are the average of three experiments of identical design. B: Bars show the SD CD4+CD25− T-cells were stimulated with anti-CD3/CD28 beads alone or in the presence of MSCs (MSC:T-cell ratio of 1:10) for 72 h. CD4+ T-cells were purified by MACs anti-CD4 beads and cultured with 50 IU/ml IL-2 for 72 h. T-cell proliferation was measured by 3H-Tdr incorporation added during the last 8 h of culture. C: T-cells stimulated with anti-CD3/CD28 beads in the presence or absence of MSC and harvested after 24 and 48 h. Surface expression of CD69, CD44, and CD25 was evaluated by flow cytometry. Histograms are representative of three independent experiments.
Activated T-cells lose surface expression of CD25 in the presence of MSCs.
In an attempt to explore this phenomenon further, we examined the expression of three surface markers that are upregulated as a consequence of activation (CD69, CD44, and CD25). As shown in Fig. 2C, while the presence of MSCs had no effect on the upregulation of CD69 or CD44, the addition of MSCs resulted in a significant reduction in CD25 expression after 2 days. This effect seemed to be specific to CD25 since the expression of CD122 (IL-2Rβ chain) and CD132 (IL-2Rγ chain) were unaffected by coculture (data not shown). Taken together, these data suggest that the MSC-dependent inhibition of both T-cell proliferation and responsiveness to IL-2 may be due to a downregulation of CD25.
The reduced expression of CD25 on T-cells by MSCs is not related to receptor internalization or transcriptional regulation.
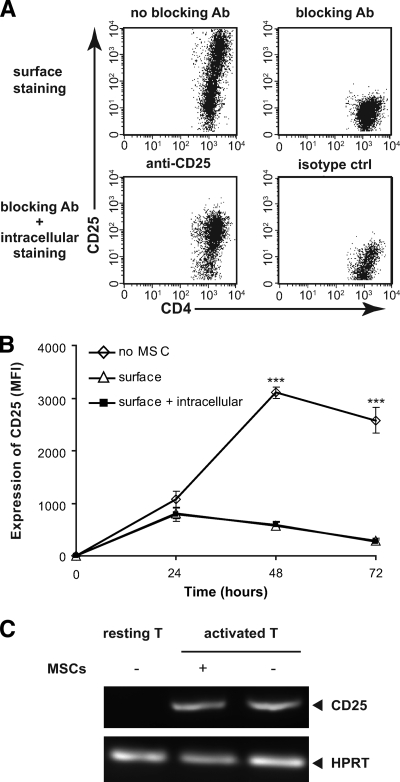
Receptor internalization of surface receptors into endocytic vesicles following ligand-induced activation serves as an important mechanism in the autonomous regulation to ligand stimulation and can dramatically reduce receptor expression (26). To ask whether this could explain the loss of surface CD25 on T-cells in the presence of MSCs, T-cells were stimulated with anti-CD3/CD28 beads and cocultured with MSCs followed by extracellular staining or extracellular plus intracellular staining for CD25. As a positive control for the detection of intracellular CD25, activated T-cells were incubated in the absence of MSCs with an excess of unconjugated anti-CD25 antibody (PC61) to block surface expression then stained with PC61-Pe with or without permeabilization. This approach completely abolishes cell surface staining (Fig. 3A, upper right panel), and thus the staining shown in Fig. 3A, lower left panel is attributable entirely to the detection of intracellular CD25. As shown in Fig. 3B, while surface CD25 expression was upregulated in the absence of MSCs, and reduced significantly in the presence of MSC, this was not explained by an increase in intracellular CD25 since the surface and surface plus intracellular profiles were essentially identical. Thus, receptor internalization does not appear to explain the MSC-dependent reduction in CD25 expression.
FIG. 3.
Reduced expression of surface CD25 in the presence of MSCs is not related to receptor internalization or reduced transcriptional activity. A: As a positive control for intracellular CD25 detection, activated T-cells were incubated with unconjugated PC61 then stained with PC61-Pe with or without permeabilization or with an isotype control. B: CD4+CD25− T-cells from naïve C57BL/6 mice were stimulated with anti-CD3/CD28 beads and cocultured with MSCs for 24, 48, and 72 h followed by extracellular staining or extracellular plus intracellular staining for CD25. The surface expression of CD25 (MFI) was significantly reduced at 48 and 72 h in the presence of MSCs, which was not explained by an increase in intracellular CD25, since the surface and surface plus intracellular profiles are essentially identical. C: T-cells were stimulated with anti-CD3/CD28 beads in the presence or absence of MSCs at the MSC:T-cell ratio of 1:10. Total mRNA was extracted from bead-purified T-cells after coculture or from naïve T-cells, and cDNA was synthesized using CMV reverse transcriptase. For PCR, CD25 primers were as follows: CD25 forward primer 5′-GAA GCC AAC ACA GTC TAT GCA CC-3′ and CD25 reverse primer 5′-TGT CCT TCC ACG AAA TGA TAG ATT C-3′; and HPRT forward primer 5′-ATC ATT ATG CCG AGG ATT TGG AA-3′ and HPRT reverse primer 5′-TTG AGC ACA CAG AGG GCC A-3′.
We then asked whether the reduced expression of CD25 induced by coculture with MSCs was related to a reduction of CD25 transcripts during T-cell activation. Total RNA was extracted and cDNA synthesized from either resting T-cells or T-cells stimulated with anti-CD3/CD28 beads in the presence or absence of MSCs. The transcriptional level of CD25 was determined by RT-PCR. Significantly, the reduction expression of CD25 induced by coculture with MSCs was not reflected by changes at the transcript level (Fig. 3C), suggesting that the effect of MSCs on CD25 expression appears to be restricted to the cell surface.
MSC-mediated suppression is completely reversed by blocking MMP-2 and MMP-9.
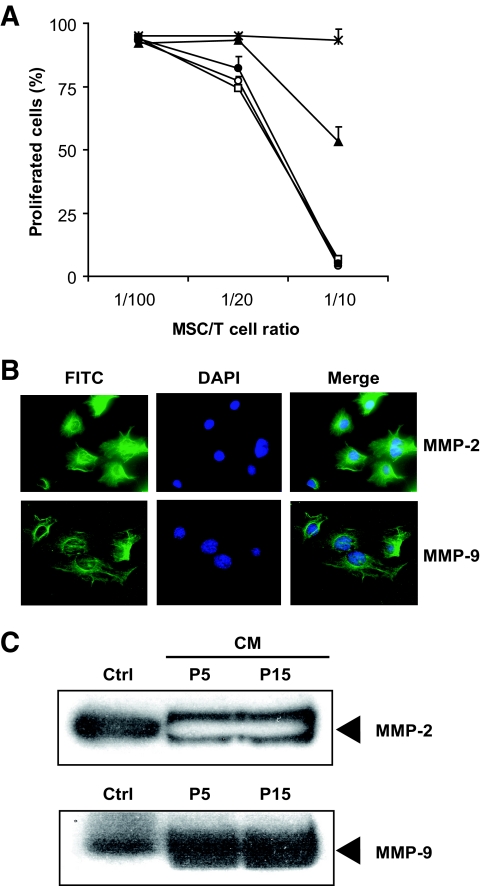
It has previously been suggested that the suppression of T-cell proliferation mediated by MSCs is dependent on IDO (27), NO (10), and HO-1 (11). To determine whether these molecules play a role in the current system, specific inhibitors were added to the cultures of T-cells stimulated in the presence of graded numbers of MSCs. The inhibitors were used at concentrations previously reported to compromise suppression by MSCs. Inhibition of IDO (1-MT) and HO-1 (SnPP) had no effect individually on MSC-mediated T-cell suppression (Fig. 4A). Addition ofl-NG-monomethyl arginine citrate (l-NMMA), an inhibitor of NO synthase, partially restored proliferation at an MSC:T-cell ratio of 1:10, indicating that the MSC effect is at least partially dependent on NO. Significantly, however, the inhibition of proliferation was completely reversed by addition of SB-3CT, a specific inhibitor of MMP-2 and MMP-9, implicating these enzymes as necessary and sufficient for the inhibitory activity of MSCs.
FIG. 4.
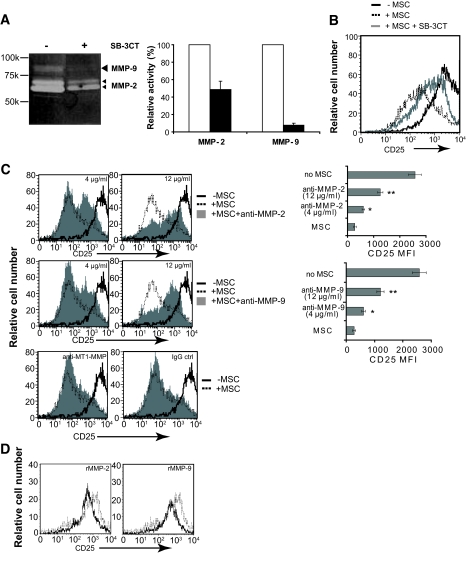
MMP-2 and MMP-9 are involved in the immunosuppression by MSCs. A: CFSE-labeled CD4+CD25− T-cells stimulated with anti-CD3/CD28 were cultured for 72 h in the presence or absence of MSCs. Cell cultures were treated with 1 mmol/l 1-MT or 1 mmol/l l-NMM or 1 mmol/l SnPP or 6 μmol/l SB-3CT. Proliferation was accessed by dilution of CFSE fluorescent intensity. Data from three independent experiments each performed in triplicate are represented as means ± SD. □, control; ○, 1-MT; ▴, l-NMMA; ●, SnPP; *SB-3CT. B: MSCs were seeded on 13-mm glass coverslips and fixed, permeabilized, and blocked with serum and incubated with mouse anti–MMP-9 or anti–MMP-2 monoclonal antibodies. Binding was visualized using an Alexa Fluro 488 FITC-conjugated secondary, and cells were counterstained with Hoechst 33342. Sections were examined using a Leica epifluorescence microscope. C: To collect conditioned medium, MSCs were washed with PBS three times and resupplied with serum-free medium for 24 h. After harvest, the MSC-conditioned medium was spun at 1,500g to remove cellular debris, loaded on SDS-PAGE gels, and blotted with monoclonal anti–MMP-2 or –MMP-9 antibodies. Recombinant mouse MMP-2 and MMP-9 were used as markers. Data shown are representative of three independent experiments. A high-quality digital representation of this figure is available in the online issue.
MMP-2 and MMP-9 are expressed and secreted into the culture by MSCs.
To determine whether the MSC used in this study express MMP-2 or MMP-9, MSCs were stained with specific antibodies and examined by fluorescence microscopy. As shown in Fig. 4B, intracellular MMP-2 and MMP-9 on MSCs were readily detected in MSC cultures. To determine whether these enzymes were secreted as soluble forms, we collected the medium conditioned by MSCs that had undergone 5 (P5) or 15 passages (P15) and performed a Western blot for MMP-2 and MMP-9. As shown in Fig. 4C, MSCs secrete both isoforms as early as passage 5.
MMP-2 and MMP-9 from MSCs contribute to the loss of expression of CD25 on T-cells.
MMPs are zinc-dependent enzymes that bind histidine-containing motifs in components of the extracellular matrix and play a crucial role in embryogenesis, wound healing, and tissue remodeling (28–30). It has been determined that MMP-2 and MMP-9 may also play a role in immunological evasion of cancer cells by clearing CD25 from T-cell surfaces (31). The data in Figs. 4B and C confirm that the MSCs used in the present study contain and secrete MMP-2 and MMP-9, and the data in Fig. 4A are consistent with a role for these enzymes in MSC-dependent inhibition of T-cell responses by cleavage of CD25 from the cell surface. To verify that in our hands inhibition of MMP-2 and MMP-9 results in a detectable inhibition of MMP-dependent proteolysis, conditioned medium from MSC cultures was separated by SDS polyacrylamide electrophoresis in a gel containing gelatin as an MMP substrate and analyzed by zymography (32). The resultant gel was then incubated overnight at 37°C and the position-specific gelatin content determined by densitometry. Addition of SB-3CT at a concentration of 6 μmol/l resulted in a 52.4 and 90.5% inhibition of MMP-2 and MMP-9, respectively (Fig. 5A). To determine whether MMP-2 and/or MMP-9 can influence the surface expression of CD25 (Fig. 2C), bead-stimulated T-cells were cultured with MSCs in the presence or absence of 6 μmol/l SB-3CT and CD25 expression evaluated by flow cytometry. As shown in Fig. 5B, the inhibitor partially reversed the loss of CD25, and although the effect was not complete, the fact that the same concentration of inhibitor caused a complete abrogation of MSC dependent T-cell inhibition (Fig. 4A) suggests that maximal levels of CD25 expression are not required for optimal responses to IL-2.
FIG. 5.
MSC-dependent reduction in CD25 expression by T-cells is mediated by MMP-2 and MMP-9. A: Enzymatic activities of MMP-2 and MMP-9 in medium conditioned by MSCs were analyzed by zymography. Conditioned medium was electrophoresed under nonreducing conditions and without heating through a 8% SDS-PAGE containing 0.1% gelatin. Following electrophoresis, the gels were incubated overnight in developing buffer containing SB-3CT to inhibit MMP activity. Gels were stained with Coomassie Blue, and densitometric quantification of MMP-2 and MMP-9 was performed using Scion software. Data were obtained from three individual experiments, and inhibition of MMP-2 and MMP-9 was determined by setting the mock treatment as 100%. □, control; ■, SB-3CT. B: To block activities of MMP-2 and MMP-9 in the MSC/T-cell coculture, SB-3CT was added at a concentration of 6 μmol/l. Stimulated T-cells alone (black curve) or cocultured with MSCs (dashed curve) or MSCs treated with SB-3CT (gray curve) were examined for surface expression of CD25. C: Stimulated T-cells were either cultured alone (black curve) or in the presence of MSCs (MSC/T-cell ratio of 1:10) (dashed curve). In some experiments, neutralizing monoclonal anti–MMP-2 or –MMP-9 antibodies were added (gray shaded). Neutralizing antibody was used to block the activity of MT1-MMP. IgG1 was used as an isotype control. T-cells were harvested at 72 h, stained with CD25-PE, and analyzed by flow cytometry. D: CD4+CD25− T-cells were activated by 10 μg/ml ConA for 24 h, washed three times to remove residual ConA, and resuspended in serum-free medium at final concentration of 2 × 105/ml. MMP-2 and MMP-9 recombinant proteins were added at final concentration of 1 mg/ml. T-cells were incubated with MMP recombinant proteins for an additional 6 h. MMP-2 or MMP-9 treated cells (solid curve) and untreated cells (dotted curve) were analyzed for CD25 expression by flow cytometry. Histograms of CD25 expression on T-cells are representative of three independent experiments.
To determine whether both MMP isoforms contribute to this effect, monoclonal antibodies specific for either MMP-2 or MMP-9 were added to bead-stimulated T-cells cultured with MSCs. As shown in Fig. 5C, while the CD25 median fluorescence intensity (MFI) of T-cells cultured in the absence of MSCs was 2,577 ± 244, this fell to 281 ± 55 in the presence of MSC. Significantly, addition of anti–MMP-2 resulted in a dose-dependent partial reversal of this effect with 4 and 12 μg/ml restoring CD25 expression to an MFI of 605 ± 45 and 1,252 ± 102, respectively (Fig. 5C, upper panel). A similar result was obtained by addition of anti–MMP-9 antibody (Fig. 5C, middle panel). Importantly, addition of an antibody to MT1-MMP, an isoform that has previously been shown to be expressed by MSCs (33), had no effect on CD25 expression. A direct effect of MMP-2 and MMP-9 on CD25 expression is shown by the fact that addition of purified recombinant proteins led to a modest but detectable loss of CD25 expression by T-cells (Fig. 5D). Taken together, these data clearly implicate both MMP-2 and MMP-9 in the MSC-mediated downregulation of CD25 expression and the inhibition of T-cell responses.
Inhibition of MMP-2 and MMP-9 restores T-cell responses to IL-2.
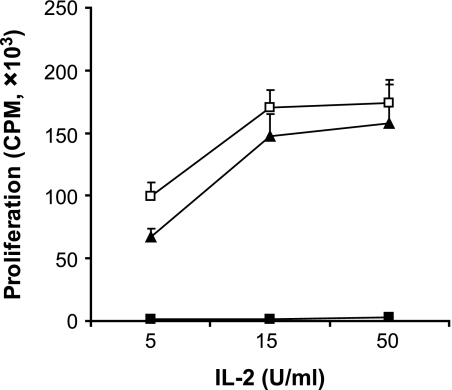
To determine whether inhibition of MMP-2 and MMP-9 has a direct functional effect on T-cell responses, CD4+ T-cells stimulated in primary culture in the presence of MSCs were reisolated and recultured for 72 h in the presence of 5, 15, and 50 IU/ml exogenous IL-2. As shown in Fig. 6, T-cells stimulated in the primary culture without MSCs proliferated vigorously in response to IL-2 in secondary culture, in clear contrast to the poor proliferation of cells stimulated initially in the presence of MSCs. However, inhibition of MSC MMP-2 and MMP-9 in the primary culture resulted in essentially normal secondary responses to IL-2, providing a direct functional link between the suppressive effect of MSC MMP activity and CD25 expression, thereby suggesting that these enzymes may contribute to the mechanism of MSC-mediated immunosuppression.
FIG. 6.
Inhibition of MMP-2 and -9 reverses MSC-induced T-cell nonresponsiveness to IL-2. Anti-CD3/CD28–stimulated T-cells were cultured alone or in the presence of untreated MSCs or MSCs treated with SB-3CT. After 72 h, CD4+ T-cells were purified using MACs anti-CD4 beads and incubated with IL-2 at concentrations of 5, 15, and 50 IU/ml. Proliferation was measured by 3H-Tdr incorporation. Data from three independent experiments, each performed in triplicates, are presented as means ± SD. □, −MSC; ■, +MSC; ▴, +MSC + SB-3CT.
MMP-2 and MMP-9 are critical in MSC-mediated suppression in vivo.
To test the hypothesis that MMPs play a functional role in MSC-mediated suppression in vivo, we used two models of T-cell responses to alloantigens. In the first, we used the trans vivo DTH assay, where ear swelling provides a read-out of T-cell responses (34), and in the second, we used life-sustaining allogeneic islet transplants in diabetic mice (35). In the DTH assay, allogeneic stimulator and responder cells were injected into the ear pinnae of immune-deficient mice in the presence or absence of MSCs and with or without the MMP inhibitor SB-3CT. As shown in Fig. 7A, coinjection of responders and stimulators resulted in a threefold increase in ear swelling compared with that induced by responders only. This DTH response was virtually abolished by coinjection of MSC, thus demonstrating a clear functional effect of these cells in modulating allogeneic responses in vivo (***P = <0.001). Significantly, however, administration of the SB-3CT (6 μmol/l) in the presence of MSCs reversed this effect, resulting in virtually unmodified DTH responses (***P = <0.001).
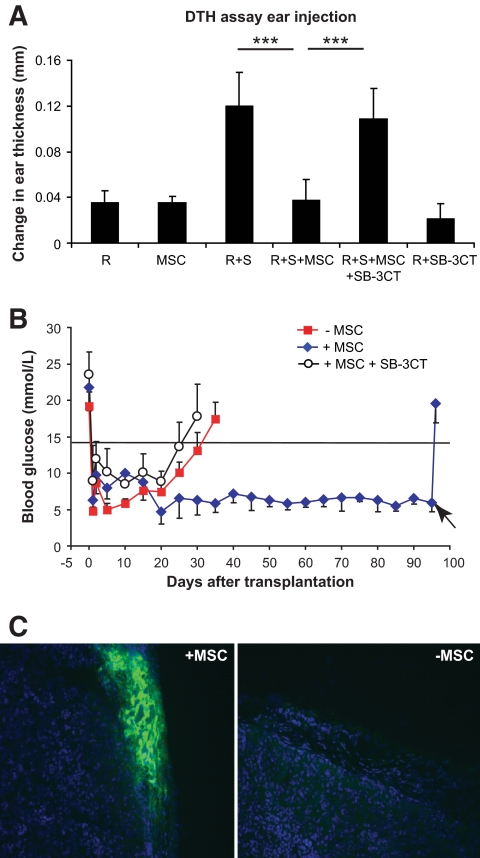
FIG. 7.
Prevention of DTH responses and protection of allogeneic islet grafts by MSCs is dependent on MMP-2 and MMP-9. A: 7 × 106 responder cells (C57BL/6) or 7 × 106 irradiated stimulator cells (CBA.Ca) were injected into the ear pinnae of BALB/c Rag−.−γ−.− mice either alone or together in the presence or absence of 1 ×105 MSCs. DTH response–induced ear swelling was calculated by subtracting the thickness before injection from the thickness after 48 h. Administration of MSCs led to profound reduction of the alloreactive DTH response, which was reversed in the presence of the inhibitor SB-3CT. Data shown are means ± SD of a representative of at least three independent experiments. B: BALB/c Rag−.−γ−.− were rendered diabetic by a single intravenous injection of 200 mg/kg STZ. Mice had an average blood glucose concentration of 20 mmol/l immediately prior islet transplantation. A total of 500 IE pancreatic islets from CB57/B6 mice (H2b) alone or in the presence of 4 × 105 BALB/c MSCs (H2d) were transplanted under the kidney capsules of STZ-induced diabetic BALB/c Rag−.−γ−.−. In some experiments, MSCs were pretreated with 6 μmol/l SB-3CT for 48 h and mice receiving treated MSCs also received SB-3CT (25 μg/mouse) intraperitoneally once every 4 days from the day of islet transplant until rejection. All islet transplant recipients were reconstituted with 1 × 105 naïve BALB/c CD4+CD25− T-cells as an effector T-cell population. Rejection was defined as a blood glucose >14.5 mmol/l for at least 2 consecutive days. Continued function of the islet allografts was confirmed by removal of the islet-bearing kidneys and a return to hyperglycemia. C: Kidneys transplanted with islets grafts were excised at the time of rejection or the end point of this study. Immunofluorescence examination of islet grafts in MSC-treated recipients revealed intensely staining insulin producing islets beneath the kidney capsule, whereas in the MSC-untreated recipients the insulin-positive islet tissue was not detected at the time of rejection. A high-quality digital representation of this figure is available in the online issue.
One of the striking characteristics of MSC-mediated suppression is the capability of these cells to prolong the survival of skin allografts (18) and heart (19). However, these grafts are physiologically passive and thus provide only a limited indication of the potential of MSCs. As a more rigorous test, we asked whether the immunosuppressive effects of MSCs would lead to the survival of life-sustaining islet allografts in streptozotocin (STZ)-induced diabetic recipients. C57BL/6 (H2b) pancreatic islets were transplanted under the kidney capsule of diabetic BALB/c Rag−.−γ−.− mice, which were reconstituted intravenously with 1 × 105 CD4+CD25− BALB/c T-cells as an effector population. Blood glucose was monitored daily, and diabetes was defined by a level of 14.5 mmol/l on 2 successive days. Transplantation of 500 islets resulted in a rapid return to normoglycemia (Fig. 7B), but mice transplanted with islets alone become profoundly diabetic within 30–35 days (median survival time [MST] = 30 days, n = 6) as a result of unmodified allograft rejection. In striking contrast, administration of 1 × 105 syngeneic MSCs mixed with the islets at the time of transplantation prevented rejection, and all animals remained normoglycemic long term (n = 6). That this was due solely to survival of the transplanted islets and not to endogenous islet regeneration is demonstrated by the fact that removal of the transplanted islets by nephrectomy at day 95 (Fig. 7B, arrow) resulted in an abrupt return to hyperglycemia. Significantly, insulin-positive islets were readily detected in these excised kidneys but were virtually absent from mice transplanted without administration of MSCs (Fig. 7C).
To ask whether this protective effect is dependent on the activity of MMP-2 and MMP-9, a third group of diabetic recipients was transplanted with allogenic islets reconstituted with effector cells and MSCs, but these animals also received the MMP-2 and MMP-9 inhibitor SB-3CT (25 μg/mouse) intraperitoneally once every 4 days from the day of islet transplant until rejection. All animals in this group became profoundly diabetic within 30 days (MST = 30 days, n = 4), again implicating MMP-2 and MMP-9 in MSC-mediated immunosuppression in vivo (Fig. 7B).
DISCUSSION
Emerging evidence indicates that MSCs can suppress T-cell responses both in vitro and in vivo, but the mechanisms underlying these effects are not yet clearly defined. In this study, we have confirmed the ability of MSCs to inhibit T-cell proliferation and have shown that coculture with MSCs results in impaired T-cells responses to IL-2. The basis of this hyporesponsiveness appears to be due to a preferential loss of CD25 (the α-chain of the IL-2 receptor) from the cell surface, and, significantly, this was not explained either by receptor internalization or a reduction in CD25 expression at the transcriptional level. In an examination of candidate molecules that have been implicated in MSC-mediated suppression (9–11), we were intrigued by the observation that addition of the MMP-2– and MMP-9–specific inhibitor, SB-3CT, reversed the suppression by MSCs. Whereas inhibition of IDO, HO-1, or NO led to either no effect or only partially restored T-cell proliferation, inhibition of MMP-2 and MMP-9 in the primary coculture of T-cells with MSCs restored T-cell responses to IL-2 in secondary MSC-free cultures, and this was associated with restored levels of CD25 expression. Critically, the role of MMP-2 and MMP-9 in MSC-mediated suppression in vivo was demonstrated by the DTH assay, which provides an indirect read-out of T-cell responses. More persuasively, however, the effect of MSC was also demonstrated in a life-sustaining islet transplant model, where sustained normogylcemia provides a direct and physiologically relevant indication of attenuated T-cell responses. Overall, the data suggest that one of the principal explanations for the immunosuppressive capacity of MSCs is the cleavage of CD25 from the T-cell surface by MSC-secreted MMPs. Precisely where this takes place in this model is not clear at present, but given the fact that the MSCs are colocalized at the graft site, we would argue that their major effect is mediated within the graft itself. We predict, therefore, that the MSCs may have little effect on T-cell sensitization in secondary lymphoid tissues but rather that MMP-dependent cleavage of CD25 from the T-cell surface is restricted to the peritransplant area and thus limits prolonged T-cell activation. We are currently testing this hypothesis by looking for differential levels of CD25 on T-cells from the graft site and the draining lymph nodes.
Besides their important role in modification of the extracellular matrix, cell–extracellular matrix interactions, and cell mobilization, MMPs have been identified to possess a broader range of substrate specificity that includes cytokines and other inflammatory mediators, with consequent effects on regulation of the immune response and cell proliferation (30,36). In the field of cancer, MMP-2 and MMP-9 have been shown to play a direct role in tumor-mediated immunosuppression by cleaving CD25 from the T-cell surface (31), and our study suggests that while these enzymes that have a detrimental effect on tumor immunity, they may explain the beneficial effects of MSCs on the control of GvHD and allograft rejection.
In view of their immunosuppressive effects on the immune system, MSCs have significant therapeutic potential for the treatment of immune-related diseases that are refractory to conventional medical therapy. For example, pilot clinical studies have shown that MSCs are effective in the treatment of refractory acute GvHD, a severe complication that can occur following allogeneic bone marrow transplantation (12). MSCs have also been shown to be useful in the treatment of autoimmune diseases including experimental autoimmune encephalomyelitis (14), collagen-induced arthritis (15), and autoimmune enteropathy (16). Despite the well-documented inhibitory activities of MSCs, and the clinical trials investigating the therapeutic potential of these cells in the treatment of immune-mediated disorders, little is known about the potential of MSCs in solid organ transplantation, and, furthermore, the mechanisms of MSC-mediated immunosuppressive effects in vivo remained to be elucidated (37). Thus far, Bartholomew et al. (18) have shown a single intravenous administration of donor-type MSCs into major histocompatibility complex–mismatched baboons prolonged the survival of skin grafts to 11.8 ± 1.4 days compared with 7.0 ± 0 days in untreated controls. In a mouse allograft model, Casiraghi et al. (19) demonstrated that intraportal administration of MSCs prolong heart allograft survival to a median survival time of 40 days compared with 10 days in untreated controls.
The therapeutic potential of MSCs in islet transplantation could address both aspects of these immune responses by suppression of the alloreactivity and autoimmunity (38,39). The current study shows that administration of MSCs can result in the prolonged survival of allogeneic islets and lead to long-term stable normoglycemia. Moreover, inhibition of the activity of MMP-2 and MMP-9 in vivo after cotransplantation of MSCs and allogeneic islet grafts abrogated the protective effect of the MSCs, resulting in rejection of the transplanted islets. Although our studies do not address the autoimmune component of responses to transplanted islets, we would argue on the basis of the relative precursor frequency of direct-pathway alloreactive T-cells and self-restricted autoreactive T-cells that control of the rejection response might also limit a reoccurrence of the original disease, especially when MSC delivery was combined with transient immunosuppression to target self-restricted memory T-cells. Taken together, our data demonstrate that MSCs can inhibit the immune response to allogeneic tissue in vivo and that MMP-2 and MMP-9 contribute to the molecular mechanism of suppression mediated by MSCs in vitro and in vivo and thus cement the rationale for their therapeutic implication in allogeneic islet transplantation.
Acknowledgments
This work was supported by grants from The Wellcome Trust (grant no. 082519) and the Optistem Consortium, an Integrated Project from the European Union FP7 Program (grant no. 223098).
No potential conflicts of interest relevant to this article were reported.
Y.D. and D.X. contributed to the conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. G.F. contributed to the conception and design, provision of study material, and data analysis and interpretation. A.B. contributed to data interpretation and manuscript writing. R.M. and K.W. contributed to the conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
The authors are grateful to the staff at the Biomedical Services Unit for animal care and welfare, Nick Jones (Nuffield Department of Surgery, the University of Oxford, Oxford) for instructive discussions, and other members of Transplantation Research Immunology Group for help throughout the project.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1728.
REFERENCES
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143– 147 [DOI] [PubMed] [Google Scholar]
- 2.Woodbury D, Schwarz EJ, Prockop DJ, Black IB: Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 2000; 61: 364– 370 [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Gehron Robey P: Marrow stromal stem cells. J Clin Invest 2000; 105: 1663– 1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N: Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol 2004; 32: 657– 664 [DOI] [PubMed] [Google Scholar]
- 5.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL: Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841– 848 [DOI] [PubMed] [Google Scholar]
- 6.Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E, Daum-Woods K, Goetchius GW, Fu P, Welniak LA, Murphy WJ, Laughlin MJ: Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(-) early progenitors cultured over human MSCs as a feeder layer. Stem Cells 2002; 20: 573– 582 [DOI] [PubMed] [Google Scholar]
- 7.Nauta AJ, Fibbe WE: Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499– 3506 [DOI] [PubMed] [Google Scholar]
- 8.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH: The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 2008; 26: 1047– 1055 [DOI] [PubMed] [Google Scholar]
- 9.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D: Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619– 4621 [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K: Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007; 109: 228– 234 [DOI] [PubMed] [Google Scholar]
- 11.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC: A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007; 110: 3691– 3694 [DOI] [PubMed] [Google Scholar]
- 12.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O: Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439– 1441 [DOI] [PubMed] [Google Scholar]
- 13.Bolanos-Meade J, Vogelsang GB: Novel strategies for steroid-refractory acute graft-versus-host disease. Curr Opin Hematol 2005; 12: 40– 44 [DOI] [PubMed] [Google Scholar]
- 14.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005; 106: 1755– 1761 [DOI] [PubMed] [Google Scholar]
- 15.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G: Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 2007; 56: 1175– 1186 [DOI] [PubMed] [Google Scholar]
- 16.Parekkadan B, Tilles AW, Yarmush ML: Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells 2008; 26: 1913– 1919 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S: Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther 2008; 326: 523– 531 [DOI] [PubMed] [Google Scholar]
- 18.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R: Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42– 48 [DOI] [PubMed] [Google Scholar]
- 19.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M: Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008; 181: 3933– 3946 [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318– 1330 [DOI] [PubMed] [Google Scholar]
- 21.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230– 238 [DOI] [PubMed] [Google Scholar]
- 22.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM: Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001; 98: 2615– 2625 [DOI] [PubMed] [Google Scholar]
- 23.Warnecke G, Chapman SJ, Bushell A, Hernandez-Fuentes M, Wood KJ: Dependency of the trans vivo delayed type hypersensitivity response on the action of regulatory T cells: implications for monitoring transplant tolerance. Transplantation 2007; 84: 392– 399 [DOI] [PubMed] [Google Scholar]
- 24.Essery G, Feldmann M, Lamb JR: Interleukin-2 can prevent and reverse antigen-induced unresponsiveness in cloned human T lymphocytes. Immunology 1988; 64: 413– 417 [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KA: Interleukin-2: inception, impact, and implications. Science 1988; 240: 1169– 1176 [DOI] [PubMed] [Google Scholar]
- 26.Neel NF, Schutyser E, Sai J, Fan GH, Richmond A: Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev 2005; 16: 637– 658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gieseke F, Schutt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Muller I: Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood 2007; 110: 2197– 2200 [DOI] [PubMed] [Google Scholar]
- 28.Page-McCaw A, Ewald AJ, Werb Z: Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007; 8: 221– 233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens LM, Fingleton B, Matrisian LM: Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002; 295: 2387– 2392 [DOI] [PubMed] [Google Scholar]
- 30.Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161– 174 [DOI] [PubMed] [Google Scholar]
- 31.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH: A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 2001; 61: 237– 242 [PubMed] [Google Scholar]
- 32.Xu D, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, Kessler BM: Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics 2008; 7: 2215– 2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P: MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007; 109: 4055– 4063 [DOI] [PubMed] [Google Scholar]
- 34.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG: Human allograft acceptance is associated with immune regulation. J Clin Invest 2000; 106: 145– 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin D, Tao J, Lee DD, Shen J, Hara M, Lopez J, Kuznetsov A, Philipson LH, Chong AS: Recovery of islet β-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleen. Diabetes 2006; 55: 3256– 3263 [DOI] [PubMed] [Google Scholar]
- 36.Parks WC, Wilson CL, Lopez-Boado YS: Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004; 4: 617– 629 [DOI] [PubMed] [Google Scholar]
- 37.Crop M, Baan C, Weimar W, Hoogduijn M: Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH: Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 2008; 57: 1759– 1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005; 23: 447– 485 [DOI] [PubMed] [Google Scholar]