Abstract
Cell motility is a highly coordinated multistep process. Uncovering the mechanism of myosin II (MYO2) activation responsible for the contractility underlying cell protrusion and retraction provides clues on how these complementary activities are coordinated. Several protein kinases have been shown to activate MYO2 by phosphorylating the associated myosin light chain (MLC). Recent work suggests that these MLC kinases are strategically localized to various cellular regions during cell migration in a polarized manner. This localization of the kinases together with their specificity in MLC phosphorylation, their distinct enzymatic properties and the distribution of the myosin isoforms generate the specific contractile activities that separately promote the cell protrusion or retraction essential for cell motility.
Key words: myosin, MLCK, ROK, MRCK, phosphorylation, cell migration
Cell movement is a fundamental activity underlying many important biological events ranging from embryological development to immunological responses in the adult. A typical cell movement cycle entails polarization, membrane protrusion, formation of new adhesions, cell body translocation and finally rear retraction.1 A precise temporal and spatial coordination of these separate steps that take place in different parts of the cell is important for rapid and efficient movement.2
One major event during eukaryotic cell migration is the myosin II (MYO2)-mediated contraction that underlies cell protrusion, traction and retraction.1,3 An emerging theme from collective findings is that there are distinct myosin contractile modules responsible for the different functions which are separately regulated by local myosin regulatory light chain (MLC) kinases. These kinases contribute to contractile forces that connect adhesion, protrusion and actin organization.2 Unraveling the regulation of these contractile modules is therefore pivotal to a better understanding of the coordination mechanism.
At the lamellipodium, the conventional calcium/calmodulin-dependent myosin light chain kinase (MLCK) has been shown to play an essential role in a Rac-dependent lamellipodial extension.4 Inhibition of calmodulin or MLCK activity by specific photoactivatable peptides in motile eosinophils effectively blocks lamellipodia extension and net movement.5 Furthermore, there is a strong correlation between activated MLCK and phosphorylated MLC within the lamellipodia of Ptk-2 cells as revealed by fluorescence resonance energy transfer (FRET) analysis.6 More recent studies showed MLCK to regulate the formation of focal complexes during lamellipodia extension.7,8 Functionally, MLCK is thought to play a critical role in the environment-sensing mechanism that serves to guide membrane protrusion. It mediates contraction that exerts tension on integrin-extracellular matrix (ECM) interaction, which, depending on the rigidity of the substratum, will lead to either stabilization of adhesion resulting in protrusion or destabilization of attachment seen as membrane ruffling on non-permissive surfaces.8,9
As a Rho effector, Rho-associated kinase (ROK/ROCK/Rho-kinase) has been shown to regulate stress fibers and focal adhesion formation by activating myosin, an effect that can be blocked by the specific ROK inhibitor Y-27632.10,11 Myosin activation by ROK is the effect of two phosphorylation events: the direct phosphorylation on MLC and the inhibition of myosin phosphatase through phosphorylation of its associated myosin-binding subunit (MBS).11 Consistent with this notion of a localization-function relationship, ROK and MBS, which can interact simultaneously with activated RhoA,11 have been shown to colocalize on stress fibers.12,13 In migrating cells, Rho and ROK activities have been mostly associated with the regulation of tail retraction, as inhibition of their activities often results in trailing tails due to the loss of contractility specifically confined to the cell rear.14,15 Tail retraction requires high contractile forces to overcome the strong integrin-mediated adhesion established at the rear end, an event which coincides with the strategic accumulation of highly stable and contractile stress fibers that assemble at the posterior region of migrating cells.
MRCK was previously shown to phosphorylate MLC and promote Cdc42-mediated cell protrusion.16 More recently, it was found to colocalize extensively with and regulate the dynamics of a specific actomyosin network located in the lamella and cell center, in a Cdc42-dependent manner but independent of MLCK and ROK.17 The lamellar actomyosin network physically overlaps with, but is biochemically distinct from the lamellipodial actin meshwork.9,18 The former network consists of an array of filaments assembled in an arrangement parallel to the leading edge, undergoing continuous retrograde flow across the lamella, with their disassembly occurring at the border of the cell body zone sitting in a deeper region.17–19 Retrograde flow of the lamellar network plays a significant role in cell migration as it is responsible for generating contractile forces that support sustained membrane protrusion and cell body advancement.17–19
It is therefore conceivable that these three known MLC kinases are regulated by different signaling mechanisms at different locations and on different actomyosin contractile modules. The coordination of the various modules will ensure persistent directional migration (Figure 1). Phosphorylation of MLC by PAK and ZIP kinase has also been reported, but their exact roles in this event have yet to be determined.20,21 It is also noteworthy that individual kinases can work independently of each other, as amply shown by evidence from inhibitor treatments. This is particularly true for MRCK in the lamella, whose activity on lamellar actomyosin flow is not affected by ML7 and Y-27632, the inhibitors of MLCK and ROK respectively.17 These findings further indicate that although both ROK and MRCK have been shown to upregulate phosphorylated MLC levels by inhibiting the myosins phosphatases,11,22 they are likely to act as genuine MLC kinases themselves, without the need of MLCK as previously suggested.11
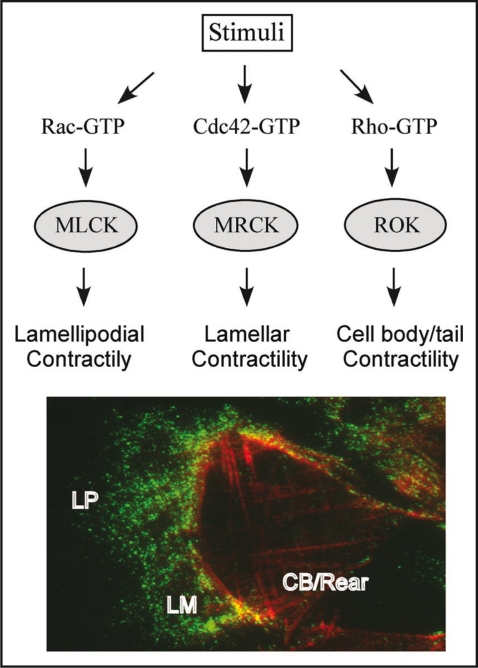
Figure 1.
Upper panel depicts a model for the specific activation of the different MLC kinases at various locations in the cell. In response to upstream signals, MLC kinases MLCK, MRCK and ROK are activated and localized to different regions. In the case of MRCK and ROK, the interaction of the GTP-bound Rho GTPase binding domain will determine the specific action of the downstream kinase, resulting in actomyosin contractility at different locations. The coordination of these signalling events is crucial for directional cell migration. Lower panel shows a typical front-rear location for Myosin 2A and 2B in a migrating U2OS cell.
In conjunction with their differences in localization, the three MLC kinases show apparent individual preferences and specificity towards the MYO2 isoforms that they associate with. The two major MYO2 isoforms MYO2A and 2B are known to have distinct intracellular distributions that are linked to their individual functions (Figure 1).23,24 In motile cells, MYO2A localization that is skewed towards the protruding cell front is consistent with it being the major myosin 2 component of the lamellar filaments regulated by MRCK as well as its regulation by MLCK in lamellipodial contraction.8,17,19 In contrast, the enrichment of MYO2B at retracting cell rear conforms well with the requirement of thick and stable stress fibers capable of causing tail contraction and prevention of protrusion under the control of Rho/ROK signaling.23,25 The selection for MYO2B filaments in the cell rear stems from their more contractile and stable nature compared with MYO2A, a consequence of their higher time-averaged association with actin.26,27 Conversely, the lower tension property of MYO2A filaments suggests that they are more dynamic in nature,26,27 a characteristic which fits well with the dynamic actomyosin activities at the leading edge and lamella that regulate protrusion.
It deserves special mention that the three MLC kinases display subtle differences in their specificity towards MLC. While MLCK and MRCK phosphorylate only a single Ser19 site (monophosphorylation),18,28 ROK is able to act on both Thr18 and Ser19 residues causing diphosphorylation of MLC,29 MLCK only causes diphosphorylation when present at higher concentrations.30 By further increasing its actin-activated ATPase activity, diphosphorylation of MLC has been shown to induce a higher myosin activation and filament stability.30–32 The use of specific antibodies that can differentiate between the two populations of phosphorylated MLC has been instrumental in revealing their localization and correlation with the activity of the MLC kinases. The emerging picture from these experiments is that mono and diphosphorylated MLC exhibit distinct distributions in migrating cells, with the monophosphorylated MLC localized more towards the protrusive region, while the diphosphorylated form is more enriched at the posterior end.21,33 Taking into account their biochemical properties, the polarized distributions of these differentially phosphorylated MLC coincide functionally with the segregation of the MYO2 isoforms and their corresponding regulators. These findings provide further support for the existence of segregated contractile modules in migrating cell and their distinctive regulation.
The mechanisms that determine the specific segregation of the contractile modules and their regulation are unclear. However, some clues have emerged from recent studies. It has been shown that the C-terminal coiled-coil region of MYO2B is important for determining its localization in cell rear25 and which requires Rho/ROK activity as their inhibition resulted in the loss of this specific localization.23 Correspondingly, the inhibition of MRCK activity resulted in the loss of lamella-localized MYO2A.17 These findings suggest that activation of MYO2 filaments by their upstream regulators is important for their functional segregation and maintenance. It is noteworthy that both ROK and MRCK have distinct regulatory domains including the pleckstrin homology domains which have been shown to be essential for their localization, a process which may involve myosin interaction and lipid-dependent targeting as has been respectively shown for ROK and MRCK.11,13,16 Further, the specificity of MRCK for lamellar actomyosin is believed to be largely determined by the two proteins it forms a complex with: the adaptor LRAP35a, and the MYO2-related MYO18A. Activation of MYO18A by MRCK, a process bridged by LRAP35a, is a crucial step which facilitates MRCK regulation on lamellar MYO2A.17
The mechanisms responsible for segregating the contractile modules and their regulators may also comprise a pathway that parallels the microtubule-modulatory Par6/aPKC/GSK3β signalling pathway which regulates cellular polarization. This notion is supported by both Cdc42 and Rho being common upstream regulators of these two pathways.34 GTPase activation may determine the localized activities of the separate contractile modules and create an actomyosin-based asymmetry across the cell body, which together with the microtubule-based activities, result in the formation of a front-back axis important for directional movement. The involvement of MRCK in MTOC reorientation and nuclear translocation events,35 and our unpublished observation that LRAP35a has a GSK3β-dependent microtubule stabilizing function are supportive of a possible cross-talk between these two pathways.
In conclusion, the complex regulation of contractility in cell migration emphasizes the importance of the localization, specificity and enzymatic properties of the different MLC kinases and myosin isoforms involved. The initial excitement and confusion caused by the emergence of the different MLC kinases are fading, being now overtaken by the curiosity about how they cooperate and are coordinated while promoting cell motility.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: www.landesbioscience.com/journals/celladhesion/article/8212
References
- 1.Lauffenburger DA, Horwitz AF. Cell migration: a physical integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TD, Borisy GG. Cell motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 4.Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- 5.Walker JW, Gilbert SH, Drummond RM, Yamada M, Sreekumar R, Carraway RE, et al. Signaling pathways underlying eosinophil cell motility revealed by using caged peptides. PNAS. 1998;95:1568–1573. doi: 10.1073/pnas.95.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol. 2002;156:543–553. doi: 10.1083/jcb.200110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannone G, Dubin-Thaler BJ, Döbereiner H, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 9.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKa is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 12.Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKalpha-implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- 14.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 16.Leung T, Chen XQ, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan I, Yong J, Dong JM, Lim L, Leung L. A tripartide complex containing MRCK modulates lamellar actomyosin retrograde flow. Cell. 2008;135:123–136. doi: 10.1016/j.cell.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1785. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuazon PT, Traugh JA. Activation of actin-activated ATPase in smooth muscle by phosphorylation of myosin light chain with protease-activated kinase. J Biol Chem. 1984;259:541–546. [PubMed] [Google Scholar]
- 21.Komaisu S, Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J Cell Biol. 2004;165:243–254. doi: 10.1083/jcb.200309056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan I, Ng CH, Lim L, Leung T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem. 2001;276:21209–21216. doi: 10.1074/jbc.M102615200. [DOI] [PubMed] [Google Scholar]
- 23.Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol Biol Cell. 2003;14:4745–4757. doi: 10.1091/mbc.E03-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, Kovács M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of nonmuscle myosin IIB. J Biol Chem. 2003;278:27439–27448. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- 27.Kovács M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin II. J Biol Chem. 2003;278:38132–38140. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher PJ, Herring BP, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda K, Murata-Hori M, Tatsuka M, Hosoya H. Rho-kinase contributes to diphosphorylation of myosin II regulatory light chain in nonmuscle cells. Oncogene. 2002;21:5852–5860. doi: 10.1038/sj.onc.1205747. [DOI] [PubMed] [Google Scholar]
- 30.Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- 31.Mizutani T, Haga H, Koyama Y, Takahashi M, Kawabata K. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. J Cell Phy. 2006;209:726–731. doi: 10.1002/jcp.20773. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Hosoya H, Yonemura S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation od its light chain in epithelial cells. Mol Biol Cell. 2007;18:605–616. doi: 10.1091/mbc.E06-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoh T, Takemura S, Ueda K, Hosoya H, Nagayama M, Haga H, et al. Differential localization of non-muscle myosin II isoforms and phosphorylated regulatory light chains in human MRC-5 fibrablasts. FEBS Lett. 2001;509:365–369. doi: 10.1016/s0014-5793(01)03186-6. [DOI] [PubMed] [Google Scholar]
- 34.Basu R, Chang F. Shaping the actin cytoskeleton using microtubule tips. Curr Opin Cell Biol. 2007;19:88–94. doi: 10.1016/j.ceb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]