Abstract
DOCK180 is an atypical guanine nucleotide exchange factor of Rac1 identified originally as one of the two major proteins bound to the SH3 domain of the Crk adaptor protein. DOCK180 induces tyrosine phosphorylation of p130Cas, and recruits the Crk-p130Cas complex to focal adhesions. Recently, we searched for DOCK180-binding proteins with a nano-LC/MS/MS system, and found that ANKRD28, a protein with twenty-six ankyrin domain-repeats, interacts with the SH3 domain of DOCK180. Knockdown of ANKRD28 reduced the migration velocity and altered the distribution of focal adhesion proteins such as Crk, paxillin and p130Cas. On the other hand, the expression of ANKRD28, p130Cas, Crk and DOCK180 induced hyper-phosphorylation of p130Cas, which paralleled the induction of multiple long cellular processes. Depletion of ELMO, another protein bound to the SH3 domain of DOCK180, also retarded cell migration, but its expression together with p130Cas, Crk and DOCK180 induced extensive lamellipodial protrusion around the entire circumference without 130Cas hyperphosphorylation. These data suggest the dual modes of DOCK180-Rac regulation for cell migration.
Key words: ankyrin, Rac, p130Cas, DOCK180, paxillin, Crk, migration, focal adhesion
DOCK180 was originally identified as one of the two major binding proteins of the adaptor protein Crk.1 The orthologs of DOCK180 in C. elegans and D. melanogaster are Ced-5 (cell death abnormal 5) and Mbc (Myoblast city), respectively, and together with DOCK180 comprise an evolutionarily conserved protein group.2 Depletion or mutation of DOCK180 in C. elegans reduces engulfment of dying cells and distal tip cell migration,2 and mutations of DOCK180 in D. melanogaster cause embryos to exhibit defects in dorsal closure and cytoskeletal organization in the migrating epidermis.3 In zebrafish, DOCK180 and DOCK5 are both required for fast-twitch myoblast fusion.4 Mice with disrupted DOCK180 show defects in breathing and posture due to defects in myoblast fusion, which were not observed in DOCK5 null mice.5 A spontaneous mutation in DOCK5 was found in mice exhibiting rupture of lens cataract.6 A series of studies using gene-targeting techniques in mice provided evidence for the role of DOCK2, a haematopoietic specific DOCK family member, in lymphocytes and neutrophil motility7,8 and T cell proliferation and development9 Genetic and biochemical studies have revealed that DOCK180 functions as a guanine-nucleotide exchange factor (GEF) for a small GTPase Rac.10,11 In addition, studies in C. elegans have identified orthologs of Crk (Ced-2) and Rac (Ced-10) to show that the Crk-DOCK180-Rac signaling axis is evolutionally conserved.12
Recently we showed that ANKRD28 binds to the N-terminus of DOCK180 containing the SH3 domain.13 The same region of DOCK180 was previously reported to interact with ELMO (engulfment and cell motility). Since the ortholog of ELMO in C. elegans, Ced-12, is required for apoptotic cell clearance and cell migration, as are DOCK180 and Rac,14,15 the signaling pathway of ELMO-DOCK180-Rac is evolutionary conserved and used for cell motility. Both depletion of ANKRD28 and depletion of ELMO retard cell migration in mammalian cells, and they compete with each other for DOCK180 interaction, suggesting that they independently regulate DOCK180 for Rac activation. Whether or not the binding of ELMO directly increases DOCK180 GEF activity remains a matter of controversy,16,17 but it has been established that ELMO regulates DOCK180 in two different modes: One is stabilizing DOCK180 expression by preventing it from ubiquitylation,18 and the other is bridging RhoG and DOCK180 for Rac activation.19 ANKRD28 does not possess these functions; instead, it is suggested to regulate focal adhesion distribution, and thereby to regulate cell migration.
p130Cas was identified as the major substrate of Src. In a subsequent study, it was shown that the phosphorylated tyrosines of p130Cas recruit Crk to transmit signals from integrins.20,21 Independently from this observation, retrovirus insertion mutagenesis identified p130Cas as one of the genes involved in tamoxifen-resistance and estrogen-independent growth in breast cancer.22 In agreement with this discovery, it has been demonstrated that p130Cas phosphorylation is enhanced in tamoxifen-treated estrogen receptor-positive MCF-7 breast cancer cells.23 We have previously shown that DOCK180 expression enhances p130Cas-CrkII complex formation and the tyrosine phosphorylation of p130Cas.24 The p130Cas-Crk-DOCK180 complex formation and p130Cas phosphorylation may occur in focal adhesion, since the phosphorylation was not induced when the cells were cultured in suspension,13 and these proteins were recruited to the focal adhesion only in the presence of all three proteins.24 Since the DOCK180 mutants without the SH3- or Crk-binding domain were deficient in p130Cas phosphorylation, we speculate that DOCK180 somehow stabilizes the p130Cas-Crk complex with the Crk- and SH3-binding proteins, or that these domains are required for the proper conformation of DOCK180 to interact with the binding partners. In addition to the phosphorylated p130Cas-Crk interaction, the SH3 domain of p130Cas also binds directly to DOCK180,25 which might be effective for tight interaction of p130Cas-Crk-DOCK180. Interestingly, ANKRD28, but not ELMO, enhances p130Cas phosphorylation induced by the DOCK180-Crk complex.13 In accordance with this observation, depletion of ANKRD28 or DOCK180 relocates CrkII, p130Cas and paxillin from focal adhesions to the cytosol or other unidentified structures within the cells. As expected, ELMO depletion also induces mis-localization of focal adhesions, probably by reducing the DOCK180 protein expression.
The distinct roles of ANKRD28 and ELMO have also been addressed by peculiar cell morphologies upon their expression. Plasma membrane-targeted DOCK180 alters the morphology of COS cells from spindle-shaped to flat and polygonal1 due to activation of Rac throughout the entire plasma membrane. In contrast, coexpression of DOCK180, Crk and p130Cas induces a limited cellular extension; that is, elongated cellular branches with local membrane protrusion.13,24 Additional expression of ANKRD28 in cells expressing p130Cas, Crk and DOCK180 increases the length and numbers of the cellular branches. In contrast, expression of ELMO induces marked lamellipodial formation along the entire cell circumference, as does the plasma membrane-tagged DOCK180 or activated Rac.13 Since DOCK7 regulates axon development through Rac activation and microtubule stabilization,26 we initially surmised that the branches were generated by outgrowth from the cell body. However, real-time imaging revealed that the extended cellular branches are the tails of randomly migrating cells. Since the magnitude of the phosphorylation paralleled the length of the cellular branches, we speculate that p130Cas hyperphosphorylation induces retarded retraction.
Using purified pseudopodia from migrating cells, Klemke and colleagues have shown that the Crk-130Cas complex is formed in the growing pseudopodia to upregulate Rac.27–29 Although they have not examined whether DOCK180, ELMO and/or ANKRD28 are present in these purified pseudopodia, it is likely and understandable that the p130Cas-Crk-DOCK180 complex localizes and functions at the front of the cell to reorganize the actin cytoskeleton via Rac1 activation, since the cell front of migrating cells exhibits significant membrane ruffling and lamellipodia formation.30,31 The recent finding that phosphorylation of p130Cas initiates Rac activation and membrane ruffling supports this model.32 Compared to these studies on the leading edge formation, we have very limited knowledge on the trailing edge biogenesis and regulation.33 In the literature, one can find figures of cells with elongated branches that resemble ANKRD28-expressing cells; these include myosinIIA-null mouse ES cells34 and HEF1-expressing MCF7 cells treated with ROCK inhibitor.35 Interestingly, A375M2 melanoma cells exhibit an elongated shape in three-dimensional environments containing ROCK or myosin II inhibitors.36 This elongated shape is due to mesenchymal-type movement, which is correlated to the level of active Rac, and is dependent on the expression of DOCK3/MOCA and NEDD9/HEF1, a member of the DOCK180 family and a member of p130Cas family, respectively.36 In addition, the urokinase-type plasminogen activator receptor, together with integrinβ3, drives formation of the p130Cas-Crk-DOCK180 signaling complex for Rac activation, which elevates cell motility and elongated cell shape.37 Although these reports have indicated the roles of DOCK180-Rac in cell invasion and leading edge protrusion, they do not rule out the possibility that the same complex also functions at the trailing edge. The GEF activity of DOCK180 is required for p130Cas phosphorylation;13 expression of p130Cas and Crk enhances DOCK180-GEF activity toward Rac1;10 and depletion of p130Cas perturbs paxillin disassembly at focal adhesions.38 These observations suggest that ANKRD28 alters local Rac activity and p130Cas kinetics at the elongated region of the cell, which should be confirmed experimentally in the future.
We speculate that ANKRD28 functions as a scaffold protein for the components of focal adhesion proteins (Fig. 1), because ANKRD28 is mostly comprised of ankyrin repeat domains and intercalating linker domains. The ankyrin repeat, a 33-residue sequence motif, folds the canonical helix-loop-helix-β-hairpin/loop and mediates protein-protein interactions.39 Tandem ankyrin repeats exhibit tertiary-structure-based elasticity and behave as a liner and fully reversible spring.40 It should be noted that mechanical strain applied to cells induces p130Cas extension and tyrosine phosphorylation at the cell periphery.41 Therefore, it is tempting to speculate that ANKRD28 may cooperate with p130Cas to sense the strain and to regulate focal adhesion dynamics and cell migration.
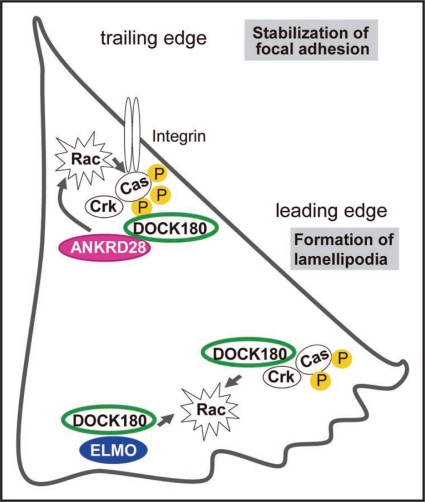
Figure 1.
Dual-mode of DOCK180 functions in the migrating cell. ANKRD28 forms a complex with p130Cas-Crk-DOCK180 at the trailing edges to enhance p130Cas phosphorylation, while ELMO binds to RhoG and DOCK180 at the leading edges to induce membrane ruffling. Both ANKRD28 and ELMO are required for cell migration.
A variant of ANKRD28, known as Phosphatase Interactor Targeting K protein (PITK), has been reported as a PP1-targeting subunit that modulates the phosphorylation of the transcriptional regulator hnRNP K.42 Another variant of ANKRD28, PP6-ARS-A, has been identified as one of the PP6-binding partners.43 In addition, it has recently been reported that the chromosomal translocation in a patient with adult myelodysplastic syndrome/acute myelogenous leukemia resulted in ANKRD28-NUP98 fusion protein, which localized to the nucleus.44 In our hands, ANKRD28 mRNA was expressed ubiquitously and the majority of ANKRD28 protein was localized at the cytosol. Further examination of the localization of ANKRD28 and its binding partners for the purpose of mediating focal adhesion dynamics would provide more clues as to how ANKRD28 functions in cell migration.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/8857
References
- 1.Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996;16:1770–1776. doi: 10.1128/mcb.16.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 3.Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city Encodes a Conserved Protein That Is Essential for Myoblast Fusion, Dorsal Closure and Cytoskeletal Organization. J Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CA, Parkin CA, Bidet Y, Ingham PW. A role for the Myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development. 2007;134:3145–3153. doi: 10.1242/dev.001214. [DOI] [PubMed] [Google Scholar]
- 5.Laurin MA, Fradet N, Blangy A, Hall A, Vuori K, Cote JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Pro Nat Acad Sci. 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omi N, Kiyokawa E, Matsuda M, Kinoshita K, Yamada S, Yamada K, et al. Mutation of Dock5, a member of the guanine exchange factor Dock180 superfamily, in the rupture of lens cataract mouse. Exp Eye Res. 2008;86:828–834. doi: 10.1016/j.exer.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 8.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, et al. Sequential Regulation of DOCK2 Dynamics by Two Phospholipids during Neutrophil Chemotaxis. Science. 2009:1170–1179. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, et al. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–129. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 10.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan KM, Barrett K, Lu Y, Hu KQ, Vincent S, Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana M, Kiyokawa E, Hara S, Iemura Si, Natsume T, Manabe T, et al. Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp Cell Res. 2009;315:863–876. doi: 10.1016/j.yexcr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, et al. CED-12/ELMO, a Novel Member of the CrkII/Dock180/Rac Pathway, Is Required for Phagocytosis and Cell Migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 15.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, et al. Dock180 and ELMO1 Proteins Cooperate to Promote Evolutionarily Conserved Racdependent Cell Migration. J Biol Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 16.Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, et al. An Alpha-Helical Extension of the ELMO1 Pleckstrin Homology Domain Mediates Direct Interaction to DOCK180 and Is Critical in Rac Signaling. Mol Biol Cell. 2008;8:4. doi: 10.1091/mbc.E08-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Kinchen JM, Rossman KL, Grimsley C, deBakker C, Brugnera E, et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol. 2004;11:756–762. doi: 10.1038/nsmb800. [DOI] [PubMed] [Google Scholar]
- 18.Makino Y, Tsuda M, Ichihara S, Watanabe T, Sakai M, Sawa H, et al. Elmo1 inhibits ubiquitylation of Dock180. J Cell Sci. 2006;119:923–932. doi: 10.1242/jcs.02797. [DOI] [PubMed] [Google Scholar]
- 19.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 20.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trend Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman A, van der Flier S, Kok EM, Dorssers LCJ. BCAR1, a Human Homologue of the Adapter Protein p130Cas, and Antiestrogen Resistance in Breast Cancer Cells. J Natl Cancer Inst. 2000;92:112–120. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- 23.Cowell LN, Graham JD, Bouton AH, Clarke CL, O'Neill GM. Tamoxifen treatment promotes phosphorylation of the adhesion molecules, p130Cas//BCAR1, FAK and Src, via an adhesion-dependent pathway. Oncogene. 2006;25:7597–7607. doi: 10.1038/sj.onc.1209747. [DOI] [PubMed] [Google Scholar]
- 24.Kiyokawa E, Hashimoto Y, Kurata T, Sugimura H, Matsuda M. Evidence that DOCK180 upregulates signals from the CrkII-p130Cas complex. J Biol Chem. 1998;273:24479–24484. doi: 10.1074/jbc.273.38.24479. [DOI] [PubMed] [Google Scholar]
- 25.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watabe-Uchida M, John KA, Janas JA, Newey SE, van Aelst L. The Rac Activator DOCK7 Regulates Neuronal Polarity through Local Phosphorylation of Stathmin/Op18. Neuron. 2006;51:727–739. doi: 10.1016/j.neuron.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk Coupling Serves as a “Molecular Switch” for Induction of Cell Migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho SY, Klemke RL. Extracellular-regulated Kinase Activation and CAS/Crk Coupling Regulate Cell Migration and Suppress Apoptosis during Invasion of the Extracellular Matrix. J Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishioka T, Aoki K, Hikake K, Yoshizaki H, Kiyokawa E, Matsuda M. Rapid Turnover Rate of Phosphoinositides at the Front of Migrating MDCK Cells. Mol Biol Cell. 2008;19:4213–4223. doi: 10.1091/mbc.E08-03-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Mayer B. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biology. 2008;9:50. doi: 10.1186/1471-2121-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rid R, Schiefermeier N, Small JV, Kaverina I. The Last but not the Least: The Origin and Significance of Trailing Adhesions in Fibroblastic Cells. Cell Motil Cytoskeleton. 2005;61:161–171. doi: 10.1002/cm.20076. [DOI] [PubMed] [Google Scholar]
- 34.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 35.Bargon SD, Gunning PW, O'Neill GM. The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochimica et Biophysica Acta (BBA) 2005;1746:143–154. doi: 10.1016/j.bbamcr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac Activation and Inactivation Control Plasticity of Tumor Cell Movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 37.Smith HW, Marra P, Marshall CJ. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol. 2008;182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 39.Mosavi LK, Cammett TJ, Desrosiers DC, Peng Zy. The ankyrin repeat as molecular architecture for protein recognition. Prot Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature. 2006;440:246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 41.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force Sensing by Mechanical Extension of the Src Family Kinase Substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwiek NC, Thacker DF, Datto MB, Megosh HB, Haystead TAJ. PITK, a PP1 targeting subunit that modulates the phosphorylation of the transcriptional regulator hnRNP K. Cell Signal. 2006;18:1769–1778. doi: 10.1016/j.cellsig.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Stefansson B, Ohama T, Daugherty AE, Brautigan DL. Protein Phosphatase 6 Regulatory Subunits Composed of Ankyrin Repeat Domains. Biochemistry. 2008;47:1442–1451. doi: 10.1021/bi7022877. [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa M, Yagasaki F, Okamura D, Maeda T, Sugahara Y, Jinnai I, et al. A Novel Gene, ANKRD28 on 3p25, Is Fused with NUP98 on 11p15 in a Cryptic 3-Way Translocation of t(3;5;11)(p25;q35;p15) in an Adult Patient with Myelodysplastic Syndrome/Acute Myelogenous Leukemia. Int J Hematol. 2007;86:238–245. doi: 10.1532/IJH97.07054. [DOI] [PubMed] [Google Scholar]