Abstract
Cell-cell adhesion is a critical process for the formation and maintenance of tissue patterns during development, as well as invasion and metastasis of cancer cells. Although great strides have been made regarding our understanding of the processes that play a role in cell-cell adhesion, the precise mechanisms by which diverse signaling events regulate cell and tissue architecture is poorly understood. In this commentary we will focus on the Eph/ephrin signaling system, and specifically how the ephrinB1 transmembrane ligand for Eph receptor tyrosine kinases sends signals affecting cell-cell junctions. In a recent study using the epithelial cells of early stage Xenopus embryos, we have shown that loss- or gain-of function of ephrinB1 can disrupt cell-cell contacts and tight junctions. This study reveals a mechanism where ephrinB1 competes with active Cdc42 for binding to Par-6, a scaffold protein central to the Par polarity complex (Par-3/Par-6/Cdc42/aPKC) and disrupts the localization of tight junction-associated proteins (ZO-1, Cingulin) at tight junctions. This competition reduces aPKC activity critical to maintaining and/or forming tight junctions. Finally, phosphorylation of ephrinB1 on specific tyrosine residues can block the interaction between ephrinB1 and Par-6 at tight junctions, and restore tight junction formation. Recent evidence indicates that de-regulation of forward signaling through EphB receptors may play a role in metastatic progression in colon cancer. In light of the new data showing an effect of ephrinB reverse signaling on tight junctions, an additional mechanism can be hypothesized where de-regulation of ephrinB1 expression or phosphorylation may also impact metastatic progression.
Key words: ephrin, Eph, cell-cell adhesion, Par-6, tight junctions
Although cell-substrate adhesion plays an important role in cell migration, cell-cell adhesion maintains intercellular connections between neighboring epithelial cells for normal morphogenetic movements during development. Disruption of this critical process is also one of the earliest steps in metastatic progression of cancer cells. Cell-cell adhesion depends upon specific junctional complexes such as the tight junctions, adherens junctions, gap junctions, and desmosomes.1 Tight junctions mediate adhesion between the most apical polarized epithelial cells and control paracellular permeability of ions and molecules across epithelial sheets. Tight junctions also form a barrier to control the diffusion of integral membrane proteins between apical and basolateral membrane surfaces. Other junctional complexes that are critical to maintain cell-cell adhesion are the adherens junctions that are located more basal than tight junctions. Adherens junctions are associated with the actin cytoskeleton that encircles the cell just inside the membrane of the epithelial cells. Cadherins are central transmembrane proteins of the adherens junctions and are required for binding and localization of a critical group of cytoplasmic proteins, known as catenins, that form a link between the cadherin complex and the actin cytoskeleton.2,3 Loss of E-cadherin for example, is linked to tumor progression and invasion.4 In this commentary, we will focus on the tight junctions and how ephrinB signaling through its intracellular domain may exert influence on these junctions by regulating complexes critical to their establishment and maintenance.
Eph/ephrin signaling affects cell-cell adhesion5 and cell movement during development,6 and during tumorigenesis has been shown to play an instructive role in angiogenesis, as well as invasion.4 The cell-cell adhesion system is mediated by cadherin and plays a critical role in morphogenesis during development. This process depends upon the ability to form and disassemble cell-cell contacts and disruption of this adhesion system has been shown to play a critical role in cancer cell invasion and metastasis. A plethora of evidence indicates that Eph receptor tyrosine kinases (RTKs) and their ephrin ligands are either regulated by or control cell-cell adhesion complexes.6 The de-regulation of this signaling system is linked to the promotion of more aggressive and metastatic tumor phenotypes in a large variety of human cancers, including breast, lung, prostate, melanoma, and leukemia.7
Eph is the largest family of RTKs and they are divided into A and B subclasses (EphA1 - A10 and EphB1 - B6) by sequence similarities and binding specificity towards two subclasses of ligands (ephrinA1 – A6 and ephrinB1 – B3) known as ephrins. The ligands are all membrane-bound proteins with the A subclass being glycosylphosphatidylinositol anchor-linked to the membrane and the B subclass being transmembrane proteins having a short cytoplasmic domain. Generally, the A-type receptors have specificity toward A type ligands, while B-types bind to their cognate receptors, although there are exceptions to this rule.6,8,9
Eph receptors can associate with proteins involved in regulating the Rho family of GTPases, which are intimately involved in regulating cell morphology, cell adhesion, and cell migration. Several guanine nucleotide exchange factors (RhoGEFs) that activate RhoA, Cdc42 or Rac have been shown to associate with the Eph receptors and become activated upon ligand binding.10–14 Also, a negative regulator of the small GTPase Ras, p120 RasGAP, has been shown to bind phosphorylated EphB2 and this protein can also associate with p190 RhoGAP, a negative regulator of RhoA activity.15
Eph receptors and ephrin ligands signal in a bi-directional manner, where both molecules transmit intracellular signals upon cell-cell contact. These interactions induce cell repulsive or attractive responses in several cell types, and may have different effects within a sub-population of cells. Although ephrin ligands are bi-directional signaling molecules, much of the focus has been on Eph receptor signaling and function.
Unlike the receptors, the B-type transmembrane ephrin ligands do not possess any intrinsic catalytic activity for signaling. Thus, they rely upon a scaffolding activity that recruits signaling molecules to transmit an effect on cell function. It has been shown that ephrinBs utilize both phosphorylati on-dependent and -independent signaling pathways, which may be viewed as different modes of reverse signaling: (1) one mode where tyrosine phosphorylation of the intracellular domain of ephrinB leads to recruitment of signaling molecules that exert a functional effect; (2) another mode where unphosphorylated ephrinB associates with a protein complex that transduces a signal, but upon tyrosine phosphosphorylation, the interaction of ephrinB with the signaling complex is disrupted or modulated.
In response to an interaction with clustered Eph receptors, phosphorylation-dependent reverse signaling is initiated through a Src family kinase that phosphorylates tyrosines within the cytoplasmic domain of ephrinBs.16,17 In addition, alternative growth factor receptors (ie. FGF receptor, PDGF receptor, TIE-2) residing within the same cell as ephrinB can induce the phosphorylation event in cis.17–20 Upon ephrinB phosphorylation, Grb4, an adaptor protein, and STAT3, a signal transducer and activator of transcription, have been shown to associate with ephrinB1 in a phosphorylation-dependent manner and mediate a functional effect. The ephrinB/Grb4 association results in increased focal adhesion kinase (FAK) catalytic activity in cell culture21 and modulation of dendritic spine morphogenesis through the G protein-coupled receptor kinase-interacting protein (GIT) 1.11,22 Grb4 can also associate with other proteins implicated in cytoskeletal regulation including Cbl-associated protein (CAP/ponsin), the Abl-interacting protein-1 (Abi-1), dynamin, p21-activated kinase (PAK 1), heterogeneous nuclear ribonucleoprotein K (hnRNPK) and axin.21 The specific function of the STAT3 association with ephrinB is less clear, but it can lead to Jak2-dependent activation and transcription of reporter targets in Cos-1 cells and murine neuroepithelial cells, revealing a signaling pathway from ephrinB1 to the nucleus.23
Phosphorylation-independent reverse signaling is observed with PDZ-RGS3, a cytoplasmic protein that interacts B-type ephrins. This protein binds the cytoplasmic tail of B ephrins through a PDZ domain, and has a regulator of heterotrimeric G protein signaling (RGS) domain. Stromal cell-derived factor-1 (SDF-1), a chemokine with a G protein-coupled receptor, act as a chemoattractant for cerebellar granule cells, and this action is selectively inhibited by engagement of a soluble EphB receptor.24 Another protein, Dishevelled, which is a scaffold protein critical for the Wnt signaling pathway has also been shown to bind B-type ephrins and mediate signals affecting cell sorting and movement via the Rho small GTPase pathway.25,26 However, phosphorylation of ephrinB1 modulates or abrogates the signaling that controls retinal progenitor cell movement within the eye field by disrupting the ephrinB1/Dishevelled interaction.18 Thus, there are several modes and functions ascribed to ephrinB reverse signaling, and possible links from Eph receptors and ephrins to regulators of cytoskeletal architecture.
EphrinB reverse signaling may also occur through cytoplasmic release of the intracellular domain. A recent study showed that ephrinB1 can be sequentially cleaved by MMPs and γ-secretase, and the resulting C-terminal fragment can re-localize from the cell surface to the nucleus when the proteasome system is inhibited.27 However, the functional significance of this event is still unclear. Another report demonstrated that ephrinB2 can also be processed by MMPs and PS1/γ-secretase to a 12 kDa C-terminal fragment that binds to the Src kinase, inducing its autophosphorylation. Moreover, the γ-secretase system is required for EphB-induced ephrinB2 reverse signaling that regulates endothelial cell sprouting.28
Although the above mentioned studies have given insight into ephrinB signaling that affects cell movement, tissue boundaries and dendritic morphogenesis, the precise mechanism by which the ephrinB1 molecule signals through its intracellular domain to regulate cell-cell contacts has remained elusive. However, it is the ability to regulate cell-cell adhesion and motility that makes Eph/ephrin signaling a formidable system for regulating tissue separation and morphogenesis.
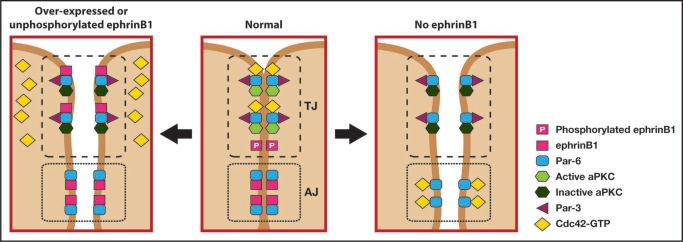
A particularly interesting aspect of ephrinB reverse signaling that is beginning to emerge is a role affecting cell-cell junctions. One study demonstrated a physical interaction between ephrinB1 and claudins, where upon cell-cell contact claudin induces a cis-phosphorylation of ephrinB1 that may regulate cell-cell adhesion and paracellular permeability.29 In a recent study from our laboratory, it was found that ephrinB1 signaling might regulate cell-cell junctions through a cell polarity complex in vivo.30 This study focused on assessing whether ephrinB1 may be a mediator or modulator of cell-cell junction signaling in epithelial cells using the Xenopus system. Evidence was presented that the Par polarity complex protein, Par-6, which is a major scaffold protein required for establishing tight junctions, associates with ephrinB1 and results in the loss of tight junctions. Using exogenous expression in the Xenopus system, along with endogenous immunoprecipitation analysis in a human colon carcinoma cell line (HT29), it was shown that an interaction exists between ephrinB1 and Par-6. Par-6 constitutively binds atypical protein kinase C (aPKC), and upon binding an active Cdc42-GTP undergoes a conformational change that leads to aPKC activation. The Par-6/aPKC/Cdc42-GTP complex localizes to the apical cell junctions where it regulates tight junction formation, and tight junction complexes may associate with the actin cytoskeleton, which is reorganized for the formation and maintenance of cell-cell contacts.31 Over-expression of ephrinB1 in embryonic ectoderm caused the loss of tight junctions, as evidenced by ultrastructural analysis and localization of tight junction proteins (ZO-1 and Cingulin). Expression and immunoprecipitation analysis in Xenopus oocytes demonstrated that ephrinB1 can compete with the small GTPase Cdc42 for association with the Par-6 protein. This competition model (Fig. 1) was tested and confirmed in vivo, where tight junction formation was rescued in ectoderm over-expressing ephrinB1 when an active form of Cdc42 was also expressed at the appropriate level.30
Figure 1.
EphrinB1 regulates tight junction formation through an interaction with Par-6. Unphosphorylated ephrinB1 may compete with Cdc42-GTP for Par-6 binding and inhibit aPKC activation in the Par complex, leading to tight junction disruption (left panel). Upon tyrosine phosphorylation ephrinB1 fails to interact with Par-6, which is now available to interact with Cdc42-GTP and establish tight junctions (middle panel). Loss of ephrinB1 may allow Par-6 that is localized at adherens junctions and lateral cell borders compete with tight junction-associated Par-6 for Cdc42-GTP. The resulting reduction in Cdc42-GTP localized at the apical border may reduce aPKC activity and disrupt tight junctions (right panel).
EphrinB1 is known to be tyrosine phosphorylated (through a Src family kinase) upon interacting with the extracellular domain of its cognate EphB receptor, and phosphorylated in cis by an active FGF receptor. Immunoprecipitation analysis in the Xenopus oocyte system, as well as the HT29 human colon carcinoma cell line, demonstrates that tyrosine phosphorylation of the intracellular domain of ephrinB1 disrupts the interaction with Par-6. Furthermore, phosphorylation of ephrinB1 rescues the interaction between active Cdc42 and Par-6, supporting a model where unphosphorylated ephrinB1 and active Cdc42 compete for Par-6 binding (Fig. 1). Moreover, it was demonstrated that phosphorylation on tyrosine 310 rescues tight junction formation in embryonic ectoderm that is over-expressing ephrinB1. In vivo evidence for this phosphorylation event disrupting the ephrinB1/Par-6 complex and thus maintaining tight junctions during normal ectoderm development comes from ephrinB1 replacement experiments. In these studies, translation of endogenous ephrinB1 was blocked by the ephrinB1MO (ephrinB1 antisense morpholino oligonucleotide), and wild-type or tyrosine 310 mutant ephrinB1 RNAs that are resistant to the MO were introduced at carefully titrated concentrations. While wild-type ephrinB1 was able to rescue the localization of the tight junction-associated protein ZO-1 in the presence of the ephrinB1MO, expression of the ephrinB1Y310F mutant in the presence of ephrinB1MO failed to restore appropriate localization of ZO-1. This data is consistent with the observed enrichment of ephrinB1 tyrosine phosphorylation at the apical lateral domain at cell junctions.29,30,32 These experiments provide critical in vitro and in vivo evidence for a mechanistic model (Fig. 1) of how ephrinB1 controls tight junction formation.30
We propose a model where unphosphorylated ephrinB1 possesses a competitive advantage for binding to Par-6, thus displacing or preventing Cdc42-GTP from interacting with Par-6 at apical lateral borders. Since the Cdc42/Par-6 interaction is inhibited, aPKC activity is reduced and tight junctions are disrupted. In contrast, upon cell-cell contact a cognate Eph receptor (or possibly an active FGF receptor or claudin) can induce phosphorylation of ephrinB1 at the apical junctions, and dissociate ephrinB1 from Par-6. Thus, Cdc42-GTP is free from competition with ephrinB1 and can now bind to Par-6, inducing aPKC activation and establishing tight junctions.30
One of the questions that remains to be resolved is why loss of ephrinB1 disrupts tight junction formation? In our study, loss of ephrinB1 expression via the introduction of an ephrinB1MO causes a loss of tight junction assembly.30 In addition, Cortina and colleagues reported that conditional loss of ephrinB1 in intestinal epithelia of the mouse shows a substantial reduction of tight junctions.4 One possibility within the confines of our model (Fig. 1) is that loss of ephrinB1 may result in more available Par-6 along the lateral borders of the cell. For example, phosphorylated ephrinB1 appears to be enriched in apical junctions,29,30 while more unphosphorylated ephrinB1 appears to reside along the lateral borders and adherens junctions.30 Thus, it may be possible that loss of ephrinB1 allows Par-6 at these locations to compete for an interaction with Cdc42-GTP, effectively displacing a portion of the Cdc42-GTP from the apical junction region where aPKC resides and its activity required for tight junction formation.
Alternatively, it is possible that ephrinB1 may affect adherens junctions through the Par polarity complex. Several recent papers have linked Cdc42 to adherens junction stability. One report by Harris and Tepass identifies the Par complex as an effector for Cdc42 in controlling the endocytosis of apical proteins (ie. crumbs) that are critical for stabilizing basolateral adherens junctions.33 Two other studies show that Cdc42 functions with Par-6 and aPKC to regulate E-Cadherin endocytosis through the Arp2/3 complex, and this regulation affects adherens junction stability.34,35
It is also possible that ephrinB1 may regulate cell-cell adhesion independent of the tight junction-associated Par complex. For example, ephrinB1 may have another interacting partner that plays a critical role in adherens junction formation or maintenance. In this case, loss of ephrinB1 may affect adherens junction or even gap junction formation, leading to an unraveling of cell-cell adhesion that results in tight junction dissolution. Supporting this possibility, we have observed by electron microscopy that a small portion of the ectoderm cells injected with ephrinB1MO, show loss of adherens junctions, but not tight junctions (Lee and Daar, unpublished results).
It has been reported that gap junction communication may be regulated by ephrinB1 through an interaction with Connexin 43 (Cx43), which is a major component of the gap junction complex.32 A gap junction is an intercellular membrane composed of two hemi-channels that connect cells across the intercellular space and permit cell-cell communication by allowing small molecules to pass between cells. Gap junctions play an important role in morphogenetic processes during development. Studies with zebrafish ectodermal explants have shown that bi-directional signaling between EphB receptors and ephrinB ligands prevents cell intermingling through gap junction communication. In contrast, unidirectional signaling through Eph receptors or ephrins restricts cell-cell communication through gap junctions.36 Recent insight has been gained from a report showing that a mouse heterozygous for loss of ephrinB1 results in calvarial defects, and that gap junction communication is inhibited by ectopic ephrinB1 expression at cell boundaries. Moreover, biochemical support for a role of ephrinB1 in gap junction communication was obtained by co-immunoprecipitation analyses showing that ephrinB1 interacts with Cx43 and regulates its localization.32
Is it possible that an interaction between ephrinB1 and gap junction proteins may play a role in cell-cell adhesion beyond a role in gap junction communication? It has been recently reported that gap junctions do not mediate neuronal migration by acting as an aqueous channel, but rather, act as adhesive contacts that interact with the internal cytoskeleton of the migrating neurons.37 In this report, the gap junction subunits Cx26 and Cx43 are expressed at the contact points and mediate adhesion between radial fibers and migrating neurons of cerebral cortex. An alternative role for Cx43 in adhesive events has been proposed in a model in which Cx43 and N-cadherin may modulate neural crest cell motility by engaging in cross-talk through p120 catenin and/or integrin signaling.38,39 It remains to be tested whether ephrinB1 may regulate cell adhesion through an interaction with connexin or another gap junction-associated protein in a manner independent of gap junction communication. It will require a systematic and thorough identification of the ephrinB interacting partners and their localization during morphogenetic events to unravel the full extent of ephrinB involvement in cell-cell adhesion. Thus, it is interesting to consider the case of cancer progression, where de-regulation of the Eph/ephrin signaling system may lead to invasion and metastasis.
In colorectal cancer, intestinal adenomas are early stage precursors of metastatic disease, and express high levels of certain Wnt target genes, such as EphB2 and B3 receptors.40 However, during invasive progression of these adenomas, the EphB receptors become down-regulated. It was also shown in the Apc/Min mouse model system that loss of forward signaling through the EphB receptor leads to invasion and malignant adenomas.4,40 Using colorectal cancer cell lines, the mechanism of action has been identified, where activation of EphB enhances E-cadherin-dependent adhesion. This cell-cell adhesion prevents colorectal cells from invading the adjacent ephrinB+ territory. The situation in vivo reflects this signaling, and EphB+ tumor cells are restricted by contact with normal intestinal cells expressing high levels of ephrinB1. In contrast, there is loss of EphB along with a redistribution of E-cadherin from the adenoma cell surface as these cells become invasive.4
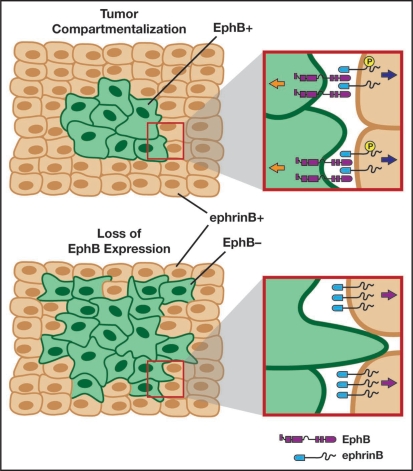
This begs the question of whether ephrinB1 in the surrounding epithelial tissue only plays a role as ligand for the EphB+ tumor cells, or might ephrinB1 reverse signaling through its intracellular domain contribute to metastasis as well. Our study offers the hypothesis that reverse signaling through ephrinB1 in the surrounding epithelial cells may be de-regulated, and that ephrinB1 may be unphosphorylated at the apical junctions due to loss of interaction with the EphB receptor in the tumor cells. If such a situation exists in colorectal cancer, it might lead to ephrinB1 competing with Cdc42 for Par-6 binding in the adjacent surrounding tissue, and the tight junctions and cell-cell boundaries may be compromised, allowing for invasion of tumor cells into this adjacent territory (Fig. 2). It will require high resolution imaging and immunocytochemistry in tumor models and patient samples to refute or validate this hypothesis.
Figure 2.
Loss of Eph-ephrin interactions may result in tumor invasion. Adenoma cells (green) may be compartmentalized and restricted from invasion through bi-directional signaling (upper left panel) established by the expression of ephrinBs in the surrounding normal tissue (flesh tone cells). During metastatic progression, EphB expression is lost in the adenoma cells (bottom left panel) and leads to unidirectional signaling (bottom right panel). This loss of contact with the EphB receptor may lead to an accumulation of unphosphorylated ephrinB1 in the surrounding cells (bottom right panel). The unphosphorylated ephrinB1 may disrupt the Par complex (see Fig. 1), leading to tight junction dissolution and allowing tumor cells to invade the surrounding tissue. Adapted in part from Clevers and Batlle, 2006.41
Abbreviations
- Eph
Erythropoietin producing hepatoma
- ephrin
erythropoietin producing hepatoma interactor
- FGF
fibroblast growth factor
- Par
Partitioning defective
- PDZ
PSD-95/Dlg/ZO-1
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: www.landesbioscience.com/journals/celladhesion/article/8211
References
- 1.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherincatenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 5.Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, et al. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 7.Wimmer-Kleikamp SH, Lackmann M. Eph-modulated cell morphology, adhesion and motility in carcinogenesis. IUBMB Life. 2005;57:421–431. doi: 10.1080/15216540500138337. [DOI] [PubMed] [Google Scholar]
- 8.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 10.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 11.Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- 12.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Irie F. [Roles of Eph receptors and the cell surface heparan sulfate proteoglycan syndecan-2 in dendritic spine morphogenesis] Seikagaku. 2002;74:391–395. [PubMed] [Google Scholar]
- 14.Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 15.Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, et al. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. Embo J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 17.Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 18.Lee HS, Mood K, Battu G, Ji YJ, Singh A, Daar IO. FGF Receptor-Induced Phosphorylation of EphrinB1 Modulates its Interaction with Dishevelled. Mol Biol Cell. 2009;20:124–133. doi: 10.1091/mbc.E08-06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore KB, Mood K, Daar IO, Moody SA. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 20.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- 22.Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- 23.Bong YS, Lee HS, Carim-Todd L, Mood K, Nishanian TG, Tessarollo L, Daar IO. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci USA. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. Embo J. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- 27.Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M, Kamata R, Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. EMBO J. 2005;24:3700–3711. doi: 10.1038/sj.emboj.7600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HS, Nishanian TG, Mood K, Bong YS, Daar IO. EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat Cell Biol. 2008;10:979–986. doi: 10.1038/ncb1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007 doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- 32.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiou M, Marinari E, Burden J. Baum B, Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 36.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 37.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, et al. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154:217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 41.Clevers H, Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res. 2006;66:2–5. doi: 10.1158/0008-5472.CAN-05-3849. [DOI] [PubMed] [Google Scholar]