Abstract
Purpose
Recent data in postmenopausal women indicate that current and past use of exogenous hormones is related to urinary incontinence (UI) risk. Little is known about exogenous hormones and UI risk in younger women. We investigated the association between oral contraceptive pills (OCPs) and incident UI in premenopausal women enrolled in the Nurses' Health Study II.
Materials and Methods
Participants reported use of OCPs from 1989 to 2001. Among 21,864 premenopausal women, aged 37-54 years, reporting no UI in 2001, we identified 749 cases with incident UI at least weekly between 2001 and 2003. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression.
Results
Women who had ever used OCPs had statistically significant 27% (95% CI 1-59%) increased odds of developing UI at least weekly compared with women who never used OCPs. Among women with 10 or more years of use, the odds ratio increased to 1.48 (95% CI 1.13-1.95). Ever use of OCPs was specifically associated with urgency UI (OR 2.48, 95% CI 1.07-5.76) and not stress UI (OR 1.04, 95% CI 0.78-1.40). Although we had limited information on urinary tract infection, control for urinary tract infection did not alter these findings.
Conclusions
Use of OCPs may be associated with a modest increase in odds of UI among premenopausal women. However, this is one of the first reports of such an association and thus, further research is needed to confirm our findings and investigate possible mechanisms.
Keywords: urinary incontinence; contraceptives, oral; epidemiology
INTRODUCTION
Recent large-scale prospective studies, including randomized trials, in postmenopausal women have found increased risks of urinary incontinence (UI) among both current and former users of hormone therapy.1-3 In the Nurses' Health Study, we found a significant 50% increased risk of UI among current users of oral hormone therapy and significant 18-39% increases in risk in past users up to 9 years after stopping therapy.1 Although a biologic mechanism for this association has not yet been established, the association of hormone use with changes in the collagen composition of the bladder and increased bladder contractility have been proposed as some potential explanations.2, 3
Because few studies have examined use of oral contraceptive pills (OCPs) in relation to UI risk,4-8 whether exogenous hormone use is associated with an increase in UI risk in premenopausal women is largely unknown. Additional knowledge of the relation of OCPs to UI, especially by UI type, will be important for beginning to understand possible ways in which exogenous hormone use may influence incontinence mechanisms. Thus, we examined the association of OCP use with incident UI in premenopausal women aged 37-54 years.
MATERIALS AND METHODS
Study population
The Nurses' Health Study (NHS) II, modeled after the original NHS,9 was established when 116,671 female nurses aged 25-42 years provided written informed consent by responding to a mailed questionnaire in 1989. Information from participants is updated using biennial questionnaires. Responses are identified by identification number only to provide participant confidentiality. To maximize participation during each questionnaire cycle, the full-length questionnaire is sent for initial mailings, after which an abbreviated version of the questionnaire is sent to non-responders. In 2003, the follow-up rate was 88%. This study was approved by the Institutional Review Board of Brigham and Women's Hospital.
Questions about UI frequency and quantity were included on the 2001 and 2003 full-length questionnaires. Women who completed both full-length questionnaires (n=70,712) were similar to those who did not in mean age, mean body mass index (BMI), parity, and use of OCPs.
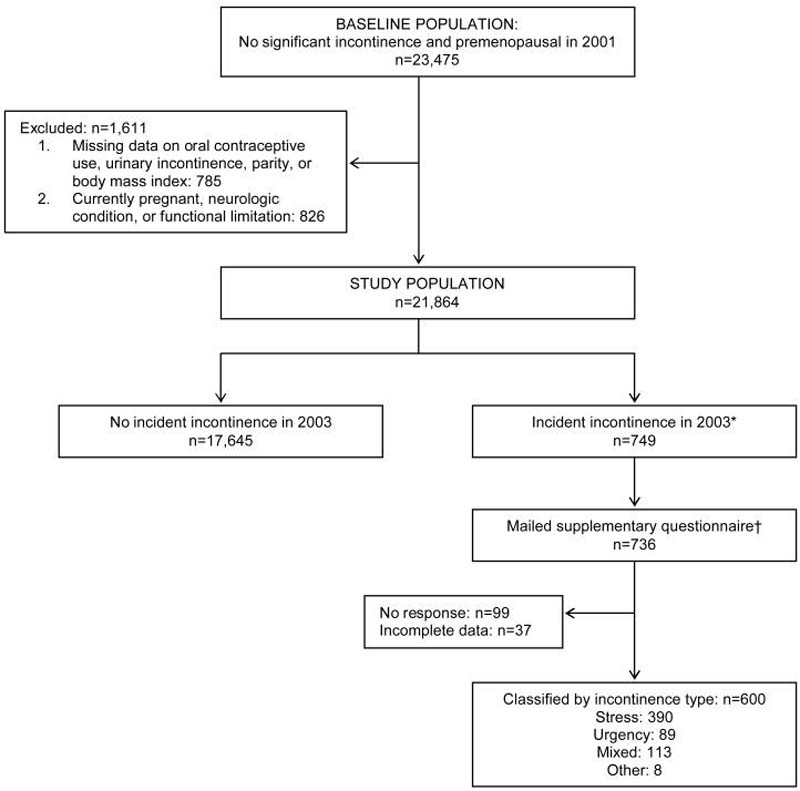
To ensure that only continent women were included at baseline, we excluded women reporting UI at least once per month or more than a few drops of urine leakage less than once per month in 2001 (n=36,704). Of the 34,008 women at risk for incident UI in 2001, 33,952 (99.8%) also provided UI data in 2003. We restricted these analyses to women who were premenopausal and not currently pregnant as of the 2001 questionnaire (n=23,286). Premenopausal women were defined as women reporting that their natural periods had not ceased permanently or women aged ≤45 years reporting having their uterus removed, but with at least 1 ovary retained.10 we excluded women missing data on OCP use (n=99) and key UI risk factors, including parity (n=462) and BMI (n=168). To avoid potential confounding by health status, women with major health conditions, including stroke, multiple sclerosis, and Parkinson's disease (n=217), or functional limitations, defined as difficulty climbing a flight of stairs, walking 1 block, or bathing or dressing (n=476), were also excluded. Thus, 21,864 premenopausal participants were at-risk for incident UI in 2001 (Figure 1).
Figure 1. Flow diagram of study participants.
* Analysis did not include 3,470 women with less than weekly incident incontinence in 2003.
† Supplementary questionnaires were not mailed to 13 incident cases identified late in the follow-up cycle.
Measurement of urinary incontinence
In 2001 and 2003, participants were asked, “During the last 12 months, how often have you leaked or lost control of your urine?” Response options were: never, less than once per month, once per month, 2-3 times per month, about once per week, and almost every day. Participants reporting incontinence were then asked, “When you lose your urine, how much usually leaks?” Response options were: a few drops, enough to wet your underwear, enough to wet your outer clothing, and enough to wet the floor. High reproducibility of responses to these questions was demonstrated in a similar group of nurses.11
We defined incident incontinence as leakage occurring at least once per week in 2003. In all analyses, non-cases were women reporting no UI or leakage of a few drops less than once per month again in 2003.
Supplementary questionnaires including validated questions to assess UI type12 were mailed to 98% (n=736) of incident cases toward the close of the 2003 follow-up cycle (13 cases were identified after we had already mailed the supplementary questionnaire and thus did not receive a mailing). After excluding 99 non-responders and 37 women with incomplete data, there were 600 women with information on UI type. Mean BMI, mean age, parity, and ever use of OCPs were similar in incident cases with frequent UI who did and those who did not provide information on UI type. On the supplementary questionnaire, women were asked about the frequency of UI (never, rarely, sometimes, or often) during coughing or sneezing, lifting things, laughing, and exercise (stress UI symptoms) as well as after experiencing a sudden feeling of bladder fullness or having an urge to urinate when a toilet could not be found or was occupied (urgency UI symptoms). Women reporting stress UI symptoms more often than urgency UI symptoms were classified as stress UI and those reporting urgency UI symptoms more often then stress UI symptoms were classified as urgency UI. Women were classified as mixed UI when stress and urgency UI symptoms were reported at the same frequency on the supplementary questionnaire.
Measurement of oral contraceptive use
In 1989, participants were asked to report whether they used an OCP for at least 2 months at each age from 13 years or younger to the present. Questions about OCP use during the previous 2 years, including number of months of use, have been included on each subsequent biennial questionnaire. From these reports, we classified each participant's use of OCPs in 2001 (current, past, never) and determined duration of use (<5, 5-9, ≥10 years) among ever users. Because a relatively small percent of the women were currently taking OCPs in 2001, our primary analyses focused on ever use of OCPs. A validation study found 99% agreement for ever use of OCPs and a high correlation for duration of use (r=0.94) comparing questionnaire reports with data from a detailed telephone.13
Statistical analysis
Women with less than weekly incident UI were excluded from these analyses since they met neither our case nor non-case definition (n=3,470). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using separate multivariable logistic regression models for each case definition (i.e., any, stress, urgency, and mixed UI). To ensure that women with incontinence were not included with non-cases, each analysis included only cases meeting the specific definition of interest (e.g., stress UI cases were excluded from urgency UI analyses). Covariates were variables previously identified in the literature as UI risk factors, including age (≤40, 41-45, 46-50, >50 years), parity (0, 1-2, ≥3 births), BMI (continuous), race/ethnicity (white, black, Hispanic, Asian, other/missing), cigarette smoking (never, past, current), hysterectomy, and type 2 diabetes. Additional adjustment for hypertension and use of diuretics did not change results and, thus, were not included in the final models. All covariate information was self-reported as of the 2001 questionnaire.
Tests for linear trend in UI odds with increasing duration of OCP use were performed by using the median value of each OCP duration category in a continuous variable in the logistic regression model, excluding women who never used OCPs.
Because we were concerned about potential residual confounding by cigarette smoking, we conducted a secondary analysis restricted to women who never smoked (n=15,458). Also, because parity may have an overwhelming impact on risk of stress UI in younger women, we repeated the analysis of OCP and stress UI among nulliparous women (n=4,537).
RESULTS
In 2001, study participants were age 37-54 years. Compared with never users, women who had ever used OCPs were more likely to be parous, cigarette smokers, and to have undergone hysterectomy (Table 1). Among those who had used OCPs, 15% (n=2,823/18,592) were current users in 2001. The 2-year incidence rates for UI were 2.9% (94/3,272) among women who never used OCPs and 3.5% (655/18,592) among women who had used OCPs.
Table 1.
Characteristics of premenopausal women at risk for incident urinary incontinence by oral contraceptive use in 2001
| Oral Contraceptive Use |
||
|---|---|---|
| Characteristics | Never (n=3,272) | Ever (n=18,592) |
| Mean age (years) | 45.1 | 44.7 |
| Mean body mass index (kg/m2) | 25.5 | 25.2 |
| Race or ethnicity (%) | ||
| White | 90.6 | 93.5 |
| Black | 0.8 | 1.1 |
| Hispanic | 1.3 | 1.1 |
| Asian-American | 3.8 | 1.3 |
| Other/missing | 3.5 | 3.0 |
| Parity (%) | ||
| 0 live births | 28.1 | 19.5 |
| 1-2 live births | 40.2 | 52.6 |
| ≥ 3 live births | 31.8 | 27.9 |
| Cigarette smoking (%) | ||
| Never | 81.9 | 68.8 |
| Past | 14.1 | 24.2 |
| Current | 4.0 | 7.0 |
| Hysterectomy (%) | 3.3 | 4.9 |
| Diabetes mellitus (%) | 2.6 | 1.5 |
After multivariable adjustment, ever use of OCPs was significantly associated with modest odds of incident UI (OR 1.27, 95% CI 1.01-1.59) (Table 2). In addition, the odds of UI increased significantly with increasing duration of OCP use (p for trend=0.03). Findings were generally similar in analyses limited to women who never smoked cigarettes (OR 1.42, 95% CI 1.09-1.85 for ever use; OR 1.68, 95% CI 1.21-2.33 for at least 10 years of OCP use).
Table 2.
Odds ratios for urinary incontinence by status and duration of oral contraceptive use among premenopausal women in the Nurses' Health Study II*
| Incident Incontinence |
||||
|---|---|---|---|---|
| Oral contraceptive use | Noncases | Cases | Age-adjusted OR (95% CI) | Multivariable OR (95% CI)† |
| Never | 2699 | 94 | 1.00 | 1.00 |
| Ever | 14,946 | 655 | 1.29 (1.04-1.61) | 1.27 (1.01-1.59) |
| Duration of use‡ | ||||
| < 5 years | 7,166 | 300 | 1.21 (0.95-1.53) | 1.17 (0.92-1.48) |
| 5-9 years | 3,994 | 183 | 1.37 (1.06-1.76) | 1.31 (1.01-1.70) |
| ≥ 10 years | 2,991 | 139 | 1.42 (1.08-1.85) | 1.48 (1.13-1.95) |
| P Value for Trend | 0.11 | 0.03 | ||
CI, confidence interval; OR, odds ratio. Incontinence is defined as leaking at least once per week. Women who reported less than weekly incident incontinence are excluded from these analyses (n=3,470).
Adjusted for age, parity, body mass index, race/ethnicity, cigarette smoking, hysterectomy, diabetes mellitus
Women with missing data on duration of oral contraceptive use are excluded from these analyses (n=828)
Separate analyses of current versus never use of OCPs and past versus never use (data not shown in table) also suggested an increase in UI odds with long duration of use, although the relatively small number of cases who were current users (n=78) limited the statistical power for this comparison. For example, the odds ratio for UI among those with at least 10 years of OCP use was 1.37 (95% CI 0.97-1.96) in current users and 1.55 (95% CI 1.15-2.11) in past users compared with never users. As expected, among past users, the increase in odds appeared to be highest in the more recent users (OR 1.88, 95% CI 1.37-2.59 for last OCP use within 2 years; OR 1.23, 95% CI 0.97-1.55 for last OCP use 10 or more years ago).
When we examined the odds of specific UI types, ever use of OCPs was associated with urgency UI (OR 2.48, 95% CI 1.07-5.76) and not stress UI (OR 1.04, 95% CI 0.78-1.40) (Table 3). Odds ratios for mixed UI were not statistically significant, although there was a trend of increasing odds with increasing duration of use (p for trend=0.02). In analyses restricted to nulliparous women, there was a suggestion that ever use of OCPs was associated with stress UI (OR=5.40, 95% CI 1.66-17.50); however, given the relatively small number of nulliparous women, the confidence interval was wide and this finding should be interpreted cautiously.
Table 3.
Odds ratios for stress, urgency, and mixed urinary incontinence by status and duration of oral contraceptive use among premenopausal women in the Nurses' Health Study II*
| Stress Incontinence |
Urgency Incontinence |
Mixed Incontinence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oral contraceptive use Noncases | Cases | Age-adjusted | Multivariable† | Cases | Age-adjusted | Multivariable† | Cases | Age-adjusted | Multivariable† | |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |||||
| Never | 2,699 | 56 | 1.00 | 1.00 | 6 | 1.00 | 1.00 | 15 | 1.00 | 1.00 |
| Ever | 14,946 | 334 | 1.11 (0.83-1.47) | 1.04 (0.78-1.40) | 83 | 2.48 (1.08-5.70) | 2.48 (1.07-5.76) | 98 | 1.22 (0.71-2.11) | 1.29 (0.74-2.24) |
| Duration of use‡ | ||||||||||
| < 5 years | 7,166 | 167 | 1.13 (0.83-1.53) | 1.05 (0.77-1.44) | 35 | 2.20 (0.92-5.23) | 2.23 (0.93-5.36) | 38 | 0.96 (0.53-1.74) | 0.97 (0.53-1.79) |
| 5-9 years | 3,994 | 85 | 1.07 (0.76-1.50) | 0.98 (0.69-1.38) | 21 | 2.35 (0.95-5.84) | 2.24 (0.89-5.65) | 36 | 1.71 (0.93-3.14) | 1.81 (0.98-3.35) |
| ≥ 10 years | 2,991 | 66 | 1.13 (0.79-1.62) | 1.14 (0.79-1.64) | 22 | 3.28 (1.32-8.12) | 3.30 (1.31-8.28) | 22 | 1.45 (0.75-2.81) | 1.68 (0.86-3.29) |
| P Value for Trend | 0.98 | 0.76 | 0.17 | 0.22 | 0.08 | 0.02 | ||||
CI, confidence interval; OR, odds ratio. Women who reported less than weekly incident incontinence (n=3,470), incident cases missing data on incontinence type (n=149), and incident cases whose incontinence type was unclassifiable (n=8) are excluded from these analyses.
Adjusted for age, parity, body mass index, race/ethnicity, cigarette smoking, hysterectomy, diabetes mellitus
Women with missing data on duration of oral contraceptive use are excluded from these analyses (stress: n=811; urgency: n=800; mixed: n=797)
DISCUSSION
Among these premenopausal women aged 37 to 54 years, we found modest increases in the odds of UI in women with a history of OCP use for 5 years or more. OCP use was specifically associated with elevated odds of urgency UI. However, these results should be interpreted cautiously since, to our knowledge, they are the first large-scale, prospective data on the relation of exogenous hormones to UI in premenopausal women. Further confirmation in additional large, prospective studies is needed.
Of the limited previous studies that have investigated use of OCPs and risk of UI,4-8 the majority4-6 found no association. However, these investigations generally utilized broad UI definitions and did not consider UI frequency or type. In our study, OCP use was primarily associated with an increase in more frequent UI and urge UI. In addition, few studies have considered current or past OCP use separately. In a cross-sectional study of 8,689 twins aged 20-46 years, Iliadou et al reported a lower prevalence of stress (OR 0.57, 95% CI 0.41-0.79) and urgency UI (OR 0.36, 95% CI 0.14-0.92) among current OCP users compared with past and never users.8 However, past and never users were combined in the reference group and duration of use was not examined. Moreover, this was not a prospective study, which could increase the possibility of bias. Although we had limited power to assess risks specifically in current users, in contrast to the Iliadou study, our data suggested an elevated UI risk in current users with long duration of use and in those who had recently stopped using OCPs. Thus, again, more detailed research is needed in large-scale prospective studies of premenopausal women.
Overall, the link between exogenous hormone use and an increase in UI risk are relatively recent,1-3 and the mechanisms underlying this association are not yet understood. Our finding of a relation between OCPs and urgency UI may help in beginning to identify mechanisms, as could our observation that the highest odds of UI appeared to be among long-term OCP users. For example, these observations could be consistent with an adverse neurologic effect of OCP use that requires many years to develop. In addition, although our observation of an increase in odds of stress UI among nulliparous women using OCPs was a sub-group analysis and should be interpreted cautiously, this result may be attributed to mechanisms similar to those proposed for postmenopausal hormone therapy in stress UI.2, 3 Specifically, hormone therapy has been associated with an increase in collagen turnover,14, 15 which may lead to an increased risk of stress UI development. In premenopausal women, these factors may be notable only in nulliparous women, whose risk of stress UI is not primarily determined by childbirth.
Our study has several limitations. First, all information on UI and OCP use was self-reported. However, previous studies have established the reliability11 and validity16 of self-reported UI. Also, Sandvik and colleagues17 found high specificity of self-reported stress and urgency UI symptoms compared with clinical diagnoses, which is most important to obtain valid results in prospective epidemiologic studies.18 In a sub-sample of NHS II participants, reports of OCP use on the questionnaires were highly valid compared with reports provided during a detailed telephone interview.13 In addition, in a prospective study, misclassification of OCP use is likely non-differential and would tend to lead to an underestimation of odds ratios.
Because relatively few cases had never used OCPs, precision of the UI type-specific odds ratios was limited and confidence intervals were wide. In addition, although we considered multiple potential confounding variables in our analyses, the possibility of residual confounding cannot be eliminated from an observational study. In particular, we did not have detailed data on history of infections such as urethritis or cystitis which are associated with both sexual activity and urinary urgency. However, such potential confounders would need to be very strongly associated with both OCP use and urgency UI to fully explain the two to three-fold elevations we observed for urgency UI. Moreover, although we only have limited data on self-reported urinary tract infections from the participants, additional adjustment for UTI did not meaningfully affect our results. Also, while contraception was likely the main indication for OCP use, we did not collect data on reason for use and, thus, could not account for OCP use that was related to a condition that increases risk of UI. Finally, because only a small proportion of the NHS II participants are non-white, it is possible that our findings are not generalizable to minority populations in addition to women with major neurologic conditions.
CONCLUSIONS
In conclusion, our study findings suggest that OCP use may be associated with a modestly increased risk of UI among premenopausal women. To our knowledge, these are the first large-scale, prospective data to report such a relation, thus it is important that our results are confirmed in additional studies, particularly those with data on urethritis and sexual activity, before they are considered in clinical practice. However, these results, in combination with observations of increased UI risk in women using postmenopausal hormone therapy, suggest that further research to identify mechanisms by which exogenous hormone use may increase incontinence could be helpful in fully understanding incontinence development, and eventual prevention.
FINANCIAL SUPPORT
This research was supported by grants DK62438 and CA50385 from the National Institutes of Health. M. K. Townsend was supported by the Yerby Postdoctoral Fellowship Program.
REFERENCES
- 1.Grodstein F, Lifford K, Resnick NM, Curhan GC. Postmenopausal hormone therapy and risk of developing urinary incontinence. Obstet Gynecol. 2004;103:254. doi: 10.1097/01.AOG.0000107290.33034.6f. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 3.Steinauer JE, Waetjen LE, Vittinghoff E, Subak LL, Hulley SB, Grady D, et al. Postmenopausal hormone therapy: does it cause incontinence? Obstet Gynecol. 2005;106:940. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milsom I, Ekelund P, Molander U, Arvidsson L, Areskoug B. The influence of age, parity, oral contraception, hysterectomy and menopause on the prevalence of urinary incontinence in women. J Urol. 1993;149:1459. doi: 10.1016/s0022-5347(17)36415-7. [DOI] [PubMed] [Google Scholar]
- 5.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983. doi: 10.1016/s0029-7844(97)00537-1. [DOI] [PubMed] [Google Scholar]
- 6.Burgio KL, Zyczynski H, Locher JL, Richter HE, Redden DT, Wright KC. Urinary incontinence in the 12-month postpartum period. Obstet Gynecol. 2003;102:1291. doi: 10.1016/j.obstetgynecol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Hvidman L, Foldspang A, Mommsen S, Bugge Nielsen J. Menstrual cycle, female hormone use and urinary incontinence in premenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:56. doi: 10.1007/s00192-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 8.Iliadou A, Milsom I, Pedersen NL, Altman D. Risk of urinary incontinence symptoms in oral contraceptive users: a national cohort study from the Swedish Twin Register. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.07.002. doi:10.1016/j.fertnstert.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 10.Schernhammer ES, Tworoger SS, Eliassen AH, Missmer SA, Holly JM, Pollak MN, et al. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14:721. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses' Health Study. Am J Obstet Gynecol. 2003;189:428. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 12.Diokno A, Yuhico M., Jr. Preference, compliance and initial outcome of therapeutic options chosen by female patients with urinary incontinence. J Urol. 1995;154:1727. [PubMed] [Google Scholar]
- 13.Hunter DJ, Manson JE, Colditz GA, Chasan-Taber L, Troy L, Stampfer MJ, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception. 1997;56:373. doi: 10.1016/s0010-7824(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 14.Falconer C, Ekman-Ordeberg G, Ulmsten U, Westergren-Thorsson G, Barchan K, Malmstrom A. Changes in paraurethral connective tissue at menopause are counteracted by estrogen. Maturitas. 1996;24:197. doi: 10.1016/s0378-5122(96)82010-x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG. 2002;109:339. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 16.Diokno AC, Brown MB, Brock BM, Herzog AR, Normolle DP. Clinical and cystometric characteristics of continent and incontinent noninstitutionalized elderly. J Urol. 1988;140:567. doi: 10.1016/s0022-5347(17)41720-4. [DOI] [PubMed] [Google Scholar]
- 17.Sandvik H, Hunskaar S, Vanvik A, Bratt H, Seim A, Hermstad R. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48:339. doi: 10.1016/0895-4356(94)00147-i. [DOI] [PubMed] [Google Scholar]
- 18.Rothman KJ, Greenland S. Modern Epidemiology. Lippincott Williams & Wilkins; Philadelphia (PA): 1998. [Google Scholar]