Abstract
Directional cell migration requires cell polarization and asymmetric distribution of cell signaling. Focal adhesions and microtubules are two systems which are essential for these. It was shown that these two systems closely interact with each other. It is known that microtubule targeting stimulates focal adhesion dissociation. Our recent study shows that focal adhesions, in turn, specifically induce microtubule catastrophe via a biochemical mechanism. We were able to track down one of the focal adhesion proteins paxillin which is involved in this process. Paxillin phosphorylation was previously shown to be the key component in the regulation of focal adhesion assembly or disassembly. Since microtubule catastrophe dynamic differs at the leading edge and cell rear, similar to paxillin phosphorylation levels, we suggest a model connecting asymmetric distribution of focal adhesions and asymmetric distribution of microtubule catastrophes at adhesion sites as a feedback loop.
Key words: microtubule catastrophe, focal adhesion, microtubules, paxillin, cell motility
Cell migration is important for many biological processes. It requires organized asymmetric dynamics of focal adhesions (FAs), sites where cells interact with extra cellular matrix. FAs appear at the leading edge as small transient dot-like structures termed focal complexes (FXs).1,2 FX assembly and disassembly is regulated by phosphorilation status of paxillin a major FX protein.3,4 Most of FXs form and disassemble rapidly. However, some adhesions mature in a force-dependent manner, into larger late adhesions. This process, involves both an increase in size and change in molecular composition3,5 and is accompanied by a reduction in local paxillin phosphorylation.4 Late adhesions are more stable, immobile and undergo forced disassembly by multiple microtubule targeting events6 only underneath the approaching cell body or transform into fibrillar adhesions by a Src-dependent mechanism.7
Similarly to the leading edge, proper adhesion patterns at the cell rear are also essential. Most trailing adhesions are initiated in protrusions at the rear and flanks of the cell as FX rapidly mature in response to tension and transform into sliding trailing adhesions.8 The process of sliding is complex. While adhesion proteins coupled with the actin cytoskeleton can be translocated relative to substratum, those that are associated with the membrane are thought to undergo treadmilling within the adhesion site.9,10 Treadmilling, which includes disassembly of adhesion proteins at the distal end and reassembly at the proximal end,10 is accompanied by fusion with new adhesions formed in front of the sliding one.6 Thus, despite a protein composition similar to late adhesions, sliding adhesions are more dynamic. Not surprisingly, sliding adhesions have high paxillin phosphorylation at the distal end of the adhesion site, indicating very dynamic assembly/disassembly rates.4
Several mechanisms have been proposed for the regulation of adhesion turnover (reviewed in ref. 11). However, these have not accounted for the observed asymmetry of adhesion turnover. Understanding this requires examining the connection with another asymmetric intracellular system, the microtubule network. This dynamic network closely interacts with FAs. Microtubules play an essential role in cell migration and polarized distribution of signals within the cell. Multiple microtubule targeting to FA leads to their disassembly both at the leading edge and at the cell rear.6
Unlike microtubule growth in other cell regions, growth at its leading edge is persistent, characterized by short periods of shrinkage.8 Simultaneous observation of microtubules and FAs show that microtubules specifically target adhesion sites.12 More detailed analysis of microtubule dynamics reveals that FAs are preferable sites for microtubule catastrophes.13 Although FAs cover only about 5% of cell area more than 40% of catastrophes occur at these sites. The likelihood of microtubule catastrophe is seven times higher when a microtubule grows through a FA rather than through an adhesion-free area13 and about 90% of microtubules approaching adhesion sites undergo catastrophe. Although most of the catastrophes occur at late adhesions, due to their increased stability and lifespan, there is no difference in efficiency of catastrophe induction between small focal complexes and large rigid late adhesions.13 As FX do not have dense adhesion or actin plaque, it is likely that microtubule catastrophe is triggered by a biochemical mechanism rather than mechanical rigidity. This is also supported by the fact that mechanical obstacles in a cell do not necessarily cause microtubule catastrophe.13
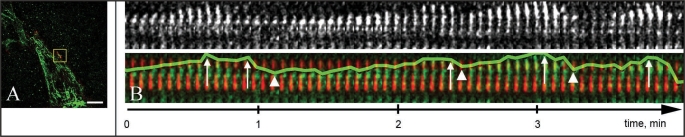
At the cell rear, microtubule dynamics differ from those at the leading edge. Microtubules spend less time in a growing phase and more time in pauses and shrinkage.8 Polymerization and depolymerization occur within a very limited area close to the cell edge.8 Live-cell imaging of cells expressing both microtubule and focal adhesion markers show that this complex dynamic sequence often happens within a single sliding adhesion. Microtubules that are captured at the proximal end of adhesion undergo multiple repetitive catastrophes at the distal end (Fig. 1) accompanied by rescue at the capture site. Thus, the capture mechanism significantly increases the lifetime of a microtubule and ensures that repetitive catastrophes occur at the single adhesion. This scenario leads to high catastrophe frequency at the cell rear, resulting in intensive catastrophe-dependent regulation in this cell region.
Figure 1.
Multiple microtubule catastrophes at the sliding adhesion. (A) Frame from TIRF video sequence of a fish fibroblast cell (CAR) co-transfected with GFP-tubulin (green) to visualize microtubules and Cherry-Zyxin (red) to mark focal adhesions. The boxed region is presented in the kymograph in (B). Bar, 10 µm. (B) Kymograph of microtubule dynamics at a trailing end focal adhesion. Top panel shows microtubule (MT) only. Bottom panel shows life history plot of MT (green line shows movement of MT end) in relation to focal adhesions (red). Arrows show catastrophes at the distal end of adhesion, arrowheads show capture at the proximal end of adhesion.
Detailed analysis of microtubule catastrophe localization shows that they occur at the areas of FAs where paxillin is enriched and highly phosphorylated.4,13 Paxillin was shown to interact with microtubules through its Lim2/Lim3 domain.14 Purified GST-Lim2/Lim3 fragment injected into the cell localizes to FAs, displacing endogenous paxillin.13 This leads to a 40% decrease in the number of microtubule catastrophe events at adhesion sites,13 indicating that paxillin is needed for catastrophe initiation.
In summary, we conclude that microtubule catastrophes at focal adhesions are specific events that are triggered by a biochemical mechanism. This process involves the focal adhesion protein paxillin, which may serve as a docking site for microtubules and/or microtubule catastrophe factors. The nature of catastrophe factors remains to be clarified. Possible mechanisms include molecules which induce microtubule catastrophe directly, such as stathmin,15 or molecules which regulate catastrophe-inducing factors activity. Alternatively, catastrophe factors at adhesion sites could act by removing stabilizing factors from microtubule tips. Thus, allowing already active catastrophe-inducing molecules such as kinesin-13 family member MCAK16,17 to complete their function. Furthermore, microtubule catastrophe at paxillin-enriched areas, followed by release of microtubule-associated factors, may be involved in paxillin phosphorylation. This local regulation of adhesion disassembly would close the feed-back loop to microtubule regulation of FA turnover.
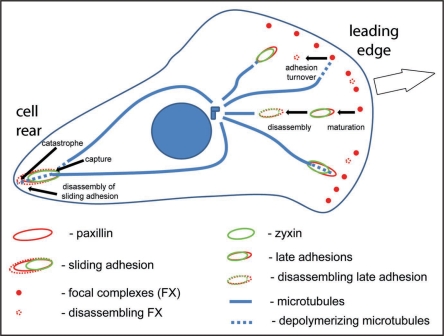
In this model, asymmetric distribution of microtubule catastrophes is tightly linked to asymmetric regulation of FA. Since asymmetric FA dynamics in a cell are critical for organization of the actin cytoskeleton, tensile force distribution and directional cell migration, we conclude that microtubule catastrophes serve as important regulatory events for asymmetric signaling and dynamics of the whole cell (Fig. 2).
Figure 2.
Model for asymmetric focal adhesion and microtubule dynamics. Focal complexes at the leading edge either disassemble or mature in response to tension. Microtubules undergo catastrophe both at focal complexes and late adhesions. Late adhesions disassemble in response to multiple microtubule targeting. At the cell rear a microtubule is captured at the proximal end of sliding adhesion and undergoes multiple catastrophes at its distal end, supporting disassembly of this region.
Acknowledgements
This work was supported by NIH NIGMS grant 1RO1GM078373-01A2 to I.K.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/8858
References
- 1.Nobes CD, Hall A. Rho rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 8995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 2.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in thecontrol of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 3.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 5.Rottner K, Krause M, Gimona M, Small JV, Wehland J. Zyxin isnot colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tipsand exhibits different dynamics to vinculin, paxillin and VASP in focal adhesions. Mol Biol Cell. 2001;12:3103–3113. doi: 10.1091/mbc.12.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rid R, Schiefermeier N, Grigoriev I, Small JV, Kaverina I. The last but not the least: the origin and significance of trailing adhesions in fibroblastic cells. Cell Motil Cytoskeleton. 2005;61:161–171. doi: 10.1002/cm.20076. [DOI] [PubMed] [Google Scholar]
- 7.Volberg T, Romer L, Zamir E, Geiger B. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J Cell Sci. 2001;114:2279–2289. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- 8.Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil. Cytoskeleton. 1999;42:48–59. doi: 10.1002/(SICI)1097-0169(1999)42:1<48::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 10.Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Kaverina I, Rottner K, Small JV. Targeting, capture and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efimov A, Schiefermeier N, Grigoriev I, Ohi R, Brown MC, Turner CE, et al. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J Cell Sci. 2008;121:196–204. doi: 10.1242/jcs.012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MC, Turner CE. Roles for the tubulin- and PTP-PEST-binding paxillin LIM domains in cell adhesion and motility. Int J Biochem Cell Biol. 2002;34:855–863. doi: 10.1016/s1357-2725(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 15.Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 16.Wordeman L. Microtubule-depolymerizing kinesins. Curr Opin Cell Biol. 2005;17:82–88. doi: 10.1016/j.ceb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Moore AT, Rankin KE, von Dassow G, Peris L, Wagenbach M, Ovechkina Y, et al. MCAK associates with the tips of polymerizing microtubules. J Cell Biol. 2005;169:391–397. doi: 10.1083/jcb.200411089. [DOI] [PMC free article] [PubMed] [Google Scholar]