Abstract
Deficiencies in polyunsaturated essential fatty acids (PUFA) are implicated in mood disorders, although mechanisms of action and regional specificity in the brain are unknown. We hypothesized that plasma phospholipid PUFA levels are correlated with regionally specific relative cerebral metabolic rates of glucose (rCMRglu). 29 medication-free depressed subjects were studied using [18F]-fluoro-2-deoxyglucose positron emission tomography. Docosahexaenoic acid (22:6n-3), arachidonic acid (20:4n-6), and eicosapentaenoic acid (20:5n-3) were assessed as a percentage of total phospholipid PUFA (DHA%, AA%, EPA%, respectively). DHA% and AA% correlated positively with rCMRglu in temporoparietal cortex. In addition, DHA% correlated negatively with rCMRglu in prefrontal cortex and anterior cingulate. No correlations were seen with EPA%. Thus, under conditions of low plasma DHA, rCMRglu was higher in temporoparietal cortex and lower in anterior cingulate/prefrontal cortex. Opposing effects of DHA on these regions is a hypothesis that could be addressed in future prospective studies with n-3 supplementation. This pilot study is the first to demonstrate fatty acid- and regionally specific correlations in the brain between plasma PUFA and rCMRglu in humans.
INTRODUCTION
The major brain species of essential polyunsaturated fatty acids (PUFA) are docosahexaenoate (DHA, 22:6n-3) and arachidonate (AA, 20:4n-6). DHA, in particular, is important for normal brain development [1, 2] and mature brain functioning [3]. Abnormal levels of PUFA also are implicated in mood disorders. Depressive episodes are associated with lower blood levels of n-3 PUFA in Major Depressive Disorder (MDD) [4–8] and Bipolar Disorder (BD) [9]. Higher ratios of n-6 to n-3 fatty acids are seen in MDD [4, 6, 10], in suicide attempters [11, 12], in first-degree relatives of patients with BD [13], and in correlation with severity of manic symptoms [14]. Levels of n-6 fatty acid AA metabolites (prostaglandins) also are higher in MDD [15–19]. A functional opposition of n-3 and n-6 PUFA in mood disorders [20] is consistent with competition of n-3 and n-6 in regard to a number of biochemical processes [21, 22], including long-chain PUFA production from precursors, breakdown to active metabolites, and membrane insertion. Meta-analysis of randomized controlled trials of EPA and DHA supplements in major depression found a large treatment effect size, as reported in treatment recommendations for n-3 fatty acids issued by the American Psychiatric Association. [23]
Although PUFA play a vital role in brain structure and performance, only a few studies have examined relationships between PUFA status and human brain functioning in healthy volunteers [24–28] or in subjects with mood disorders [29–33]. We therefore performed an exploratory study of correlations between plasma DHA and AA levels and rCMRglu in medication-free depressed adult subjects. Eicosapentaenoate (EPA, 20:5n-3) was included as a control, since EPA is a plasma precursor to DHA that is not found in brain in significant amounts. In designing the study, we made the assumption that DHA and AA are each in a steady-state equilibrium between plasma and brain, as brain DHA and AA levels come from circulating DHA and AA entering the brain, equaling their rate of consumption therein [34]. Synthesis of DHA and AA from precursors in the brain is minimal even under conditions of deficiency [34, 35]. Dietary deficiency of n-3 PUFA has been shown to differentially alter regional brain PUFA concentrations in rats: frontal cortex [36–38] and temporal lobe [37] were among the regions with the largest depletion of DHA. In postmortem infants, erythrocyte membrane PUFA levels correlate positively with brain PUFA levels [39].
We further assumed that PUFA can influence brain functioning in part through effects on glucose utilization in a regionally specific manner, since rats on a diet low in n-3 PUFA exhibited 30–50% decreases in DHA in membrane phospholipids and a 30% decrease in relative regional cerebral glucose utilization (rCMRglu) in brain regions studied (fronto-parietal cortex, hippocampus and suprachiasmatic nucleus). [40] Furthermore, in primary cultures of astrocytes from neonatal mice, AA, but not palmitic or arachidic acids, had a time- and concentration-dependent effect of increasing 2-deoxy-D-[1-3H]glucose uptake. [41] One potential mechanism for effects of PUFA deficiency on brain glucose metabolism is post-transcriptional reduction of glucose transport: n-3 deficient rats exhibited decreased immunoreactivity of the glucose transporter GLUT1 but not the corresponding mRNA message in cerebral cortex homogenates and brain microvessels. [42] GLUT1 mediates glucose uptake into astrocytes, where glucose is metabolized to lactate that, upon release, forms the primary energy substrate utilized by neurons. [43]
The primary objective for using this correlative paradigm in a limited sample was to identify brain regions vulnerable to dietary deficiencies in PUFAs. Our hypothesis was that lower levels of DHA would correlate with glucose metabolism in brain circuits known to be implicated in the pathophysiology of depression. Although erythrocyte PUFA levels provide a more long-term measure of subjects’ nutritional state, we studied plasma phospholipid PUFA for a cross-sectional assessment of PUFA dietary status, an approach that has been successfully utilized in animal and human studies [4, 6, 44].
MATERIALS AND METHODS
All subjects (N=29) gave written informed consent as approved by the Institutional Review Board of the New York State Psychiatric Institute, Columbia University. They were drawn from patients presenting for evaluation and treatment for depression in the context of a research study of the biology of mood disorders. At study entry, subjects met DSM-IV criteria [45] for a current major depressive episode in context of major depressive disorder (N=23) or bipolar disorder (N=6) based on the Structured Clinical Interview for DSM-IV [46], with scores greater than 15 on the clinician-rated 17-item Hamilton Depression Rating Scale (HDRS) [47, 48]. Subjects also completed the Beck Depression Inventory (BDI) [49], a subjective self-assessment of depression severity. Subjects were free of medical illness based on history, physical examination and laboratory tests and had not taken psychotropic medications for at least 14 days prior to PET studies (6 weeks for fluoxetine and 4 weeks for oral antipsychotics) with the exception of lorazepam, which could be taken for up to 3 mg daily during the washout phase, but not in the 3 days before scanning. We note that some subjects have been included in other FDG-PET studies with different objectives [12, 50–54].
Baseline fasting plasma samples were drawn before 10 AM on the day of the PET scan, stored at −80°C, and shipped on dry ice to the National Institute of Mental Health, where levels were determined for PUFA in phospholipids according to the following protocol. Total plasma lipids were extracted by a method modified from Folch and colleagues [55]. Samples were aliquoted into 2 mL CHCl3, 1 mL BHT-MeOH, and a known quantity of 23:0 methyl ester as an internal standard. One milliliter of 0.2 mol/L Na2HPO4 was added after brief vortexing. The samples were capped under N2 and vortexed again. After centrifugation, CHCl3 was removed and dried under N2. Total plasma phospholipids were separated using solid phase extraction as previously described [56]. The phospholipid fraction was methylated with BF3-MeOH for 60 min [57]. Samples were kept cold and under N2 throughout analysis to prevent oxidation. Gas chromatography was performed on a Hewlett-Packard (HP) 5890 series II with a flame ionization detector, an autosampler, and a FFAP capillary column (J&W Scientific). Peaks were identified using authentic standards (NuChek Prep, Elysian, MN). Fatty acids were quantified by comparison to peak areas of the 23:0 internal standard. When subjected to thawing and refreezing, within- and between-run coefficients of variance were less than 0.3% and 5%, respectively.
Before entering the scanner, subjects gazed at a uniform visual stimulus (cross hairs) in a dimmed, quiet room during the first 15 min of the 45 min 18FDG distribution phase. After another 15 minutes of resting quietly, they were transferred to the scanner, where they lay supine. The head was positioned with the lowest scanning plane parallel to and approximately 1.0 cm above the canthomeatal line. Head movement was minimized with an individually customized thermoplastic mask. A Siemens ECAT EXACT 47 scanner (in plane spatial resolution 5.8 mm, axial resolution 4.3 mm FWHM at center) acquired a 60-min emission scan in 2D mode as a series of twelve 5-min frames. The attenuation correction was based on a 15-min 68Ge/68Ga transmission scan. Images were reconstructed with a Shepp radial filter, cutoff frequency of 35 and a ramp axial filter, cutoff frequency of 0.5.
Image preprocessing included automated image coregistration [58] to align the 12 frames within each PET scan [59] that were then summed and transformed into MNI standard stereotaxic atlas space. Each image was smoothed by applying an isotropic 12 mm Gaussian kernel, to increase the signal to noise ratio.
To control for between-subject differences in total PUFA, we measured DHA, AA, and EPA as a percentage of total phospholipid PUFA (DHA%, AA%, EPA%). Statistical analyses of associations between individual PUFA and relevant demographic and clinical characteristics were conducted using SPSS Version 11.0.1 for Mac OS X (Chicago: SPSS Inc). Continuous demographic/clinical variables were independently tested for correlation with initial levels of DHA%, AA%, EPA%, using Pearson’s r; for each categorical variable, differences between PUFA means were independently tested using Student’s t.
Independent, voxel-level analyses were performed to determine correlations between rCMRglu and DHA%, AA%, and EPA%, with adjustment for nuisance variables that correlated with or trended toward correlation with individual PUFA concentrations: age, sex, and diagnosis (MDD vs. BD). Analyses were performed using the general linear model with Statistical Parametric Mapping (SPM2; Institute of Neurology, University College of London, London, England) implemented in Matlab 6.5, Release 13, service pack 1 (The Mathworks Inc, Natick, Mass) [60]. Global normalization with proportional scaling was applied to control for global cerebral rates of glucose metabolism and other global effects. For all analyses, voxel intensity and cluster extent thresholds were set a priori to P<0.01 and P<0.05, respectively, after correction for multiple comparisons by SPM. In some post-hoc exploratory analyses, cluster extent (but not voxel intensity) thresholds were relaxed as described in the text in order to detect additional trends. All SPM analyses included diagnosis (MDD vs BD), sex, and age as covariates/cofactors. No adjustments were made for smoking status or body mass index, neither of which correlated with PUFA levels. As described above, results were corrected for multiple comparisons of the voxel-wide analyses, but they were not further corrected for multiple analyses with individual PUFA, since lipid variables are not independent. Regional labelling was accomplished using the SPM-generated MNI coordinates for significant clusters, transformed by algorithm [61] into Talairach space and entered into the Talairach Client (University of Texas Health Science Center, San Antonio).[62]
RESULTS
Clinical Outcomes
Observed mean plasma PUFA levels (μg/ml ± SD) were as follows: total phospholipid fatty acids, 1040.2 ± 217.0; DHA, 36.9 ± 15.9; AA, 113.6 ± 34.6; EPA, 6.6 ± 3.0. Total plasma phospholipid PUFA correlated with individual PUFA: DHA, r = 0.71, p < 0.000; AA, r = 0.85, p < 0.000; EPA, r = 0.53, p = 0.003; this potential confound was controlled for by expressing individual PUFA as a percentage of total PUFA. Observed mean percentages of total phospholipid fatty acids were: DHA% 3.4 ± 1.2; AA% 10.8 ± 2.0; EPA% 0.6 ± 0.2.
Associations between potentially relevant demographic and clinical characteristics and PUFA nutritional status are shown in Table 1. Patients were moderately depressed at study entry and time of scan; neither self-reported (BDI) nor clinician-evaluated (HDRS) measures of depression correlated with any PUFA levels. DHA% and AA%, but not EPA% correlated positively with age and showed a trend associated with sex: DHA% higher in males, AA% higher in females. DHA% was lower in subjects with bipolar disorder compared with MDD. Smoking has been shown to cause degradation of fatty acids [63] and has been associated with reduction in serum fatty acid levels [64]; however, plasma phospholipid levels of DHA% or AA% did not differ between smokers and nonsmokers in this study. Body mass index did not correlate with any fatty acid measures. Based on these findings, we therefore included age and diagnosis as covariates of no interest (nuisance variables) in the statistical parametric analyses; although an association with sex was only a trend, we chose the conservative approach of adjusting for it as well.
Table 1.
Relationships Between Plasma Polyunsaturated Fatty Acid Levels and Clinical/Demographic Characteristics.
| Correlation of PUFA Levels (% of Total Phospholipid FA) with Characteristic | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean (SD) | Phospholipid PUFA | Pearson’s r | p | |||
| Age yrs | 41.2 (12.4) | DHA% | 0.37 | 0.05 | |||
| AA% | 0.51 | 0.004 | |||||
| EPA% | 0.20 | 0.30 | |||||
| BMI | 26.7 (5.0) | DHA% | 0.03 | 0.87 | |||
| AA% | 0.06 | 0.77 | |||||
| EPA% | 0.21 | 0.28 | |||||
| HDRS score | 21.2 (5.0) | DHA% | − 0.05 | 0.80 | |||
| AA% | 0.10 | 0.61 | |||||
| EPA% | − 0.14 | 0.47 | |||||
| BDI score | 32.1 (9.3) | DHA% | − 0.17 | 0.38 | |||
| AA% | 0.04 | 0.85 | |||||
| EPA% | − 0.14 | 0.48 | |||||
|
| |||||||
|
Phospholipid PUFA Levels (% of Total Phospholipid FA) by Characteristic Status | |||||||
| Mean PUFA Level | |||||||
| N(%) | PUFA | MDD | BD | t | df | p | |
| MDD (vs. BD) | 23 (79) | DHA% | 3.7 (1.1) | 2.4 (1.0) | 2.53 | 27 | 0.02 |
| AA% | 11.0 (1.8) | 10.0 (2.6) | 1.12 | 27 | 0.27 | ||
| EPA% | 0.6 (0.2) | 0.6 (0.2) | 0.11 | 26 | 0.91 | ||
| Male | Female | ||||||
| Male | 14 (48) | DHA% | 3.8 (1.2) | 3.1 (1.0) | 1.75 | 27 | 0.09 |
| AA% | 10.1 (2.0) | 11.4 (1.9) | −1.74 | 27 | 0.09 | ||
| EPA% | 0.6 (0.2) | 0.6 (0.2) | 0.87 | 26 | 0.39 | ||
| Smoker | Non-smoker | ||||||
| Smoker | 11 (38) | DHA% | 3.0 (1.2) | 3.6 (1.2) | 1.43 | 27 | 0.16 |
| AA% | 10.3 (2.3) | 11.1 (1.8) | 1.03 | 27 | 0.31 | ||
| EPA% | 0.6 (0.1) | 0.6 (0.3) | 0.94 | 26 | 0.35 | ||
Bolded values significant at p<0.05. Data expressed in mean (SD) or N(%) as appropriate. Individual phospholipid fatty acid values shown as a percentage of total phospholipid fatty acids. Two-tailed Student’s t-tests compare mean PUFA levels for each categorical variable, in columns labelled “+/− characteristic.” Abbreviations: PUFA, polyunsaturated fatty acid; BMI, body mass index; HDRS, Hamilton Depression Rating Scale (17-item); BDI, Beck Depression Inventory; FA, fatty acid; MDD, Major Depressive Disorder; BD, Bipolar Disorder; DHA, docosahexaenoate; AA, arachidonate.
FDG-PET
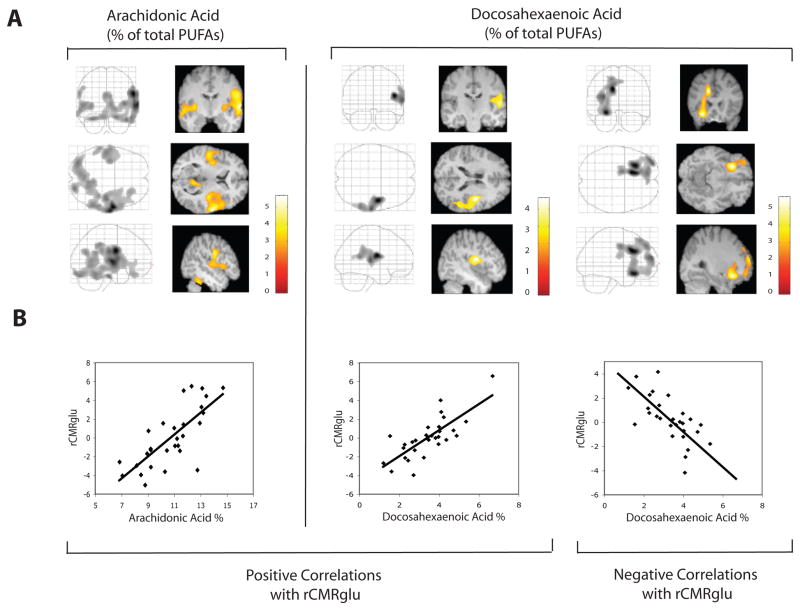
Statistical parametric mapping results of PUFA correlations with rCMRglu (after adjustment for age, sex, and diagnosis and SPM correction for multiple comparisons) are presented graphically in Figure 1 and summarized in Table 2. Both AA% and DHA% correlated positively with rCMRglu in an area of right temporoparietal cortex that included precentral gyrus, superior temporal gyrus, and inferior parietal lobule. The correlated cluster extent (number of voxels) was about five times greater for AA% compared with DHA%, extending to include inferior frontal, fusiform, inferior temporal, inferior frontal, middle temporal, postcentral and lingual gyri; insula, posterior cingulate, claustrum, and bilateral cerebellum. AA% also correlated positively with rCMRglu in left superior and middle temporal gyri. There was no left-sided positive correlation of rCMRglu with DHA% at a priori significance thresholds. However, when the cluster extent significance threshold was lowered post-hoc to pcorrected <0.5 (pcorrected = p after correction for multiple comparisons), with voxel intensity threshold maintained at pcorrected=0.01, positive correlation was found in the corresponding left precentral and superior temporal gyri (data not shown). There were no negative correlations of rCMRglu with AA%. However, DHA% correlated negatively with rCMRglu in left anterior cingulate, middle frontal, and inferior frontal gyri. At the less stringent significance for cluster extent threshold (pcorrected <0.5), the negative correlation between DHA% and rCMRglu in anterior cingulate was also bilateral. As expected, neither positive nor negative correlations were seen with EPA%.
Figure 1. Statistical parametric maps of regional cerebral metabolic rates of glucose metabolism correlated with plasma polyunsaturated fatty acid levels.
Regional cerebral metabolic rates of glucose metabolism (rCMRglu) are shown correlated with plasma phospholipid docosahexaenoate and arachidonate as a percentage (DHA%, EPA%) of total phospholipid polyunsaturated fatty acids (PUFA). All analyses have been corrected for global cerebral rates of glucose metabolism, age, diagnosis (Major Depressive or Bipolar Disorder), and sex. A. Correlations are shown in orthogonal views in a ‘glass brain’ format (left panels) and also as superimposed on relevant MRI slices (right panels). Color scales indicate the strength (t score) of the correlations. B. Correlations are graphed at the cluster global maxima for DHA% (positive, r = 0.77, p = 0.0005; negative, r = −0.77, p < 0.0000) and AA% (positive, r = 0.75, p < 0.0000).
Table 2.
Statistical Parametric Mapping of Correlations Between Plasma Polyunsaturated Fatty Acid Levels and Regional Cerebral Glucose Metabolism.
| rCMRglu Analysis | Cluster- level Pcorrected | Cluster Extent (voxels) | Anatomic Locations **Cluster Maxima | Brodmann Areas | |
|---|---|---|---|---|---|
| Positively correlated with AA% | <0.001 | 10,402 | Right | Precentral Gyrus** | 6, 44 |
| “ | Superior Temporal Gyrus | 13, 22, 42 | |||
| “ | Postcentral Gyrus | 1 | |||
| “ | Inferior Temporal Gyrus | 20 | |||
| “ | Insula | 13 | |||
| “ | Inferior Parietal Lobule | 40 | |||
| “ | Posterior Cingulate | 29, 30 | |||
| “ | Inferior Frontal Gyrus | 11, 47 | |||
| “ | Middle Temporal Gyrus | 21 | |||
| “ | Occipital Lobe, Lingual Gyrus | 19 | |||
| “ | Claustrum | ||||
| “ | Precuneus | 31 | |||
| Left | Cerebellum, Posterior Lobe, Declive, Uvula | ||||
| “ | Cerebellum, Anterior Lobe, Culmen | ||||
| Right | Cerebellum, Posterior Lobe, Declive, Tuber | ||||
| “ | Cerebellum, Anterior Lobe, Culmen | ||||
|
| |||||
| 0.050 | 1,939 | Left | Superior Temporal Gyrus** | 22 | |
| “ | Middle Temporal Gyrus | 21 | |||
| Claustrum | |||||
|
| |||||
| Positively correlated with DHA% | 0.033 | 2,137 | Right | Precentral Gyrus**, | 6 |
| “ | Superior Temporal Gyrus | 13, 22, 42 | |||
| “ | Inferior Parietal Lobule | 40 | |||
|
| |||||
| Negatively correlated with DHA% | < 0.001 | 4,957 | Left | Cingulate Gyrus**, | 24, 32 |
| “ | Middle Frontal Gyrus | 10, 11 | |||
| “ | Inferior Frontal Gyrus | 47 | |||
| “ | Superior Frontal Gyrus | 8 | |||
Abbreviations: rCMRglu, regional cerebral rate of glucose metabolism; DHA% and AA%, docosahexaenoate and arachidonate, respectively, as a percentage of total phospholipid fatty acids; pcorrected, p values after Statistical Parametric Analysis (SPM2) correction for multiple comparisons.
DISCUSSION AND CONCLUSIONS
This is the first study in depressed human subjects to examine the relationship between plasma levels of fatty acids known to have clinical effects on mood and the in vivo glucose metabolism of brain regions implicated in emotion processing. Currently there is significant interest in optimizing the levels and ratios of distinct subtypes of PUFA in depressed subjects, given the increasing usage of n-3 supplementation. We sought to identify neural targets for future research into PUFA effects on cognition and emotion regulation, by identifying specific brain regions that may be uniquely vulnerable to PUFA deficiencies. Results can be used to design region-specific cognitive tasks that can further refine our understanding of the impact of PUFAs on cognitive and emotional functioning.
We found that temporoparietal glucose usage correlated positively with both DHA% and AA%, indicating that individuals with lower PUFA levels have relative glucose hypometabolism in this region. Functions of the superior temporal gyrus include auditory processing [65], error processing in response inhibition tasks [66] and decision-making [67]. These effects may be mediated through connectivity with the limbic system [68, 69], possibly by evaluating the emotional significance of auditory stimuli [70]. In humans, lower erythrocyte DHA correlates with lower scores on the Mini-Mental State Exam among Alzheimer’s patients [71], while supplementation with DHA plus AA improves cognitive functioning in patients with MCI [72]. Prospective studies can be used in the future to test the hypothesis emerging from our findings, that low fatty acids may have an effect on depression-related cognitive problems, such as conflict resolution and decision-making, via decreased glucose metabolism in temporoparietal cortex.
We also observed that DHA% but not AA% correlated negatively with rCMRglu in frontal cortex and anterior cingulate; that is, subjects with low DHA% had relative glucose hypermetabolism in these areas. Speculatively, if DHA has inhibitory effects in the anterior cingulate and areas of prefrontal cortex, then DHA deficiency could result in disinhibition of these regions, both of which have been implicated in regional brain studies of MDD (reviewed in [73]). Previous research suggests these regions may be particularly sensitive to PUFA concentrations: 1) Postmortem studies of orbitofrontal cortex found lower DHA content in females with MDD [32] and lower DHA and AA content in subjects of both sexes with BD [33] compared with age-matched normal controls. 2) In healthy volunteers, higher dietary intake of DHA and EPA was associated with regionally-specific higher gray matter volume in the subgenual anterior cingulate, as well as in right amygdala and right hippocampus. [28]
The observed positive temporoparietal correlation and negative anterior cingulate/prefrontal correlation of rCMRglu with DHA% could indicate cross-talk between these regions. Superior temporal gyrus is known from animal studies to have neuroanatomical connections with anterior cingulate/prefrontal cortex [69, 74–79]. Functional connectivity analyses in primates also have shown that prefrontal cortex exerts inhibitory control over temporal cortex [77, 80]. The opposite directionality of the correlative effects we observed in these regions is consistent with human studies in which increased prefrontal cortical activity is coincident with decreased temporal cortical activity in patients with prefrontal cortical lesions [81, 82] and in patients with schizophrenia [83, 84].
With regard to the laterality of our findings, the meaning of positive right temporoparietal correlation and negative left anterior cingulate/prefrontal correlation of rCMRglu with DHA% is unclear. As the PUFA/rCMRglu correlations were bilateral at a lower significance threshold, the apparent laterality may not have physiological importance. On the other hand, laterality of anatomy and function has been proposed to be a characteristic of the healthy brain that is diminished in conditions of n-3 PUFA deficiency, as observed in rat striatum. [85] Our findings are consistent with this notion, as the correlative effects indicate that the greater the concentrations of PUFAs, the greater the unilateral regional changes in rCMRglu. This particular contralateral regional pairing also has been observed in context of structural changes in subjects with remitted geriatric depression, who were found to have smaller volumes of right middle temporal gyrus and larger left cingulate gyrus volumes. [86]
The lack of correlations with EPA% was expected, as EPA is not expressed in appreciable amounts in brain and therefore acted as a control for spurious findings. Since there was no comparison group, it was not possible to determine whether the observed associations are specifically related to mood disorders. However, the anterior cingulate-prefrontal cortical circuitry has repeatedly been implicated in mood regulation [51, 87–91], including Brodmann area 32 [51, 54, 89, 92], a region negatively correlated with DHA% in our study.
The study employed a between-subjects, rather than a between-groups, design, for two reasons. First, the primary hypothesis was parametric rather than categorical: that as dietary levels of PUFA increased, changes in glucose usage would vary in a PUFA subtype-specific way in brain regions known to mediate depression. A categorical comparison of depressed to non-depressed subjects would have obscured these more nuanced findings. Second, evidence supports the hypothesis that the brains of depressed patients are different, even after recovery from depression, from never-depressed healthy subjects.[93, 94] Differences in activity found in a categorical comparison of these two groups might reasonably be ascribed to baseline differences rather than PUFA levels. To improve interpretability of this initial study, we therefore used correlation between ecologically valid parametric variation in PUFA levels and glucose utilization in a relatively monolithic population, although we acknowledge that within major depression, clinical and biological heterogeneity exists.
Given our findings that PUFA status is correlated with regional brain glucose utilization, additional studies are warranted to compare PUFA-rCMRglu associations in depressed subjects vs. healthy volunteers, since PUFAs have been implicated in the etiology and treatment of depression [4–20]. Another interesting approach would be exploration of associations between PUFA status, rCMRglu, and clinical characteristics such as depression subtype, severity and suicidal ideation. Future studies should also consider associations with other neurobiological systems related to depression. Especially promising targets for study are the monoaminergic and hypothalamic-pituitary-adrenal axis (HPA) systems, as they are functionally linked with each other, with depression etiology, and with PUFA metabolism. [95, 96]
As the results of this study are correlational, they do not demonstrate causal relationships among the variables. Thus, one possible meaning of our findings is that peripheral PUFA concentrations affect regional brain function. However, alternative explanations are that brain activity is affecting peripheral PUFA levels, or that both PUFA levels and rCMRglu are changing in response to an unknown third variable. As an example of the second hypothesis, limbic system activity could trigger HPA axis activation, which is known to have effects on fatty acid desaturase genes [97], and could thus regulate peripheral production of highly unsaturated fatty acids from precursors. If this was the case, we might have observed changes in the EPA/rCMRglu correlations; however, lack of EPA effects does not prove that other PUFAs were not affected in this way. Dietary assessments were not performed in this study; therefore, this study does not address whether differences in subject plasma PUFA levels are due to dietary intake or metabolic differences [98]. In this study, we limited our objectives a priori to the study of specific polyunsaturated fatty acids on the basis of their probable clinical significance for depression. However, it would be useful in future studies to examine the full complement of PUFA in relation to regional brain activity.
Conclusions
This is the first study utilizing FDG-PET to explore relationships between plasma levels of essential PUFA and regional human brain activity during a major depressive episode. Correlations were seen between plasma PUFA levels and rCMRglu in regions including prefrontal cortex, cingulate gyrus, and temporoparietal cortex, brain regions that have been previously implicated as key players in the neurocircuitry of depression. Some distinct differences between DHA% and AA% correlational patterns were observed. Taken together, these findings suggest that future neuroimaging studies are warranted, to prospectively examine effects of n-3 supplementation on rCMRglu in depressed and healthy individuals.
Acknowledgments
Acknowledgements: None.
Sources of Support: This study was supported in part by grants MH40695, MH62185, and RR00645 from the National Institutes of Health, Bethesda, MD, and by the National Alliance for Research on Schizophrenia and Depression, Great Neck, New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 3.Rao JS, et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 4.De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73:3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 7.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 8.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 9.Ranjekar PK, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 10.Adams P, Lawson S, Sanigorski A, Sinclair A. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 11.Huan M, et al. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential Fatty Acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 13.Sobczak S, et al. Lower high-density lipoprotein cholesterol and increased omega-6 polyunsaturated fatty acids in first-degree relatives of bipolar patients. Psychol Med. 2004;34:103–112. doi: 10.1017/s0033291703001090. [DOI] [PubMed] [Google Scholar]
- 14.Sublette ME, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calabrese J, et al. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- 16.Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins and Leukotrienes in Medicine. 1983;10:361–367. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- 17.Linnoila M, Whorton A, Rubinow D, Cowdry R, Ninan P, Waters R. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983;40:405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- 18.Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- 19.Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport S, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Archives of General Psychiatry. 2002;59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 21.Rubin D, Laposata M. Cellular interactions between n-6 and n-3 fatty acids: a mass analysis of fatty acid elongation/desaturation, distribution among complex lipids, and conversion to eicosanoids. J Lipid Res. 1992;33:1431–1440. [PubMed] [Google Scholar]
- 22.Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Freeman MP, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 24.Esposito G, et al. Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage. 2007;34:1342–1351. doi: 10.1016/j.neuroimage.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovacchini G, Chang M, Bokde A, et al. Brain incorporation of [11C]arachidonic acid (AA) and palmitic acid (PA): Human studies with PET. J Nucl Med. 2001;42:65P. [Google Scholar]
- 26.Giovacchini G, et al. Brain incorporation of [11]Carachidonic acid in young healthy humans measured with positron emission tomography. Journal of Cerebral Blood Flow Metabolism. 2002;22:1453–1462. doi: 10.1097/01.WCB.0000033209.60867.7A. [DOI] [PubMed] [Google Scholar]
- 27.Giovacchini G, et al. Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004;45:1471–1479. [PubMed] [Google Scholar]
- 28.Conklin SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 29.Puri B, Counsell S, Hamilton G, Richardson A, Horrobin D. Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. Int J Clin Pract. 2001;55:560–563. [PubMed] [Google Scholar]
- 30.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21:435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 31.Hirashima F, et al. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 32.McNamara RK, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 33.McNamara RK, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Favreliere S, Barrier L, Durand G, Chalon S, Tallineau C. Chronic dietary n-3 polyunsaturated fatty acids deficiency affects the fatty acid composition of plasmenylethanolamine and phosphatidylethanolamine differently in rat frontal cortex, striatum, and cerebellum. Lipids. 1998;33:401–407. doi: 10.1007/s11745-998-0221-y. [DOI] [PubMed] [Google Scholar]
- 37.Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- 39.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- 40.Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J Neurochem. 2002;81:1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu N, Martin JL, Stella N, Magistretti PJ. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc Natl Acad Sci U S A. 1993;90:4042–4046. doi: 10.1073/pnas.90.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pifferi F, et al. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr. 2005;135:2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- 43.Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996;1:445–452. [PubMed] [Google Scholar]
- 44.Neuringer M, Connor WE, Van Petten C, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984;73:272–276. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. DSMIV; Washington, D.C: 1994. [Google Scholar]
- 46.First M, Williams J, Spitzer R, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Publishing, Inc; Washington D.C: 1997. [Google Scholar]
- 47.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 50.Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ. Regional brain responses to serotonin in major depressive disorder. J Affect Disord. 2004;82:411–417. doi: 10.1016/j.jad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Kegeles LS, et al. Response of cortical metabolic deficits to serotonergic challenge in familial mood disorders. Am J Psychiatry. 2003;160:76–82. doi: 10.1176/appi.ajp.160.1.76. [DOI] [PubMed] [Google Scholar]
- 52.Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- 53.Oquendo MA, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge in major depressive disorder with and without borderline personality disorder. Neuropsychopharmacology. 2005;30:1163–1172. doi: 10.1038/sj.npp.1300689. [DOI] [PubMed] [Google Scholar]
- 54.Oquendo MA, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 55.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 56.Kim HY, Salem N., Jr Separation of lipid classes by solid phase extraction. J Lipid Res. 1990;31:2285–2289. [PubMed] [Google Scholar]
- 57.Morrison WR, Smith LM. Preparation Of Fatty Acid Methyl Esters And Dimethylacetals From Lipids With Boron Fluoride--Methanol. J Lipid Res. 1964;53:600–608. [PubMed] [Google Scholar]
- 58.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 59.Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 60.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Francowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995:189– 210. [Google Scholar]
- 61.Brett M. The MNI brain and the Talairach atlas 1999 [Google Scholar]
- 62.Lancaster JL, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porter NA. Chemistry of lipid peroxidation. Methods Enzymol. 1984;105:273–282. doi: 10.1016/s0076-6879(84)05035-7. [DOI] [PubMed] [Google Scholar]
- 64.Simon JA, Fong J, Bernert JT, Jr, Browner WS. Relation of smoking and alcohol consumption to serum fatty acids. Am J Epidemiol. 1996;144:325–334. doi: 10.1093/oxfordjournals.aje.a008933. [DOI] [PubMed] [Google Scholar]
- 65.Hurst EM. Some cortical association systems related to auditory functions. J Comp Neurol. 1959;112:103–119. doi: 10.1002/cne.901120111. [DOI] [PubMed] [Google Scholar]
- 66.Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 67.Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 68.Gower EC. Efferent projections from limbic cortex of the temporal pole to the magnocellular medial dorsal nucleus in the rhesus monkey. J Comp Neurol. 1989;280:343–358. doi: 10.1002/cne.902800303. [DOI] [PubMed] [Google Scholar]
- 69.Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- 70.Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Shinto L, Connor WE, Quinn JF. Nutritional biomarkers in Alzheimer’s disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimers Dis. 2008;13:31–38. doi: 10.3233/jad-2008-13103. [DOI] [PubMed] [Google Scholar]
- 72.Kotani S, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–164. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 74.Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- 75.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 76.Jacobson S, Trojanowski JQ. Prefrontal granular cortex of the rhesus monkey. I Intrahemispheric cortical afferents. Brain Res. 1977;132:209–233. doi: 10.1016/0006-8993(77)90417-6. [DOI] [PubMed] [Google Scholar]
- 77.Muller-Preuss P, Newman JD, Jurgens U. Anatomical and physiological evidence for a relationship between the ‘cingular’ vocalization area and the auditory cortex in the squirrel monkey. Brain Res. 1980;202:307–315. doi: 10.1016/0006-8993(80)90143-2. [DOI] [PubMed] [Google Scholar]
- 78.Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- 79.Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 80.Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- 81.Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- 82.Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- 83.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5:175–181. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 84.Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180–182. doi: 10.1038/378180a0. [DOI] [PubMed] [Google Scholar]
- 85.Vancassel S, et al. Cerebral asymmetry and behavioral lateralization in rats chronically lacking n-3 polyunsaturated fatty acids. Biol Psychiatry. 2005;58:805–811. doi: 10.1016/j.biopsych.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 86.Yuan Y, et al. Regional Gray Matter Changes Are Associated with Cognitive Deficits in Remitted Geriatric Depression: An Optimized Voxel-Based Morphometry Study. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 87.George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 88.Gemar MC, Kapur S, Segal ZV, Brown GM, Houle S. Effects of self-generated sad mood on regional cerebral activity: a PET study in normal subjects. Depression. 1996;4:81–88. doi: 10.1002/(SICI)1522-7162(1996)4:2<81::AID-DEPR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 89.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 90.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 91.Yucel M, Wood SJ, Fornito A, Riffkin J, Velakoulis D, Pantelis C. Anterior cingulate dysfunction: implications for psychiatric disorders? J Psychiatry Neurosci. 2003;28:350–354. [PMC free article] [PubMed] [Google Scholar]
- 92.Kimbrell TA, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 93.Neumeister A, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 94.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 95.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 96.Nieminen LR, Makino KK, Mehta N, Virkkunen M, Kim HY, Hibbeln JR. Relationship between omega-3 fatty acids and plasma neuroactive steroids in alcoholism, depression and controls. Prostaglandins Leukot Essent Fatty Acids. 2006;75:309–314. doi: 10.1016/j.plefa.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Brenner RR, Ayala S, Garda HA. Effect of dexamethasone on the fatty acid composition of total liver microsomal lipids and phosphatidylcholine molecular species. Lipids. 2001;36:1337–1345. doi: 10.1007/s11745-001-0850-1. [DOI] [PubMed] [Google Scholar]
- 98.Koletzko B, Demmelmair H, Schaeffer L, Illig T, Heinrich J. Genetically determined variation in polyunsaturated Fatty Acid metabolism may result in different dietary requirements. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:35–49. doi: 10.1159/000146246. [DOI] [PubMed] [Google Scholar]