Abstract
Cell cytoskeleton proteins are fundamental to cell shape, cell adhesion and cell motility, and therefore play an important role during angiogenesis. One of the major regulators of cytoskeletal protein expression is serum response factor (SRF), a MADS-box transcription factor that regulates multiple genes implicated in cell growth, migration, cytoskeletal organization, energy metabolism and myogenesis. Recent data have demonstrated a crucial role of SRF downstream of VEGF and FGF signalling during sprouting angiogenesis, regulating endothelial cell (EC) migration, actin polymerisation, tip cell morphology, EC junction assembly and vascular integrity. Here, we review the role of SRF in the regulation of angiogenesis and EC function, integrate SRF function into a broader mechanism regulating branching morphogenesis, and discuss future directions and perspectives of SRF in EC biology.
Key words: SRF, angiogenesis, vascular morphogenesis, endothelial cell, VEGF, FGF
Angiogenesis, the extension of the vascular network from preexisting blood vessels, depends on the coordinated and efficient chemoattractant gradient sensing, polarization and motility of endothelial cells (ECs).1 This coordinated cell migration requires cell cytoskeleton proteins that play a central role in angiogenesis. Changes in the cell's cytoskeleton dynamics occur rapidly in response to extracellular stimuli, through the activation of tyrosine kinase receptors and/or G-protein coupled receptors transforming the received signal into a morphological and/or behavioural change. Downstream of receptor signalling, different regulatory proteins such as formins, cofilins, Arp2/3 complex and Rho-associated protein kinase dynamically control the cytoskeleton kinetics and finely-tune the final output of the signalling pathways.2 It is important to note, however, that the intrinsic characteristics of a cell's cytoskeleton also depend on the transcriptional program of each cell and therefore on cell-specific transcription factors. Serum response factor (SRF) is an ancient and evolutionarily conserved transcription factor of the MADS-box family that regulates multiple genes implicated in cell growth, migration, cytoskeletal organization, energy metabolism and myogenesis.3 Through specific binding sites named CArG-boxes [CC(A/T)6GG], SRF has the potential to regulate the expression of genes encoding all of the actin proteins, some of the muscular myosin heavy and light chain proteins and also proteins implicated in the regulation of actin treadmilling, including profilin-1, tropomyosin-1 and -4, calponin-1, filamin-A and actin-related protein-3.3 In addition, SRF has also been implicated in the expression of genes coding for the proteins participating in cell junction assembly and adhesion, such as zona occludens-1 (ZO-1), α1-integrin, and syndencan-2 and -4.3 Interestingly, actin dynamics in turn control SRF transcriptional activity through regulation of the sub-cellular localisation of megakaryocytic acute leukaemia (MAL/MRTF-A/MLK), a SRF cofactor. Treisman's group has shown beautifully that MAL is sequestered in the cytoplasm of unstimulated cells, through binding to globular actin (G-actin). However, an increase in RhoA-mediated actin polymerisation, triggered by different signalling pathways, decreases the cytosolic pool of G-actin leading to a nuclear accumulation of MAL. Inside the nucleus MAL binds to SRF, activating the transcription of specific genes, such as β-actin and vinculin.4
Despite this prominent role of SRF in regulating cytoskeletal protein expression, the in vivo function of SRF in ECs during vascular development has only recently been addressed. We demonstrated a requirement of SRF for correct sprouting angiogenesis and the maintenance of vascular integrity.5 Specific SRF ablation in ECs using a Tie1-Cre transgenic line leads to embryonic death at around 14 days (E14) of mouse embryonic development with mutant embryos showing a severe decrease in vascular density, oedema, aneurismal structures and haemorrhages. Our study linked the vascular defects with a decreased migratory capacity of ECs, compromised tip cell morphology, altered actin polymerisation and filopodia formation, and impaired assembly of EC junctions. We showed that SRF is important for the correct expression of structural proteins such as β-actin, VE-cadherin and several integrins in ECs, both in vitro and in vivo, with changes in expression leading to the observed defective vascular morphogenesis.5 Interestingly, EC-specific deletion of SRF does not affect the differentiation of ECs and the early stages of vascular morphogenesis and therefore SRF seems to be dispensable for vasculogenesis during mouse embryonic development. However the use of a transgenic line expressing Cre recombinase at an earlier time than Tie1-Cre will be necessary to verify this observation. As an example, Schwartz's group have recently shown a novel and earlier role of SRF in the appearance of rhythmic beating myocytes and organized sarcomeres using Nkx2.5-Cre transgenic mice, which express Cre recombinase in cardiomyocytes earlier than the mouse line previously used by the same group.6
Endothelial tip cells play a central role in regulating vascular sprouting. The biological function of SRF in these cells therefore needed clarification. It has now been established that the interaction of vascular endothelial growth factor (VEGF) and Notch pathways controls endothelial tip/stalk cell phenotype, and any defect in the correct retro-talking in this system affects proper vessel sprouting.7,8 Despite the fact that SRF is strongly expressed by ECs of sprouting capillaries, both in tip and stalk cells, SRF seems to be dispensable for tip cell induction downstream of VEGF signalling. Indeed, we observed an equal number of tip cells and similar expression of Notch signalling proteins in mutant embryos.5 Moreover, in the aortic ring assay where a mosaic pattern of SRF deletion was generated, we observed that SRF-deleted cells could be at tip and stalk positions, confirming that SRF does not appear to interfere with tip cell selection. However, SRF deletion in ECs disrupted the actin cytoskeleton, compromised tip cell morphology and filopodia formation, and consequently led to impaired cell migration.5 Importantly, both studies from our group and Tarnawsky's group demonstrate that SRF in ECs is activated by VEGF-A and basic fibroblast growth factor (bFGF) signalling through RhoA-actin and MEK-ERK pathways.5,9 In fact, the VEGF-A gradient is important for tip cell induction, but also acts as a potent guidance clue stimulating endothelial cell migration, vascular outgrowth and ramification through the activation of members of the Rho family of proteins.10 Altogether these results lead to the assumption that SRF is an important transcription factor downstream of VEGF and FGF signalling in ECs, promoting cytoskeleton remodelling and cell migration (Fig. 1C). Interestingly, SRF has a similar function in the development of the tracheal system of Drosophila melanogaster, analogous in terms of function and developmental pathways to the circulatory system of vertebrates. The tracheal system develops by sequential sprouting of primary, secondary and terminal branches from an epithelial sac of ∼80 cells in each body segment of the embryo.11 During terminal tracheal branching, the cells undergo repeated episodes of cytoplasmic extension, similar to filopodia formation in mammalian cells, and intracellular lumen formation, creating ramified networks of terminal branches.11 Notably, the Drosophila melanogaster SRF homologue, dSRF/blistered/pruned, is specifically expressed in tracheal terminal cells, and loss-of-function mutations block cytoplasmic outgrowth and terminal branching, whereas constitutively active forms of SRF and the SRF partner Elk-1 drive excessive branch outgrowth.11 Sprouting and outgrowth of terminal branches is carefully regulated to meet the oxygen needs of target tissues, and expression of branchless (bnl), the homologue of FGF, in hypoxic mesenchymal cells is regulated by Fatiga, the Drosophila homologue of mammalian hypoxia-inducible factor (HIF). Bnl signals through breathless (btl), the fly homologue of the FGF receptor (FGFR), playing a crucial role throughout primary, secondary and terminal branching. However, FGF-FGFR signalling elicits different intracellular signalling cascade at each stage thereby inducing a different cell response. In terminal cells, FGFR activates MAPK signalling which in turn activates SRF.11 Thus, the axis hypoxia-HIF-FGF-FGFR plays a crucial role in regulating SRF activity, with the SRF within this pathway participating towards the regulation of terminal branching to meet local oxygen demands (Fig. 1A). Interestingly, Drosophila larva within which Drosophila myocardin-related transcription factor (DMRTF) has been inactivated, show a very similar phenotype to those for which the SRF has been deleted, suggesting that DMRTF is a partner of SRF in the regulation of tracheal terminal branching.12
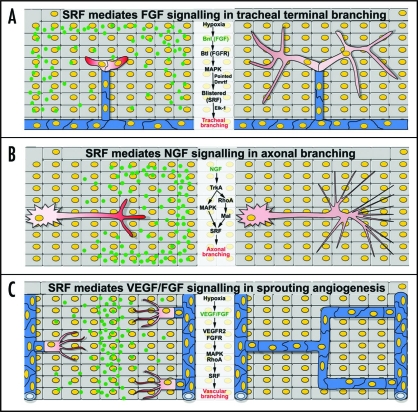
Figure 1.
Schematic representation of SRF involvement in the branching process of different tissues. (A) In terminal branching of Drosophila melanogaster tracheal system. Hypoxia induces the expression of branchless (bnl, green dots) in mesenchymal cells. Bnl signals through the breathless (btl) receptor in tracheal epithelial cells (in red, on the left), activating the MAPK pathway, which in turn activates blistered (DSRF). In cooperation with Elk-1 and DMRTF, DSRF promotes cytoplasmic extension and ramification of terminal tracheal cells (right). (B) Terminal innervation of embryonic dorsal root ganglion (DRG) sensory neurons. NGF gradient (green dots) signal interacts with TrkA receptors present in the axons of sensory neurons (red cell, on the left), and stimulates the expression of SRF via MAPK- and MAL-dependent pathways. SRF in turn regulates actin dynamics and promotes NGF-dependent axonal growth and terminal branching of sensory neurons (on the right). (C) Branching of the vertebrate vascular system. Hypoxia condition drives the expression of pro-angiogenic growth factors, such as VEGF and bFGF, in mesenchymal cells. VEGF and FGF gradients (green dots) bind to their corresponding receptors, VEGFR2 and FGFR, respectively, and promote tip cell (red cells, on the left) induction and vessel branching. Within this pathway, SRF is likely stimulated by the activation of the MAPK and Rho-actin pathways and promotes vascular branching and tissue perfusion (in blue, on right) by controlling the expression of cytoskeleton and cell adhesion molecules.
Remarkably, recent work on the role of SRF in neurons has revealed a similar signalling pathway. Alberti et al. have shown that deletion of the SRF gene in the developing mouse nervous system results in impaired migration of neurons in the rostral migratory stream.13 Moreover, Knoll et al. showed that conditional deletion of the SRF gene in mouse neurons reduces neurite outgrowth and abolishes mossy fibre segregation, and suggested that MAL participates in this event.14 More recently, Ginty's group demonstrated that SRF is an important transcription factor mediating nerve growth factor (NGF)-induced axonal growth, terminal branching and innervation of embryonic dorsal root ganglia (DRG) sensory neurons.15 Embryos specifically lacking the SRF gene in DRG do not show defects in neuronal viability or differentiation but defects in extension and arborisation of peripheral axonal projections in vivo, similar to the target innervation defects observed in mice lacking NGF.15 Moreover, the authors showed that NGF regulates SRF-dependent gene expression and axonal outgrowth through the activation of both MEK/ERK and MAL signalling pathways (Fig. 1B).
Considering all the above findings, one could imagine that SRF is an important regulator in a common and conserved mechanism that ensures the correct branching of special ramified cellular systems, driven by the local secretion of specific growth factors (GFs). The GF-induced SRF activity ensures the cytoskeletal remodelling needed to enable GF-driven chemotaxis and non-programmed branching. In mammals, other systems possess highly branched structures, such as the respiratory and ureteric systems, within which the morphogenic processes are, in part, regulated by GFs.16 It would therefore be interesting to investigate the role of SRF during the morphogenesis of these different tissues.
In addition to defects in sprouting angiogenesis, SRF deletion in ECs also compromises the vascular integrity of small vessels. Indeed, our results showed that SRF directly regulates the expression of VE-cadherin and that SRF misexpression in ECs induces defective EC junction assembly, both in vivo and in vitro.5 Furthermore, inactivation of SRF in embryonic ECs leads to the decreased expression of several integrins, ZO-1, occludin, Alk1, Alk5, endoglin and connexion-43, which could contribute to the vascular leakage of small vessels observed in SRF mutant embryos.5 It is important to note that conceptually, vascular stability is in opposition to vascular sprouting, the latter tending to destabilise EC junctions to promote EC migration.1 The precise mechanism controlling this dual role of SRF in ECs remains to be established, and more work is needed to elucidate this paradigm. Interestingly, a similar dual role of the membrane associated scaffold protein angiomotin (Amot) in the regulation of cell-cell junctions and endothelial cell migration has recently been demonstrated.17 Amot knockout mice die around E11.5 and exhibit an insufficient capillary formation and dilated vessels in the brain. In addition, knockdown of Amot in zebrafish reduced the number of endothelial tip cell filopodia and severely impaired the migration of intersegmental vessels.18 Interestingly, Amot functions additively with synectin-binding guanine exchange factor (Syx),19 a protein expressed specifically in blood vessels. Syx inactivation in the zebrafish and mouse indicates a specific role for Syx in VEGF-A-induced EC migration sprouting angiogenesis.20 Ernkvist et al. proposed a mechanism whereby endothelial migration is dependent on the formation of the Amot-Syx complex to precisely localize RhoA activity to the leading front of migrating cells. Given the similarities between the effects of deleting Syx and SRF in ECs and their link with the cell cytoskeleton, it would be interesting to investigate the possible interaction of the Amot-Syx signalling pathway with SRF activity in EC migration and vessel stability.
The dual role of SRF in sprouting angiogenesis and vascular stability could be explained through the association of SRF with different cofactors. In fact, one of the most impressive features relating to SRF is the high number of cofactors that modulate its DNA-binding and activity. SRF cofactors include members of the myocardin, GATA and Ets families.3 Interestingly, Olson's group showed that in smooth muscle cells, myocardin and Elk-1 compete for the same docking site in SRF thereby modulating smooth muscle-specific gene expression.21 Therefore, it seems reasonable to think that different cofactors could modulate SRF activity in the endothelium in a similar manner. GATA and Ets family members could be possible candidates since several works have shown that GATA and Ets members have important roles in EC biology and several members are bona fide SRF cofactors.3,22 Another possible SRF cofactor regulating SRF function in endothelial cells is MAL. It would be interesting to investigate if the MAL-actin pathway is also present in endothelial cells and, if so, to characterize its contribution to the angiogenic process. To date, expression of MAL in endothelial cells has, to our knowledge, never been reported. However, given that in the Drosophila tracheal system and mouse neurons, MRTF proteins seem to be implicated in SRF-mediated branching, a similar mechanism could exist in ECs.
Another emerging field in vascular biology is the role of microRNAs (miRs) in controlling vascular morphogenesis.23 In 2005, a study reported defects in blood vessel formation/maintenance resulting from a hypomorphic mutation in Dicer, an enzyme controlling microRNA maturation, suggesting a possible role for this enzyme in angiogenesis.24 More recently, the conditional inactivation of Dicer in ECs revealed the importance of the enzyme for normal postnatal angiogenesis.25 Furthermore, several EC-specific microRNAs have been shown to regulate EC function and angiogenesis, such as miR-222, miR-221, miR-27b, miR-21 and let-7f.23 Several recent studies have demonstrated the importance of specific microRNAs in embryonic and postnatal angiogenesis. It has been reported that miR-126 represses the expression of the p85β subunit of phosphatidylinositol-3-kinase (PI3K), sprouty-related protein with EVH-1 domain 1 (Spred1), and vascular cell adhesion protein 1 (VCAM-1) in ECs.23,26 Disruption of miR-126 expression in mice and zebrafish causes haemorrhage and loss of vascular integrity, as well as defects in EC proliferation, migration and angiogenesis.26–28 In this context, it is important to note that SRF is a well known regulator of miRNA expression and that at least 169 microRNAs in mammalian genomes, contain at least 1 CArG element in their promoter region.6,29 On the other hand, it is also known that some microRNAs can repress SRF synthesis, such as miR-133 in skeletal myoblasts.30 It will therefore be of great future interest to study the influence of SRF in the expression of target microRNAs in ECs during vascular development. A further large-scale characterization of the precise genes and microRNAs regulated by SRF in ECs is necessary and will give new important insight into the particular role of SRF during angiogenesis.
Finally, given the role of SRF in sprouting angiogenesis, it would be reasonable to expect SRF inhibition to compromise vessel sprouting and angiogenesis in adult mice as well. Tumoral angiogenesis is an important step in tumor development and malignancy and shares common signalling pathways with embryonic angiogenesis.1 For this reason, SRF inhibition in adult ECs could contribute to decreased angiogenesis and thus decreased tumor growth.
Altogether, the outcome from our study is that SRF is an important transcription factor during sprouting angiogenesis. SRF, functioning as a major regulator of cytoskeleton and cell adhesion molecules, acts as a linker between extracellular stimuli (such as VEGF, FGF, mechanic stress) and cellular phenotype (such as cell migration, cell junction assembly). Given the central role of SRF in regulating actin dynamics, its potential interaction with different cofactors, its involvement in the expression of miRs, and the different feedback control of SRF activity (such as that by actin polymerization and some miRs), we believe that a better understanding of the SRF function in endothelial cells will lead to new perspectives providing important insight into the highly complex field of vascular morphogenesis.
Acknowledgements
This work was supported by the french National Research Agency.
Abbreviations
- Amot
angiomotin
- Bnl
branchless
- Btl
breathless
- DMRTF
drosophila myocardin-related transcription factor
- DRG
dorsal root ganglia
- EC
endothelial cell
- ERK
extracellular-signal-regulated kinase
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- GF
growth factor
- HIF
hypoxia-inducible factor
- MAL
megakaryocytic acute leukaemia
- MAP
mitogen-activated protein
- MEK
MAP/ERK kinase
- MAPK
MAP kinase
- miR
microRNA
- NGF
nerve growth factor
- SRF
serum response factor
- Syx
synectin-binding guanine exchange factor
- VEGF
vascular endothelial growth factor
- ZO-1
zona occludens 1
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/8291
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 3.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 4.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 5.Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, et al. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 2008;15:448–461. doi: 10.1016/j.devcel.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Niu Z, Iyer D, Conway SJ, Martin JF, Ivey K, Srivastava D, et al. Serum response factor orchestrates nascent sarcomerogenesis and silences the biomineralization gene program in the heart. Proc Natl Acad Sci USA. 2008;105:17824–17829. doi: 10.1073/pnas.0805491105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 8.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 9.Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18:1264–1266. doi: 10.1096/fj.03-1232fje. [DOI] [PubMed] [Google Scholar]
- 10.Nagy JA, Senger DR. VEGF-A, cytoskeletal dynamics, and the pathological vascular phenotype. Exp Cell Res. 2006;312:538–548. doi: 10.1016/j.yexcr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–2064. doi: 10.1242/dev.014498. [DOI] [PubMed] [Google Scholar]
- 12.Han Z, Li X, Wu J, Olson EN. A myocardin-related transcription factor regulates activity of serum response factor in Drosophila. Proc Natl Acad Sci USA. 2004;101:12567–12572. doi: 10.1073/pnas.0405085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti S, Krause SM, Kretz O, Philippar U, Lemberger T, Casanova E, et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc Natl Acad Sci USA. 2005;102:6148–6153. doi: 10.1073/pnas.0501191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knöll B, Kretz O, Fiedler C, Alberti S, Schütz G, Frotscher M, Nordheim A. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat Neurosci. 2006;9:195–204. doi: 10.1038/nn1627. [DOI] [PubMed] [Google Scholar]
- 15.Wickramasinghe SR, Alvania RS, Ramanan N, Wood JN, Mandai K, Ginty DD. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 17.Bratt A, Birot O, Sinha I, Veitonmäki N, Aase K, Ernkvist M, Holmgren L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280:34859–34869. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 18.Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21:2055–2068. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, et al. The Amot/Patj/Syx signalling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–253. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnaas MK, Moodie KL, Liu ML, Samant GV, Li K, Marx R, et al. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in Vivo. Circ Res. 2008;103:710–716. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 22.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 24.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 25.Suárez Y, Fernández-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, et al. Dicerdependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 27.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu Z, Li A, Zhang SX, Schwartz RJ. Serum response factor micromanaging cardiogenesis. Curr Opin Cell Biol. 2007;19:618–627. doi: 10.1016/j.ceb.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]