Abstract
Metabolic signaling through the post-translational linkage of N-acetylglucosamine (O-GlcNAc) to cellular proteins represents a unique signaling paradigm operative during lethal cellular stress, and, a pathway we and others have recently shown to exert cytoprotective effects in vitro and in vivo. Accordingly, the present work addresses the contribution of the hexosaminidase responsible for removing O-GlcNAc (i.e. O-GlcNAcase) from proteins. We used pharmacologic inhibition, viral overexpression, and RNA interference of O-GlcNAcase in isolated cardiac myocytes to establish its role during acute hypoxia-reoxygenation. Elevated O-GlcNAcase expression significantly reduced O-GlcNAc levels and augmented post-hypoxic cell death. Conversely, short interfering RNA directed against, or pharmacologic inhibition of, O-GlcNAcase significantly augmented O-GlcNAc levels and reduced post-hypoxic cell death. On the mechanistic front, we evaluated post-hypoxic mitochondrial membrane potential and found that repression of O-GlcNAcase activity improves, while augmentation impairs, mitochondrial membrane potential recovery. Similar beneficial effects on post-hypoxic calcium overload were also evident. Such changes were evident without significant alteration in expression of the major putative components of the mitochondrial permeability transition pore (i.e. VDAC, ANT, CypD). The present results provide definitive evidence that O-GlcNAcase antagonizes post-hypoxic cardiac myocyte survival. Moreover, such results support a renewed approach to the contribution of metabolism and metabolic signaling to the determination of cell fate.
Keywords: O-GlcNAc, mitochondria, hypoxia, cell death, post-translational modification
Introduction
O-linked β-N-acetylglucosamine (O-GlcNAc) is a metabolic post-translational modification of nucleocytoplasmic proteins. Following its discovery in 1984 1, numerous proteins have been identified as being O-GlcNAc modified. Such targets are diverse and include transcription factors, RNA-binding proteins, cytoskeletal proteins, nuclear pore proteins, phosphatases, and kinases 2, 3. Unlike traditional N- linked protein glycosylation, O-GlcNAcylation of proteins involves the addition of one GlcNAc molecule to the Ser/Thr amino acid residues with no further elongation into more complex oligosaccharides. The GlcNAc moiety is added to serine and threonine amino acid residues by O-GlcNAc transferase (OGT), and removed by O-GlcNAcase. O-GlcNAcylation is highly inducible, dynamic, and reversible, and, the cycle of turnover of the sugar moiety exceeds the turnover of the protein itself 4. O-GlcNAc differs from phosphorylation in that the enzymes involved, OGT and O-GlcNAcase, are coded for by single genes, contrary to the numerous genes controlling protein phosphorylation/dephosphorylation.
Zachara and coworkers 5, 6 showed that O-GlcNAc levels change in response to stress and that augmentation of O-GlcNAc levels attenuated cell injury following lethal stress. We recently showed that enhanced O-GlcNAc levels attenuated injury following myocardial infarction, oxidative stress, and hypoxia 7, 8. In the present study, we address the role of O-GlcNAcase in cardiac myocyte survival following hypoxic stress. Here, we evaluate whether manipulation of O-GlcNAcase to alter O-GlcNAc levels affects sensitivity to in vitro hypoxia/reoxygenation. Our findings definitively implicate O-GlcNAcase in the pathogenesis of post-hypoxic cardiomyocyte death. In addition, this study emphasizes the significant contribution of metabolic signaling to post-hypoxic damage.
Methods
Neonatal rat cardiac myocyte isolation and culture
Neonatal rat cardiac myocytes (NRCMs) were isolated from 1-2 day old Sprague-Dawley rats and cultured according to a well characterized protocol 7-12. The first four days of culture medium contained the anti-mitotic, BrdU (0.1 mmol/L), to inhibit fibroblast growth in addition to 5% fetal bovine serum, penicillin/streptomycin, and vitamin B12. Twenty-four hours prior to experimentation, medium was changed to serum-free DMEM.
Gene transfer
NRCMs were infected with replication-deficient adenoviruses carrying O-GlcNAcase gene (AdO-GlcNAcase, 48 hours) 8, 13, or green fluorescent protein (AdGFP, Vector Biolabs) as described previously 11. Doses used include 0 and 100 multiplicity of infection (MOI) of Ad O-GlcNAcase or AdGFP. Twenty-four hours prior to experimentation, medium was changed to serum-free DMEM. Functional expression was confirmed by immunoblot analysis.
Enzyme inhibition
NRCMs were treated with O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate 14, 15 (i.e., PUGNAc: 200μmol/L) an inhibitor of O-GlcNAcase or Vehicle (0.1% ethanol) overnight and subjected to hypoxia/reoxygenation or total protein isolated. Sample size is equal to six per group per treatment.
RNA Interference
Cultured NRCMs 7, 8 were transfected with 60nmol/L short interfering (si) RNA directed against O-GlcNAcase (siRNA ID # 190811; with sense strand 5′-GCAACUUAUGACUCCAUCtt-3′ and antisense strand 3′-GAUGAGAGUCAUAAGUUGCtc-5′) or scrambled sequence as a non-silencing control (Ambion). Myocytes were transfected with Ambion's SiPORT NeoFX transfection reagent according to manufacturer's instructions. Seventy-two hours following transfection, total protein was isolated and immunoblotted for O-GlcNAcase protein and O-GlcNAc levels. Similarly treated myocytes were subjected to hypoxia/reoxygenation (H/R) and post-hypoxic media harvested to assess cell injury.
Protein expression
Total cellular proteins were isolated from NRCMs as described previously 7, 8. NRCMs were washed with PBS, harvested in ice-cold lysis buffer (containing 68 mmol/L sucrose, 200 mmol/L mannitol, 50 mmol/L KCl, 1mmol/L EGTA, 1mmol/L DTA and 5 mmol/L HEPES) with freshly added 0.2 μmol/L DTT, 0.1v/v% protease inhibitor stock, 0.4% (v/v) NP-40, 0.4% (v/v) Triton-X, and post-translational modification inhibitor stock. Extracts were sonicated and the resulting lysates centrifuged at 15000×g for 5 minutes at 4°C to remove cell debris. Forty micrograms of protein (according to Bradford assay) was applied to each lane of a 6% SDS-PAGE electroblotted onto PVDF membranes for O-GlcNAcase protein, 10% Bis-Tris gel and electroblotted onto PVDF membranes for cyclophilin D (CypD), adenine nucleotide translocase (ANT), and voltage dependent anion channel (VDAC), while fifty micrograms of protein was applied to each lane of a 4-12% gradient NuPAGE Bis-Tris gel (Invitrogen) and electroblotted onto nitrocellulose membranes (Invitrogen) for O-GlcNAc levels. Reagent-grade nonfat milk (BioRAD) 5% (w/v) in Tris buffered saline was used for blocking. Blots were incubated with anti-O-GlcNAcase (1:200), anti-O-GlcNAc antibodies (1:1000 CTD 110.6; Covance or 1:2000 RL2; Affinity BioReagents) anti-Cyclophilin D (1:1000, Affinity BioReagents), anti-ANT (1:1000, Santa Cruz), anti-VDAC (1:1000, Santa Cruz) or α-tubulin (1:1000, Santa Cruz), as primary antibodies overnight at 4°C. Blots were then incubated for one hour with 0.1μg/mL of secondary antibody (goat anti-chicken IgG-HRP conjugated, goat anti-mouse IgM-HRP conjugated, donkey anti-goat IgG-HRP conjugated or goat anti-mouse IgG-HRP conjugated) and detected with an enhanced chemiluminescent detection system (Pierce).
Densitometry
Densitometry was performed using non-saturated chemiluminescent membranes exposed and quantitated using Fuji LAS-3000 bio-imaging analyzer. Multiple exposures from every experiment were used to confirm that the signal was within the linear range. Densitometric ananlysis of O-GlcNAc levels using CTD 110.6 and RL2 antibodies were performed on the entire lane. O-GlcNAc levels were normalized to the appropriate control (Ponceau stain), and then expressed as a percentage of control (set at 100%).
In vitro hypoxia-reoxygenation injury in cardiac myocytes
Cardiac myocytes were subjected to hypoxia using 1× Esumi lethal ischemic media, pH 6.2 (containing 117 mmol/L NaCl, 12 mmol/L KCl, 0.9 mmol/L CaCl2, 0.49 mmol/L MgCl2, 4 mmol/L HEPES, 20 mmol/L sodium lactate, and 5.6 mmol/L L-glucose) by sealing the myocytes in humidified hypoxic chambers (Billups-Rothenberg, Inc) 16, flushing each chamber with a gas mixture consisting of 5% CO2 and 95% N2 for fifteen minutes, and incubating the hypoxic chamber in a modular incubator at 37°C for three hours. Following hypoxia, the media was changed to 1× Esumi control media (pH 7.4, 137 mmol/L NaCl, 3.8 mmol/L KCl, 0.9 mmol/L CaCl2, 0.49 mmol/L MgCl2, 4 mmol/L HEPES, and 5.6 mmol/L D-glucose) and culture dishes reoxygenated for one or six hours in the modular incubator or on the fluorescent microscope during imaging, as appropriate. Similarly treated NRCMs were subjected to four or 9 hours of normoxia in 1× Esumi control media to serve as normoxic/aerobic controls.
Cell death
Cell death was assessed for NRCMs as previously described 8. Normoxic or post-hypoxic LDH release was spectrophotometrically determined using a commercially available kit (Sigma) following hypoxia-reoxygenation, and, the results expressed as LDH release relative to total LDH in the cells and normalized to the appropriate controls (1hr reoxygenation data) or normoxic untreated control (for 6hrs reoxygenation data). Similarly treated NRCMs were stained with the fluorescent DNA-binding dyes Hoechst 33342, 5μg/mL and propidium iodide, 5μg/mL (Invitrogen) during the last 30 minutes of reoxygenation 8, 17. The stained nuclei were then visualized using a 20× objective on a Nikon-TE2000E2 fluorescence microscope, Xcite light source; 350/50 nm excitation and 470/40 nm emission filter for Hoechst and 560/40 nm excitation and 630/60 nm emission filter for PI. Four fields per treatment in triplicate were counted and data were expressed as % PI positive nuclei/total nuclei. Because the nuclear stain Hoechst 33342 is membrane permeable, it was used to determine total cells in each field and not as an index of apoptosis.
Assessment of mitochondrial membrane potential
Using time-lapse fluorescence microscopy 7-12, 18, detection of mitochondrial membrane potential changes was performed by following changes in tetramethylrhodamine methyl ester (TMRM) fluorescence treated with AdGFP, AdO-GlcNAcase, Vehicle, PUGNAc, Scrambled RNAi or O-GlcNAcase RNAi as previously described 8.
Assessment of calcium overload
Calcium levels were assessed in NRCMs treated with AdGFP, AdO-GlcNAcase, Vehicle or PUGNAc and subjected to 3hrs of hypoxia using time-lapse fluorescent microscopy by following the changes in Rhod-2AM fluorescence. Cardiac myocytes were plated on 35 mm glass bottom culture dishes and loaded with 2μmol/L Rhod-2AM prior to hypoxia-reoxygenation. Imaging was initiated at reoxygenation in isolated myocytes by exciting Rhod-2AM with an Xcite light source through a 546/11 nm bandpass filter and emission assessed through a 567/15 nm bandpass filter. Fluorescence intensity was monitored throughout the protocol every 90 seconds. All experimental groups were repeated in at least four separate isolations.
Results
O-GlcNAcase (AdO-GlcNAcase) overexpression exacerbates post-hypoxic cardiac myocyte death
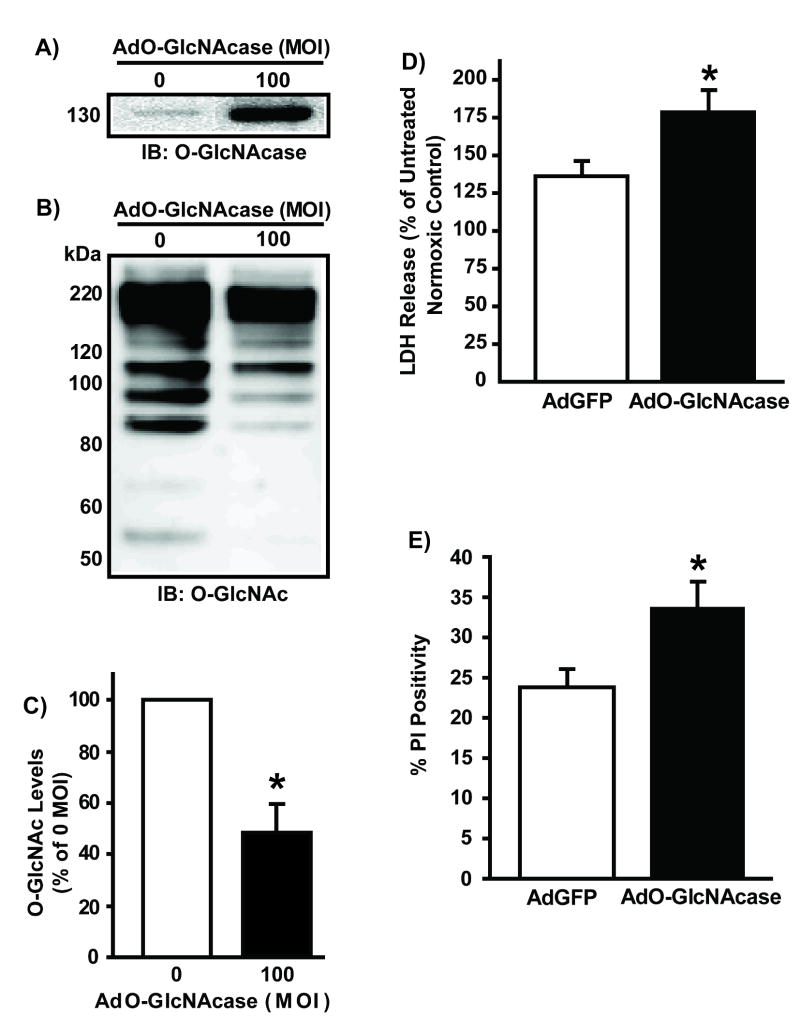
Forty-eight hours following AdO-GlcNAcase infection of isolated neonatal rat cardiac myocytes (NRCMs, n>/=5/group), total cellular proteins were isolated for O-GlcNAcase protein and O-GlcNAc levels (via western blot analysis). Adenoviral overexpression of O-GlcNAcase significantly (p<0.05) augmented O-GlcNAcase protein levels (Figure 1A). Such elevation corresponded with a significant (p<0.05) reduction in O-GlcNAc levels (48 +/- 7% of 0 MOI AdO-GlcNAcase) (Figure 1B). Similar findings were seen with another O-GlcNAc antibody, RL2 (65 +/- 8% of 0 MOI AdO-GlcNAcase, Supplemental Figure VA). Immunoblots for O-GlcNAc levels show multiple immunopositive bands because O-GlcNAc is a post-translational modification, not a single protein (Figure 1C and Supplemental Figure VA). Previous studies have demonstrated similar findings in various cell lines 5 and NRCMs 7, 8. Equal protein loading was confirmed by densitometric analysis of Ponceau-stained membranes (see Supplemental Figure VII).
Figure 1.
Myocytes (n>/=5/group) were infected with AdGFP or AdO-GlcNAcase (0 or 100 MOI) 48 hours prior to protein isolation or hypoxia-reoxygenation. A) Representative immunoblot of O-GlcNAcase protein shows significant elevation in O-GlcNAcase levels following AdO-GlcNAcase infection. Representative immunoblot (B) and Densitometric analysis (C) of O-GlcNAc levels. AdO-GlcNAcase significantly reduced O-GlcNAc levels. As expected, multiple immunopositive bands appear because the O-GlcNAc modification occurs on numerous proteins throughout the cell. D) O-GlcNAcase overexpression exacerbated post-hypoxic cardiac myocyte damage according to LDH release. E) O-GlcNAcase overexpression aggravated post-hypoxic injury according to propidium iodide positivity (n=4/group). *p<0.05 vs. 0 MOI AdO-GlcNAcase or 100 MOI AdGFP.
To evaluate the effects of O-GlcNAcase overexpression on post-hypoxic cardiac myocyte survival, similarly treated cardiac myocytes were subjected to hypoxia (3 hours) and reoxygenation (1 or 6 hours). Post-hypoxic media was harvested for LDH release, and, additional post-hypoxic NRCMs were stained with propidium iodide (PI) to assess cell death. Myocytes infected with AdO-GlcNAcase were more sensitive to hypoxia-induced injury by the first hour of reoxygenation according to LDH release (150 ± 23% of 0 MOI AdO-GlcNAcase, p<0.05, Supplemental Figure IIIA) and PI positivity (17 +/- 2% vs.10 ± 1% for AdGFP, Supplemental Figure IIID) compared to 0 MOI AdO-GlcNAcase or AdGFP respectively.
To determine if the detrimental effect of AdO-GlcNAcase on post-hypoxic cardiac myocytes was short lived, similarly treated NRCMs were reoxygenated for 6hrs after 3hrs of hypoxia. The longer duration of reoxygenation (six hours) still showed exacerbated cellular injury in AdO-GlcNAcase NRCMs, according to LDH release (179 +/- 14% vs. 136 +/- 8% for AdGFP, p<0.05 Figure 1D) and PI positivity (34 +/- 3% vs. 24 +/- 2% for AdGFP, p<0.05, Figure 1E) compared to AdGFP.
Cell damage was not significantly different among AdO-GlcNAcase, AdGFP, or uninfected NRCMs under normoxia according to LDH release (109 +/- 3% of control vs. 93 +/- 3% of control, p > 0.05, Supplemental Figure IA) and PI positivity (14 +/- 1% for AdO-GlcNAcase vs. 13 +/- 1% for AdGFP vs. 12 +/- 1% for control, p>0.05, Supplemental Figure IIA). Such results confirm that viral infection does not affect cell survival or O-GlcNAc levels in this system.
O-GlcNAcase inhibition attenuates post-hypoxic cardiac myocyte injury
NRCMs (n=6/group) were treated with PUGNAc (O-GlcNAcase inhibitor) overnight prior to protein harvest, then immunoblotted for O-GlcNAc levels. PUGNAc significantly increased O-GlcNAc levels (878 +/- 215% of control, p<0.05) compared to Vehicle (Figure 2A&B). Supplemental Figure VB contains results with an additional O-GlcNAc antibody (RL2).
Figure 2.
NRCMs were subjected to pharmacologic repression of O-GlcNAcase activity. A) Representative immunoblots for O-GlcNAc levels following PUGNAc treatment (n=6/group) show a significant increase in O-GlcNAc levels compared to Vehicle. Multiple bands occur because O-GlcNAc is a post-translational modification. B) Densitometric analyses of O-GlcNAc western blots show significantly elevated O-GlcNAc levels compared to Vehicle. C) O-GlcNAcase inhibition with PUGNAc diminished post-hypoxic injury in NRCMs (according to LDH release) compared with Vehicle. D) O-GlcNAcase inhibition with PUGNAc reduced post-hypoxic injury (per PI positivity) compared with Vehicle. *p<0.05 vs. Vehicle.
Additional NRCMs (n=6/group) were treated with PUGNAc, subjected to hypoxia-reoxygenation, and media harvested to measure LDH release. Inhibition of O-GlcNAcase (with PUGNAc) significantly attenuated post-hypoxic LDH release 70 +/- 10% of control, p<0.05, Supplemental Figure IIIB) and PI positivity (9 +/- 1% vs. 13 +/- 1% for Vehicle, p<0.05, Supplemental Figure IIIE) at the end of the first hour of reoxygenation.
The protective effect of augmented O-GlcNAc levels (with PUGNAc) was still seen at six hours of reoxygenation with LDH release (133 +/- 11% for PUGNAc, p<0.05 vs. 177 +/- 14% for Vehicle, Figure 2C) and PI positivity (21 +/- 2% vs. 36 +/- 2% of Vehicle, Figure 2D). PUGNAc or Vehicle treatment did not significantly alter normoxic/aerobic cellular viability compared with untreated NRCMs under normoxia according to LDH release (p>0.05, 90 +/- 8% of Control vs. 104 +/- 8% of Control, Supplemental Figure IB) and PI positivity (p>0.05, 12 +/- 1% for PUGNAc vs. 13 +/- 1% for Vehicle vs. 13 +/- 1% for control, Supplemental Figure IIB).
Knockdown of O-GlcNAcase reduces post-hypoxic cardiac myocyte injury
NRCMs (n=6/group) were treated with 60 nmol/L O-GlcNAcase RNAi or scrambled (Scr) RNAi for 72 hours to knockdown O-GlcNAcase expression. O-GlcNAcase knockdown significantly (p<0.05) reduced O-GlcNAcase protein levels compared to Scr RNAi, despite no change in α-tubulin levels (Figure 3A). O-GlcNAcase RNAi significantly (p<0.05) augmented O-GlcNAc levels (132 +/- 12% of control, p<0.05) compared to Scr RNAi (Figure 3B & C).
Figure 3.
A) O-GlcNAcase message knockdown (RNAi) significantly reduced O-GlcNAcase protein levels compared with Scr RNAi. B) Representative immunoblot for lysates from Scr vs. O-GlcNAcase RNAi NRCMs showing augmented O-GlcNAc levels compared to Scr. C) Densitometric analysis of O-GlcNAc immunoblots showed significant increase in O-GlcNAc levels for O-GlcNAcase RNAi compared with Scr RNAi. O-GlcNAcase RNAi-treated NRCMs (n=6/group) were more resistant to hypoxia-induced injury according to LDH release (D) and, PI positivity (E) compared to Scr RNAi. *p< 0.05 vs. Scr RNAi.
Additional NRCMs were treated with O-GlcNAcase or Scr RNAi, subjected to hypoxia-reoxygenation, and media harvested to measure LDH release. O-GlcNAcase RNAi significantly (p<0.05) reduced post-hypoxic LDH release after one (72 +/- 10% of Scr RNAi, Supplemental Figure IIIC) and six hours (130 +/- 4% for O-GlcNAcase RNAi vs. 170 +/-26% for Scr RNAi, Figure 3D) of reoxygenation compared to Scr RNAi. In addition, O-GlcNAcase RNAi significantly diminished PI positivity at six hours (22 +/- 2% for O-GlcNAcase RNAi vs. 33 +/- 2% for Scr RNAi, p<0.05, Figure 3E) compared with Scr RNAi. O-GlcNAcase or Scr RNAi treatment did not cause significant cell damage compared with untreated NRCMs under normoxia/aerobic conditions (Supplemental Figures IC and IIC).
Effect of O-GlcNAcase manipulation on mitochondrial membrane potential preservation
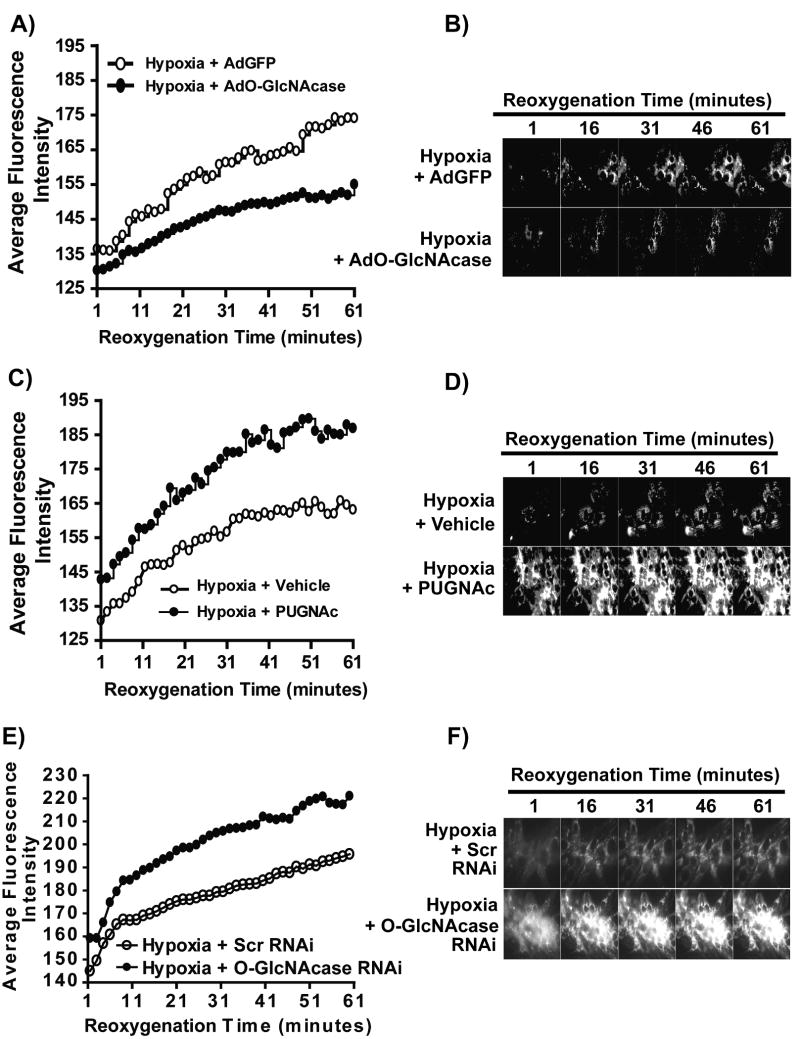
Based on our recent findings linking O-GlcNAc-mediated cardioprotection to mitochondria 7, 8, we assessed the effects of altered O-GlcNAcase activity on post-hypoxic mitochondrial membrane potential. NRCMs were treated with either AdGFP (48 hours, 100 MOI), AdO-GlcNAcase (48 hours, 100 MOI), Vehicle (overnight), PUGNAc (prior to H/R, 200 μmol/L), Scrambled RNAi (72 hrs, 60 nmol/L), or O-GlcNAcase RNAi (72 hrs, 60 nmol/L), and loaded with the mitochondrial membrane potential indicator, tetramethylrhodamine methylester (TMRM; 50 nmol/L). Cardiac myocytes were then subjected to either four hours of normoxia, or, three hours of hypoxia and one hour of reoxygenation. During the reoxygenation period, myocytes were evaluated for changes in mitochondrial membrane potential using time-lapse fluorescence microscopy. Dissipation of mitochondrial membrane potential is reflected by a loss of TMRM fluorescence. AdGFP-infected NRCMs loaded with TMRM maintained mitochondrial membrane potential over time under normoxia and showed no significant difference in mitochondrial membrane potential over time (Supplemental Figure IV). Hypoxia-reoxygenation induced mitochondrial membrane potential loss. O-GlcNAcase overexpression aggravated the loss of mitochondrial membrane potential upon reoxygenation, shown by impaired recovery of TMRM fluorescence (Figure 4A & B). Conversely, inhibition of O-GlcNAcase (via PUGNAc or O-GlcNAcase RNAi) attenuated the loss of mitochondrial membrane potential compared to Vehicle or Scrambled RNAi as shown by the significant restoration of TMRM fluorescence towards that of the normoxic control, and protected cells from post-hypoxic mitochondrial dysfunction (Figure 4C, D, E & F).
Figure 4.
Assessment of sensitivity to loss of mitochondrial membrane potential in NRCMs overexpressing O-GlcNAcase (AdO-GlcNAcase) or with inhibition of O-GlcNAcase (PUGNAc) following hypoxia. TMRM fluorescence was used to indicate mitochondrial membrane potential (n>/=6/group). A) In post-hypoxic myocytes, O-GlcNAcase overexpression (AdO-GlcNAcase) exacerbated mitochondrial membrane potential loss. B) Quantification of the average relative fluorescence intensity for AdGFP and O-GlcNAcase overexpression (AdO-GlcNAcase) showed significantly reduced recovery of mitochondrial membrane potential for AdO-GlcNAcase-treated compared to AdGFP-treated cells. C) O-GlcNAcase inhibition (PUGNAc) attenuated the loss of mitochondrial membrane potential compared to Vehicle. D) Quantification of the average relative fluorescence intensity for Vehicle and O-GlcNAcase inhibition (PUGNAc) showed significant recovery of mitochondrial membrane potential for O-GlcNAcase inhibitor (PUGNAc) compared to Vehicle. Similarly, RNAi against O-GlcNAcase improved post-hypoxic mitochondrial membrane potential recovery (E&F).
Effect of O-GlcNAcase manipulation on mPTP component expression
To test the hypothesis that O-GlcNAc-mediated protection involved alteration in expression of the putative major components of mPTP, we treated cells with the various interventions and harvested proteins. Lysates were immunoblotted for the likely mPTP components VDAC (voltage dependent anion channel), ANT (adenine nucleotide translocase), and CypD (cyclophilin D). None of the interventions significantly altered the expression of VDAC, ANT, or CypD (Figure 5).
Figure 5.
Expression of putative molecular components of mitochondrial permeability transition pore (mPTP). Expression of CypD (A), ANT (B), and VDAC (C) were not significantly affected by genetic overexpression, pharmacologic inhibition, or RNAi.
Effect of O-GlcNAcase manipulation on calcium levels
Cardiac myocytes were treated with either AdGFP (48 hours, 100 MOI), AdO-GlcNAcase (48 hours, 100 MOI), Vehicle (overnight) or PUGNAc (overnight, 200 μmol/L), loaded with the mitochondrial calcium indicator, Rhod-2AM (2 μmol/L) and subjected to three hours of hypoxia and one of reoxygenation. Time-lapse fluorescence microscopy was initiated at the beginning of reoxygenation for changes in mitochondrial calcium levels. Augmentation of calcium levels is reflected by an increase in Rhod-2AM fluorescence. Hypoxia sensitized myocytes to calcium overload. O-GlcNAcase overexpression (AdO-GlcNAcase) exacerbated the hypoxia-induced calcium overload upon reoxygenation reflected by a significant increase in Rhod-2AM fluorescence (Figure 6A&B) while O-GlcNAcase inhibition (PUGNAc) attenuated hypoxia-induced calcium overload compared to Vehicle (Figure 6C&D).
Figure 6.
Evaluation of calcium overload in post-hypoxic cardiac myocytes using Rhod-2AM ((n>/=6/group). Myocytes were treated with AdGFP or AdO-GlcNAcase (A&B), or, Vehicle or PUGNAc (C&D). Following hypoxia myocytes undergo progressive calcium overload. Genetic overexpression of O-GlcNAcase exaggerates, while pharmacologic inhibition of O-GlcNAcase attenuates post-hypoxic calcium overload.
Discussion
O-GlcNAc signaling has emerged as an integral element in the cell's armamentarium against lethal stress. Previous work from this laboratory and others suggests that pharmacologically-elevated O-GlcNAc levels are protective in cell models following hypoxia/oxidative stress 8, 19-22, and in vivo, following myocardial ischemia-reperfusion 7. However, such studies were never able to achieve the standard of genetic gain- and loss-of-function insights on O-GlcNAcase, as are available in the present work. Here, overexpression of O-GlcNAcase reduces O-GlcNAc levels, exacerbates the loss of mitochondrial membrane potential following hypoxia, and sensitizes myocytes to post-hypoxic cell death. The present results also demonstrate that inhibition of O-GlcNAcase (via siRNA or PUGNAc) augments O-GlcNAc levels, enhances the recovery of mitochondrial membrane potential following hypoxia, and attenuates post-hypoxic cell death.
Recently, Taylor et al. identified a unique response of cells to O-GlcNAc levels, namely that O-GlcNAc is not a simple ‘read out’ of glucose availability 23. These results were further confirmed by Cheung et al. in Neuro-2a cells 24. Indeed, such insights are consistent with ongoing work from this laboratory that O-GlcNAc signaling represents a stress responsive program, not simply a fuel gauge. Such insights are particularly true in light of Taylor's 23 and Cheung's 24 data indicating that complete removal of glucose significantly augments O-GlcNAc levels. Because the hexosamine biosynthetic pathway (HBP) produces the monosaccharide donor for the O-GlcNAcylation of proteins, several groups have manipulated this pathway as a means of better understanding the role of O-GlcNAc modification of proteins and have shown that enhanced HBP flux is cytoprotective following acute stress19, 21, 22, 25.
Zachara et al. first showed that enhanced O-GlcNAc levels improved cell survival following stress 5. Since then, we and others have reported on the protective role of enhanced O-GlcNAc levels in different systems 7, 8, 19-22, 25-27. In the heart, Champattanachai et al. showed that inhibition of O-GlcNAcase with PUGNAc in neonatal rat cardiac myocytes improved cardiac myocyte viability following hypoxia-reoxygenation 25. Liu et al. in several studies of the isolated perfused heart showed that enhanced O-GlcNAc levels protected the heart against injury resulting from calcium paradox and hypoxia-reoxygenation 20-22. We have also shown that pretreatment of mice with PUGNAc (an O-GlcNAcase inhibitor) reduced infarct size in vivo, and, that ischemic preconditioning augmented O-GlcNAc levels 7. Moreover, we recently showed that augmenting O-GlcNAc levels by overexpressing OGT attenuated post-hypoxic injury, while inhibition of OGT (pharmacologically or genetically) in cardiomyocytes exacerbated post-hypoxic injury at the mitochondrial level. Such findings are supported by Champattanachai's study 19, showing that overexpression of OGT attenuated loss of mitochondrial membrane potential induced by H2O2 and increased mitochondrial Bcl-2. Here, we show that manipulation of O-GlcNAcase to alter O-GlcNAc levels significantly affects cardiac myocyte survival following hypoxia, though we found no evidence for differences in apoptosis at 6 hours reoxygenation (see Supplemental Figure VII).
From a molecular vantage, we have identified voltage dependent anion channel (VDAC) 7, 8, a putative member of the mitochondrial permeability transition pore, to be O-GlcNAc modified and also showed that enhanced O-GlcNAc levels attenuated calcium-induced mitochondrial permeability transition pore (mPTP) formation in adult cardiac mitochondria. The present study supports mitochondrial involvement as a potential mechanism in O-GlcNAc mediated cardioprotection in that following hypoxia, reduction of O-GlcNAc levels (by O-GlcNAcase overexpression) diminished the recovery of mitochondrial membrane potential, while augmented O-GlcNAc levels using PUGNAc enhanced the recovery of mitochondrial membrane potential during reoxygenation. The calcium overload data further support potential involvement of mitochondria in O-GlcNAc signaling induced alterations in cell survival. Yet, there were no significant changes in total protein expression of the prominent, though putative, constituents of the mPTP. Whether the physical alteration of mPTP components by O-GlcNAc explains the protective effects observed remains the subject of ongoing investigation.
O-GlcNAcase structurally has both hexosaminidase and histone acetlytransferase domains and is functionally involved with the removal of O-GlcNAc from proteins and acetylation of free histones 28-30. Because O-GlcNAcase has been shown to be cleaved by caspase 3 30, an executioner caspase in apoptosis, into N-terminal hexosaminidase domain and an C-terminal HAT domain, we hypothesize that cleavage of O-GlcNAcase might result in the loss of internal regulation of its hexosaminidase activity, thereby increasing the rate of removal of O-GlcNAc from proteins and hence the severity of post-hypoxic injury. However, significant additional efforts should be directed toward understanding the molecular regulation of O-GlcNAc signaling.
The reductionist approach used in the present study allows clear focus on the role of O-GlcNAcase in hypoxia-reoxygenation injury. It is becoming increasingly evident that O-GlcNAc signaling exerts its influence based on context and such potentially differing effects deserve investigation 31, 32. Ongoing pursuits will continue to identify the protein targets and attempt to elucidate how O-GlcNAc signaling might be altered in diabetes and aging, in contrast to acute events such as myocardial ischemia. Clearly, O-GlcNAc signaling warrants such continued and intensive attention in the cardiovascular system.
Acknowledgments
The authors acknowledge the expert technical assistance of Ms. Linda Harrison (University of Louisville).
Sources of Funding: This work is supported by grants (Dr. Jones) from the National Institutes of Health (R01 083320), American Heart Association (National Center Scientist Development Grant, 0535270N), and Kentucky Science and Engineering Foundation (KSEF-1677-RDE-011). Ms. Ngoh is an American Heart Association Predoctoral Fellow (Great Rivers Affiliate, 0815502D). Dr. Facundo is an American Heart Association Postdoctoral Fellow (Great Rivers Affiliate, 0825643D).
Footnotes
Disclosures: None.
References
- 1.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 2.Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, Zhao Y. Global identification of O-GlcNAc-modified proteins. Analytical chemistry. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- 3.Miller MW, Caracciolo MR, Berlin WK, Hanover JA. Phosphorylation and glycosylation of nucleoporins. Arch Biochem Biophys. 1999;367:51–60. doi: 10.1006/abbi.1999.1237. [DOI] [PubMed] [Google Scholar]
- 4.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 5.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 6.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochimica et Biophysica Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 8.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshima Y, Akao M, Jones SP, Marban E. Cariporide (HOE642), a Selective Na+-H+ Exchange Inhibitor, Inhibits the Mitochondrial Death Pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 10.Akao M, O'Rourke B, Kusuoka H, Teshima Y, Jones SP, Marban E. Differential Actions of Cardioprotective Agents on the Mitochondrial Death Pathway. Circ Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- 11.Teshima Y, Akao M, Jones SP, Marban E. Uncoupling Protein-2 Overexpression Inhibits Mitochondrial Death Pathway in Cardiomyocytes. Circ Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 12.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin Attenuates Oxidant-Induced Mitochondrial Dysfunction in Cardiac Myocytes. Circ Res. 2003;93:697–699. doi: 10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 13.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the Accompanying Hyperglycemia Impairs Cardiomyocyte Calcium Cycling through Increased Nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 14.Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Greig IR, Vocadlo DJ. Analysis of PUGNAc and NAG-thiazoline as Transition State Analogues for Human O-GlcNAcase: Mechanistic and Structural Insights into Inhibitor Selectivity and Transition State Poise. J Am Chem Soc. 2007;129:635–644. doi: 10.1021/ja065697o. [DOI] [PubMed] [Google Scholar]
- 15.Haltiwanger RSGK, Philipsberg GA. Modulation of O-Linked N-Acetylglucosamine Levels on Nuclear and Cytoplasmic Proteins in Vivo Using the Peptide O-GlcNAc-beta -N-acetylglucosaminidase Inhibitor O-(2-Acetamido-2-deoxy-Dglucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 16.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 17.Bishopric NH, Zeng GQ, Sato B, Webster KA. Adenovirus E1A Inhibits Cardiac Myocyte-specific Gene Expression through Its Amino Terminus. J Biol Chem. 1997;272:20584–20594. doi: 10.1074/jbc.272.33.20584. [DOI] [PubMed] [Google Scholar]
- 18.Akao M, Ohler A, O'Rourke B, Marban E. Mitochondrial ATP-Sensitive Potassium Channels Inhibit Apoptosis Induced by Oxidative Stress in Cardiac Cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- 19.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. AJP - Cell Physiology. 2008;294:C1509–1520. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion leads to improved functional recovery and reduced calpain-proteolysis. Am J Physiol Heart Circ Physiol. 2007;293:1391–1399. doi: 10.1152/ajpheart.00285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor RP, Parker GJ, Hazel MW, Soesanto Y, Fuller W, Yazzie MJ, McClain DA. Glucose deprivation stimulates O-GlcNAc modification of proteins through upregulation of O-linked N-acetylglucosaminyltransferase. J Biol Chem. 2008;283:6050–6057. doi: 10.1074/jbc.M707328200. [DOI] [PubMed] [Google Scholar]
- 24.Cheung WD, Hart GW. AMP-activated protein kinase and P38 map kinase activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283:13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol. 2007;292:C178–187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 26.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am J Physiol. 2006;290:C57–65. doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- 27.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine Biosynthesis and Protein O-Glycosylation: The First Line of Defense against Stress, Ischemia, and Trauma. Shock. 2007;29:431–440. doi: 10.1097/shk.0b013e3181598bad. [DOI] [PubMed] [Google Scholar]
- 28.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 29.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-Glycosylation of Nuclear and Cytosolic Proteins. Cloning and characterization of a neutral, cytosolic beta -N-Acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 30.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-Glycosylation of Nuclear and Cytosolic Proteins. Further characterization of the nucleocytoplasmic beta -N-Acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 31.Jones SP. A Bittersweet Modification: O-GlcNAc and Cardiac Dysfunction. Circ Res. 2005;96:925–926. doi: 10.1161/01.RES.0000168039.61228.67. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, D B, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96.9:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]