Abstract
Based on prior clinical trials indicating that γ-aminobutyric acid (GABA) based anticonvulsant medications reduce drug craving in cocaine dependent study participants, we tested the effects of valproate treatment on cue-induced cocaine craving. Crack cocaine dependent individuals (N=20) were tested in a randomized, placebo-controlled, within-subjects, crossover study design. Valproate treatment was titrated up to 1500 mg/day by Day 6 of treatment, cue testing was completed on Day 8 of treatment, and all study participants underwent a washout period of 5 days between active and placebo medication treatment periods. Testing included both cocaine and neutral cue exposure sessions, presented in a random and counterbalanced order. Main effects of cue exposure were found for subjective ratings of “desire to use cocaine now”, the cocaine craving index, cocaine-like high, and cocaine withdrawal. Treatment interaction effects were found with “desire to use cocaine now”, which underwent a greater increase following cocaine cue exposure in the valproate condition. Main effects of medication treatment were found, in which lower blood pressure and heart rate, and higher plasma cortisol levels, were associated with valproate treatment. Valproate treatment was also associated, at a trend level, with higher pre-test cocaine craving levels. The results demonstrate that cocaine cue reactivity is a robust phenomena across two assessment sessions, but fail to support the use of valproate as a means of reducing spontaneous and cue-induced cocaine craving. The use of valproate as a treatment for cocaine dependence is not supported.
Keywords: valproate, cocaine, cue exposure, craving, cortisol
1. Introduction
Cocaine dependence is a significant public health problem associated with serious medical, psychiatric, social and economic consequences. Treatment for cocaine dependence at present is primarily psychosocial counseling (Carroll, 2000, 2005). Unlike substitution treatment for opiate or nicotine dependence, or naltrexone or acamprosate treatment for alcohol dependence, no pharmacological agent is currently approved for the treatment of cocaine dependence (Vocci and Ling, 2005).
Cocaine causes abnormalities in the γ-aminobutyric acid (GABA) system. Chronic cocaine treatment results in the down regulation of GABA-A receptor alpha, beta and gamma subunits (Suzuki et al., 2000) and a decrease in endogenous GABA release in the striatum (Jung et al., 1999) of rats. Cocaine abusers show significantly greater sensitivity to a challenge dose of lorazepam, a benzodiazepine which enhances the activation of GABA receptors, both in terms of enhanced sedative effects and greater reductions in cerebral metabolism (Volkow et al., 1998). Baclofen, a GABA receptor agonist, and gamma-vinyl GABA, which elevates endogenous GABA levels via inhibition of GABA transaminase (GABA-T), have both been shown to inhibit cocaine self-administration (Brebner et al., 2000), attenuate cocaine cue-induced behavioral activity (Franklin & Druhan, 2000) and reduce cocaine induced mesolimbic dopamine release (Gerasimov et al., 2000) in rats. Recently, two open-labels trials with gamma-vinyl GABA (Brodie et al., 2003, 2005), and placebo-controlled trials with GABAergic medications baclofen (Shoptaw et al., 2003) topiramate (Kampman et al., 2004) and tiagabine (Gonzalez et al., 2003, 2007), found evidence for efficacy in treating cocaine dependence.
Valproate (Depakote®), an anticonvulsant medication which enhances neuronal GABA levels by increasing glutamic acid decarboxylase (GAD) activity and inhibiting GABA-T (Loscher, 1993; Phillips & Fowler, 1982), has a similar pharmacological profile to gamma-vinyl GABA with regards to inhibition of GABA-T. It has also been shown to inhibit cocaine and methamphetamine induced seizures (Hanson et al., 1999; Derlet & Albertson, 1990) and block the expression of methylphenidate sensitization (Yang et al., 2000) in rats. In cocaine dependent study participants, open-label treatment with valproate (1500 mg/day) resulted in a 60%–70% decrease in self-reported cocaine craving and cocaine positive urine screens (Myrick et al., 2001). Further investigation of open-label valproate treatment (2000 mg/day) found a consistent trend towards a decrease in cocaine abuse from baseline, verified by cocaine free urine samples (Halikas et al., 2001). These findings suggest that valproate produces a reduction in cocaine craving and use, and may potentially be of therapeutic value in the treatment of cocaine abuse.
Over the past several years numerous studies have demonstrated that environmental cues previously associated with cocaine use will consistently induce cocaine craving in cocaine-dependent individuals (Ehrman et al., 1992; O’Brien et al., 1990; Berger et al., 1996; Reid et al., 1998, 2008). This behavior is readily elicited in a clinical laboratory setting by means of a standardized cocaine cue procedure involving both visual/audio presentation and cocaine paraphernalia handling (Ehrman et al., 1992; Reid et al., 1998, 2008). Numerous groups have proposed cue-induced cocaine craving studies as a method for rapid screening of potential pharmacotherapies for cocaine addiction treatment. These studies have tested the effects of bromocriptine (Kranzler & Bauer, 1992), amantadine (Robbins et al., 1992), haloperidol (Berger et al., 1996), ritanserin (Ehrman et al., 1996), nicotine and mecamylamine (Reid et al., 1998, 1999), baclofen (Brebner et al., 2002), and tryptophan depletion (Satel et al., 1995) on cocaine cue reactivity. Some studies that found a reduction in cue-induced craving (Berger et al., 1996; Reid et al., 1998, 1999; Brebner et al., 2002) were then followed-up by pilot studies investigating the same, or similar, medications for the treatment of cocaine dependence. While mecamylamine (Reid et al., 2006) and dopamine receptor antagonists (Grabowski et al., 2000; Reid et al., 2005) were found to be ineffective, studies on baclofen treatment found positive evidence for efficacy in the treatment of cocaine dependence (Shoptaw et al., 2003). The present study employed cue-induced cocaine craving assessments in crack cocaine dependent patients to test for potential therapeutic effects of valproate. Briefly, this study examined the effects of valproate (1500 mg/day) treatment on cue-induced cocaine craving following an 8 day dose titration, in a within subjects, placebo-controlled, crossover study design.
2. Methods
2.1. Participants
Cocaine dependent individuals were recruited from the New York City area by newspaper advertisement. All participants were confirmed currently cocaine dependent based on DSM-IV criteria from the SCID and verified by urine samples positive of cocaine, and the preferred route of administration for all participants was smoked crack cocaine. All participants had normal results for physical exam, clinical laboratory results, and psychiatric interview. Exclusion criteria included current axis-I psychiatric disorders which required medication treatment, and any history of significant liver disease or liver dysfunction, including hepatitis B and C. All participants gave signed informed consent prior to their entry into the study. Screening, treatment, and testing were performed at the VA New York Harbor Healthcare System (VANYHHS), Manhattan Campus, and the participants were compensated for time and travel ($20/visit). This study was approved by the VANYHHS Human Subjects Subcommittee, and was conducted in accordance with the Declaration of Helsinki. Between 1/2005 and 11/2008 total of 49 potential participants were screened for participation in this outpatient medication study, of which 23 met screening criteria and were enrolled (26 screen fails, and 3 study non-completers, were dropped mainly due to failing to attend study visits). Demographic and baseline data for enrolled participants are shown in Table 1.
Table 1.
Demographics and Baseline Measures
| Male | Female | |
|---|---|---|
| Gender | 16 | 4 |
| Race | ||
| African American | 13 | 3 |
| Hispanic | 2 | 1 |
| Caucasian | 1 | |
| Unemployed | 9 | 2 |
| Cigarette Smoker | 10 | 2 |
| Means | SEM | |
| Age | 43.6 | 1.6 |
| Years Education | 13.4 | 0.5 |
| Beck Depression Inventory (BDI) (0–63) | 14.5 | 3.1 |
| Beck Anxiety Index (BAI) (0–63) | 6.1 | 2.1 |
| Addiction Severity Index (ASI) (0–1) | 0.185 | 0.018 |
| Years Cocaine Abuse | 14.3 | 1.8 |
| Days out of last 30 Cocaine Abuse | 14.5 | 1.7 |
| Prior Cocaine Abuse Treatment Episodes | 2.6 | 0.7 |
| Brief Substance Craving Scale (BSCS) (0–12) | 2.7 | 0.7 |
| Cocaine Selective Severity Assessment (CSSA) (0–136) | 17.9 | 2.6 |
Range of scores for BDI, BAI, ASI, BSCS and CSSA are indicated in parentheses.
2.2. Treatment
Participants that met study eligibility requirements were treated with valproate in a placebo-controlled, double-blind, crossover design, with active followed by placebo medication or placebo followed by active medication. Treatment sequence was randomly selected on a 1:1 ratio. Commercially available valproate (Depakote®) and placebo medications were packaged and blinded by the research pharmacist employing an over encapsulation process with 00 size, opaque (drk blue) capsules, which were dispensed on a weekly basis. Valproate treatment was provided on an 8-day dose-escalating design, chosen to maximize study retention within a minimum time needed to establish steady state levels (4 days), and based on the tolerability of reaching 1500 mg/day by the 6th day of treatment (PDR 2001): Days 1–2, 500 mg QD; Days 3–5, 500 mg BID; Days 6–10, 500 mg TID. Placebo treatment followed the same dispensing schedule. The randomization scheme included permutation blocks of 4 and each treatment condition was subject to stratification based on gender and frequency of cocaine use over the last 30 days prior to informed consent (heavy: >15 cocaine use days; moderate: ≤ 15 cocaine use days) to assure equally balanced medication treatment sequences within the study population. All participants ingested their first dose of medication at the research clinic and a clinical safety visit was scheduled 4 days after the start of medication treatment. Other than debriefing after test days, participants were not provided with any formal psychosocial treatment for cocaine abuse while in the study. Daily ad hoc cocaine abuse behavior, and compliance with the daily medication regimen, was assessed during treatment by self report and returned medication capsule count.
2.3. Cocaine Cue Reactivity Testing
The cue exposure sessions involved a combination of tactile, olfactory, visual and audio cues and were based on prior work on nicotine (Reid et al., 1998) and mecamyalmine (Reid et al., 1999) modulation of cue-induced cocaine craving. The tests consisted of a neutral cue session (A) and an active cocaine cue session (B), presented in a random order and separated by approximately 30 min. Cue sequence randomization was counterbalanced across treatment conditions to ensure that an equal number of participants started with neutral cues vs active cues on each test day.
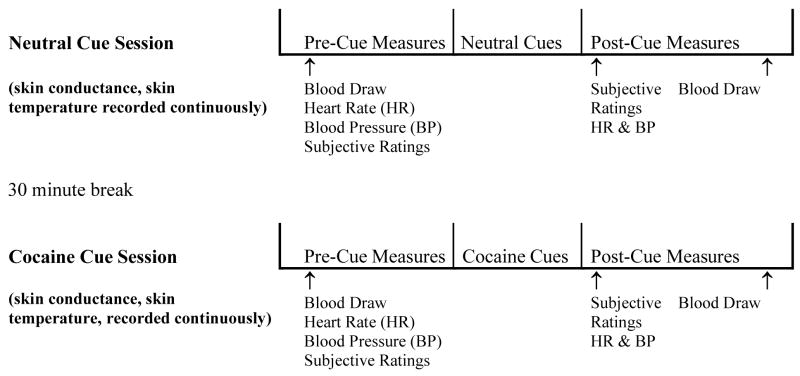
Cue testing was performed on the final day of each medication treatment period (Day 8 and Day 19). Cue testing began between 11AM and 12 noon and took approximately 2.5 hours to complete (see Figure 1). All participants were instructed to abstain from using cocaine, alcohol and other drugs of abuse for 24 hours prior to testing and not to smoke cigarettes for 1 hour prior to testing, verified by interview, on-site urine toxicology and exhaled carbon monoxide (CO) assessments. During the cocaine cue tests participants were seated in an outpatient clinic testing room in a comfortable, high back chair with arm rests. At the beginning of the test session participants completed the following psychosocial assessments: Brief Substance Craving Scale (BSCS) (Mezinskis et al., 1998), Beck Depression Index (BDI) (Beck, 1978), Beck Anxiety Index (BAI) (Beck, 1988), and the Cocaine Selective Symptoms Assessment (CSSA) (Kampman et al. 1998), and then blood samples (for plasma cortisol level determination) were taken. Once completed, electrodes for skin conductance, skin temperature, and heart rate were placed on the participant’s nonwriting finger tips, blood pressure and heart rate were measured, and then cue exposure testing commenced.
Figure 1.
Schematic diagram depicting the cue exposure study design. Sample presented is with cue order in B (neutral cue) followed by A (cocaine cue) sequence. Abbreviations: BP: Blood pressure, HR: Heart rate, SR: Subjective ratings.
Upon the beginning of cue testing procedures participants completed the Within Sessions Rating Scale and a modified version of the Positive and Negative Affect Schedule (PANAS) and then 5 minutes of baseline skin conductance, skin temperature and heart rate were collected. Following this baseline recording period, each participant underwent a 10 minute cue exposure procedure that involved viewing and handling items placed in front of him/her and viewing a 5 minute video. During the cue exposure, skin conductance and skin temperature were collected continuously. Immediately following viewing the video each participant completed the Within Sessions Rating Scale and the PANAS and blood pressure and heart rate were measured, and then 20 minutes later a second blood sample was collected. Once all procedures were completed the participant was given a 30 min rest period before proceeding with the next cue session.
2.3.1. Neutral Cues
Shells, rocks and a pinecone were placed on a tabletop directly in front to the participant. The participant was instructed to smell a stick of fragrant spice (cinnamon) and then the participant handled the shells, rocks and a pinecone and was instructed to make 2–3 patterns on the desktop with the items (5 min) then a short video (5 min) was presented. The neutral cue video tape consisted of images of pine cones and seashells being handled.
2.3.2.Cocaine Cues
Cocaine and crack paraphernalia including various crack pipes and stems, screens, lighters, scrappers, and a procaine-derived white crystalline powder designed to simulate crack “rocks” in small baggies were placed on a tabletop directly in front to the participant. The participant was asked to smell a recently used stem for any scent of crack residue (reapplied every month using pseudococaine (SIGMA, St. Louis, MO), select a stem they would typically use, and then handle each of the cocaine paraphernalia items (5 min), and then a short video (5 min) was presented. The 5 minute cocaine cue video tape contained a complex mixture of auditory and visual cues specific to the purchase and smoking of crack cocaine.
2.3.3. Post Testing Debriefing
At the end of each test day the participants underwent a debriefing procedure to minimize the impact of experimentally induced cocaine craving before being discharged for the day. Relaxation exercises and a discussion of the participant’s plans for the afternoon were involved. In addition, additional compensation for cue testing was held until the follow-up visit before it could be received.
2.4. Cue Reactivity Measures
2.4.1. Psychophysiological Measures
Physiological measures were selected based on the work of Ehrman and colleagues (1992) and Reid and colleagues (1998, 2008) demonstrating robust and reliable cue responding. Skin conductance (μMHOS) and skin temperature (F°) were recorded using fingertip electrodes connected to a PC computer via a RS232 beltpack/interface unit (Biograph, Thought Technology, Montreal, Canada). Skin conductance was recorded using Ag-Ag/Cl paste electrodes with a constant excitation set at 0.5 V d.c. and spontaneous skin conductance responses are eliminated by means of a low pass filter set at 20 μMHOS. Skin temperature was recorded using an electric thermister that monitors absolute temperature. Heart rate and blood pressure were measured using a standard blood pressure cuff and stethoscope, taken in a sitting position.
2.4.2. Plasma Sampling
Blood samples were collected into heparin containing tubes and the plasma was separated within 30 minutes by refrigerated centrifugation (1500 rpm, 15 min) followed by pipette extraction. The samples were stored at −70°C and were analyzed for cortisol content by radioimmunoassay at the Analytical Psychopharmacology Laboratories of Mr. Thomas Cooper, Nathan Kline Institute and NY State Department of Mental Health.
2.4.3. Within Sessions Rating Scale
The Within Sessions Rating Scale, based on previous cue studies (Berger et al., 1996; Reid et al., 1998, 1999), asks participants to estimate the intensity of craving and a variety of other drug-related states on a 1 to 100 mm visual analog scale (VAS) with descriptors “not at all”, “mildly”, “moderately” and “extremely” equally spaced above line from 0 to 100. Questions concerning cocaine included, “how much do you desire to use cocaine right now?” and “if you had access to cocaine right, how likely would you use it?” in the current context and when their abuse habits were greatest. Craving is quantified using the first individual item of the scale, “desire to use cocaine right now” as well as the average of all 4 craving items (Craving Index). In addition, patients rate “cocaine-like high” and “cocaine withdrawal” on a similar VAS line.
2.4.4. Positive and Negative Affect Schedule (PANAS)
The PANAS (Watson et al., 1988) is a subject rated 20-item measure with subscales for positive and negative affect. In this study we also calculated a third subscale for anxiety, based on the sum of scores for “nervous”, “jittery” and “irritable”. Items were scored on a 0–4 point Likert Scale.
2.5. Statistical Analyses
Initially, control analyses were performed to determine if ad hoc cocaine use over the preceding 7 days prior to testing were similar in each medication treatment condition. No significant differences were found. For each parameter, a multivariate approach to repeated measures ANOVA was initially used (mixed, repeated, within group analyses). Order effects of medication treatment and cue type sequence were tested for as factors in the cue reactivity ANOVA model and no effects were found so these variables were removed from the final model. The primary outcome measures were Within Sessions Rating Scale and the PANAS items for “desire to use cocaine now”, cocaine craving index, cocaine-like high, cocaine withdrawal, anxiety and plasma cortisol levels. Secondary outcome measures were heart rate, blood pressure, and the continuous physiological measures skin conductance and temperature which were assessed using mean values of 5 min recordings collected during: pre-cue, cue 1 (paraphernalia), and cue 2 (video viewing) presentation phases.
2.5.1. Effects of Valproate on Baseline and Cue-induced Cocaine Craving
Data from the Day 8 and Day 19 medication craving tests was analyzed. Pre-testing baseline mood (BDI and BIA) as well as CSSA and BSCS measures of cocaine craving and withdrawal, respectively, were compared in valproate versus placebo conditions by univariate ANOVA. For cue reactivity testing, valproate versus placebo conditions were compared in the primary and secondary outcome measures in a 2 (neutral cue, active cue) by 2 (pre-cue, post-cue) by 2 (placebo, active) repeated measures ANOVA corrected for baseline differences (physiological measures of skin temperature and conductance included 3-cue testing time points).
3. Results
3.1. Study Sample
Baseline demographic characteristics, cocaine abuse history, and related psychological assessments are displayed in Table 1. Participants were on average middle aged, high school educated, mostly unemployed, 20% were female, 60% were cigarette smokers, and 80% were African-American. Cocaine abuse histories indicated moderate addiction severity, 14 years cocaine abuse, and having been enrolled in drug rehabilitation 2–3 times prior to study participation (7 out of 20 reported no prior treatment). Cocaine craving (BSCS) and withdrawal (CSSA) levels during screening were low, and participants’ mood variables (BDI, BAI) indicated moderate levels of depressed mood and little or no anxiety among study participants.
3.2. Medication Treatment: Day 8 Pre-Test Measures
The effects of valproate treatment on self-reported craving and mood, and ad lib cocaine use over the preceding 7 days, were assessed at the beginning of each cue testing day and are shown in Table 2. Pre-test assessments of cocaine abuse, craving and withdrawal indicated, at a trend level, slightly more cocaine craving (BSCS total score) in the valproate condition (F(1,38)=2.326, p=0.103), but no effect of valproate treatment on cocaine withdrawal (CSSA) or on ad lib cocaine abuse rates. BDI and BAI symptom ratings were also unaffected by valproate treatment.
Table 2.
Treatment Day 8
| Pre-Testing Psychometric and Cocaine Abuse Measures (mean ± SEM | ||
|---|---|---|
| Valproate | Placebo | |
| Beck Depression Inventory (BDI) | 10.6±2.9 | 11.0±2.4 |
| Beck Anxiety Index (BAI) | 4.9±2.1 | 5.2±2.1 |
| Cocaine Abuse: Days use out of last 7 | 3.2±0.5 | 3.7±1.2 |
| Brief Substance Craving Scale (BSCS) | 6.2±0.6 | 5.2±0.6 |
| Cocaine Selective Severity Assessment (CSSA) | 22.2±3.6 | 21.1±3.2 |
| Cocaine Cue Testing - Physiological Measures (mean ± SEM) | ||||
|---|---|---|---|---|
| Valproate | Placebo | |||
| Cocaine Cue | Cocaine Cue | |||
| Pre-Cue | Post-Cue | Pre-Cue | Post-Cue | |
| Heart Rate (beats per minute) | 70.2±3.9 | 76.9±3.2 | 72.1±4.1 | 76.9±2.6 |
| Systolic Blood Pressure (mmHg) | 96.2±6.4 | 98.2±6.8 | 103.0±3.3 | 106.4±3.5 |
| Diastolic Blood Pressure (mmHg) | 61.1±4.1* | 64.4±4.5* | 68.7±2.4 | 69.2±2.2 |
| Plasma Cortisol (μg/dL) | 12.9±1.3* | 11.1±1.1* | 10.1±0.8 | 9.2±0.8 |
| Skin Conductance (μMHO) | 0.911±0.402 | 0.553±0.221 | 0.741±0.296 | 0.496±0.260 |
| Skin Temperature (F°) | 90.5±1.6 | 91.0±1.5 | 89.6±1.5 | 90.7±0.8 |
p<0.05 for main effect of medication treatment – valproate vs. placebo
3.3. Medication Effects on Cue-Induced Cocaine Craving and Related Subjective Measures
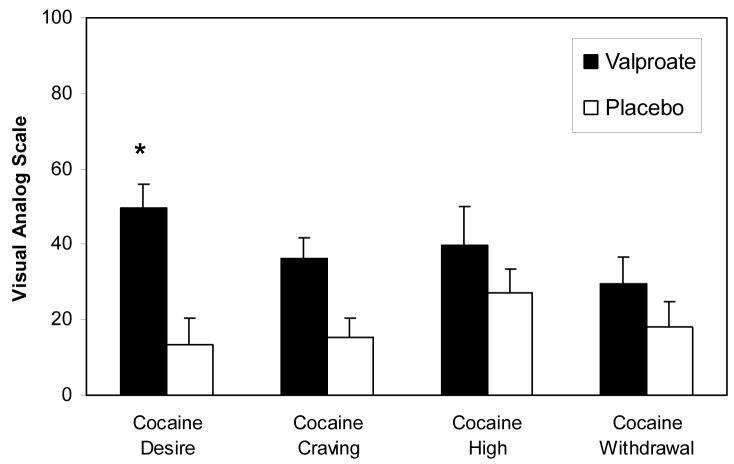
The effects of cocaine cue exposure on cocaine craving and related subjective measures in the valproate and placebo treatment conditions are displayed in Figure 2. The overall ANOVA model of cue effect found that cocaine cue exposure produced a reliable increase in ratings for “desire to use cocaine now” (F(1,19)=11.492, p<0.01), cocaine craving index (F(1,19)=10.627, p<0.05) cocaine-like high (F(1,19)=19.954, p<0.05) and cocaine withdrawal (F(1,19)=10.891, p<0.05). There were no effects of cue exposure on PANAS measures of positive or negative affect, or the subscore for anxiety (data not shown). Medication treatment interaction analyses indicated a significant effect of valproate treatment on cue-induced “desire to use cocaine now” (F(1,38)=3.916, p<0.05), in which cocaine cue-induced craving levels were higher in the valproate condition. Medication treatment interactions were statistically insignificant for all other cue-induced subjective measures.
Figure 2.
Cue-induced self-reported “desire to use cocaine now” (Cocaine Desire), cocaine craving index (Cocaine Craving), and related subjective measures (Cocaine High, Cocaine Withdrawal) assessed using the Within Sessions Rating Scale in the valproate (1500 mg/day) and placebo treatment conditions. The neutral cue corrected (cocaine cue minus neutral cue) changes in each rating from pre-to post-cue exposure are shown. Means and SEM are presented. * indicates p<0.05 for comparison of valproate versus placebo in a two (active cue, neutral cue) by two (pre-cue, post-cue) by two (valproate, placebo) ANOVA.
3.4. Medication Effects on Cue-Induced Physiological Responding
Plasma cortisol levels, heart rate, blood pressure, skin conductance, and skin temperature, measured prior to cocaine cue exposure on Day 8 of valproate or placebo treatment, are also presented in Table 2. There were no significant effects of cocaine cue exposure on plasma cortisol levels, skin conductance, skin temperature, heart rate or blood pressure. However, main effects of medication treatment indicated higher plasma cortisol levels (F(1,19)=4.132, p<0.05), reduced diastolic blood pressure (F(1,19)=6.676, p<0.05) and, at a trend level, lower heart rate (F(1,19)=2.881, p=0.090) in the valproate condition. But there were no significant cue type by medication treatment interactions for any of the physiological measures.
4. Discussion
Recent reviews of pharmacological treatments for addiction suggest that GABAergic anticonvulsants may be effective in treating cocaine dependence (Zullino et al., 2004; Vocci, & Ling, 2005; Preti, 2007; Myrick and Brady, 2003). These hypotheses were based partially on positive results obtained in single site, double-blind, placebo controlled studies with tiagabine (Winhusen et al., 2005; Gonzalez et al., 2003, 2007, but see Winhusen et al., 2007), baclofen (Shoptaw et al., 2003) and topiramate (Kampman et al. 2004). Studies with valproate in cocaine abuse patients (Myrick et al. 2001; Salloum et al., 2007) have also been cited as support for the hypothesis that anticonvulsants may be efficacious in the treatment of cocaine dependence, however, these findings were based on small, open-label studies. We completed a double-blind, placebo-controlled trial on valproate, olanzapine, or l-carnitine/coenzyme Q10 treatment for cocaine dependence (see Reid et al., 2005). Though no statistically significant evidence of treatment efficacy was found for any of the test medications, abstinence rates at the last study visit were nearly 4 times higher in the valproate (27%) versus placebo (7%) group. This study was underpowered, however, leaving the utility of valproate in cocaine dependence treatment in need of further investigation and prompting the current clinical laboratory study. In the current study valproate treatment was associated with an increase in cocaine craving, both baseline and cue-induced, in cocaine dependent participants. This does not indicate that valproate is an effective treatment for cocaine dependence.
Previous studies on the effects of cocaine cue exposure in cocaine dependent individuals have demonstrated a wide range of subjective effects including increased cocaine craving, cocaine-like high, cocaine withdrawal, anxiety and negative mood (Erhman et al., 1992; Robbins and Ehrman, 1992; Berger et al., 1996; Reid et al., 1998, 1999; Robbins et al., 1999, Sinha et al., 2000). The present study was consistent with these prior studies in that participants reported greater cocaine craving, “desire to use cocaine now”, cocaine-like high and cocaine withdrawal specific to cocaine cue exposure. However, there was no increase in anxiety or negative mood associated with cocaine cue exposure as others have reported, which could indicate a relative weakness in the subjective response profile tested in our clinical laboratory paradigm.
A significant effect of medication treatment on cue-induced cocaine craving was found; participants experienced a far greater increase in “desire to use cocaine now” during the valproate treatment condition. Consistent with this change in cue reactivity, participants also reported slightly higher levels of cocaine craving during the last day of valproate treatment. This was contrary to our hypothesis as well as prior reports of reduced drug craving in cocaine dependent patients treated with valproate (Myrick et al., 2001) or other GABAergic anticonvulsants such as gamma-vinyl GABA (Brodie et al., 2003, 2005). This discrepancy may be explained by differences in study design, in which the open label studies lacked a control group and measured craving retrospectively from patients while in treatment for drug abuse. It may also be argued that cocaine cue reactivity testing and clinical treatment trials do not measure equivalent forms of drug craving. Clinical laboratory studies on cocaine craving with similar GABAergic and anticonvulsant compounds have provided mixed results. Baclofen treatment (10–20 mg BID, 7 days) reduced (Brebner et al., 2002), while carbamazepine treatment (100–150 mg TID, 7 days) had no effect (Hersh et al., 1995), on cue-induced cocaine craving. Our results are more consistent with the effects of carbamazepine.
Clinical laboratory tests on the interaction of acute stimulants with anticonvulsant and GABAergic compounds may provide further insight into the effects of this class of medications on cocaine craving and related subjective measures. For example, topiramate pretreatment enhanced intravenous methamphetamine-induced (15mg and 30 mg) ratings of euphoria and stimulation (Johnson et al. 2007). Studies on nicotine found similar results, in which topiramate enhanced the subjective strength and rewarding effects of intravenous nicotine (0.5 mg/kg and 1.0 mg/kg) (Sofuoglu et al. 2006) and of smoked cigarettes (Reid et al., 2007). In cocaine dependent individuals, gabapentin pretreatment resulted in an increase in self-administration of low doses of smoked cocaine (12 and 25 mg) as well as an increase in cocaine-induced craving and high during the initial testing period (Hart et al., 2004), baclofen pretreatment had no effects on subjective and physiological responding to intranasal cocaine (45 mg) (Lile et al., 2004), and tiagabine pretreatment attenuated intravenous cocaine-induced (20–30 mg) ratings of stimulation and cocaine craving (Sofuoglu et al., 2005). While none of these studies tested valproate directly, the findings with topiramate and gabapentin suggest that this class of anticonvulsant medications can prime stimulant-induced drug craving. It is possible that valproate similarly primes cue-induced cocaine craving.
Previous studies on the effects of cocaine cue exposure in cocaine dependent individuals have demonstrated a wide range of physiological effects, including increased heart rate, skin conductance, and plasma cortisol and ACTH levels accompanied by a decrease in skin temperature (Erhman et al., 1992; Robbins and Ehrman, 1992; Berger et al., 1996; Reid et al., 1998, 1999, 2008; Robbins et al., 1999, Sinha et al., 2000). In the present study we did not find a significant effect of cocaine cue exposure on any of the physiological measures collected. This indicates a weakness in cue elicited physiological responding in our clinical laboratory paradigm. However, valproate treatment produced an overall increase in plasma cortisol levels, as well as a decrease in heart rate and diastolic blood pressure, which may have masked the ability of cocaine cues to affect these measures. The decrease in cardiovascular activity is consistent with previous studies on valpoate and related GABA transaminase inhibitors in animals (Tanaka et al., 1992; Loscher, 1982) and humans (Chiodera et al., 1989; Isojarvi et al., 1998; Chong et al., 2001). The increase in cortisol levels is also consistent with prior studies on chronic valproate treatment in epileptic patients (Aydin et al., 2005; Vlasov et al., 2001). Previous studies have shown that cortisol is critical to the reinstatement of drug and alcohol seeking behavior (see Weiss et al., 2001), and that elevated cortisol levels may produce changes in brain reward circuits resulting in greater sensitivity to the reinforcing properties of drugs of abuse (Piazza and LeMoal, 1998). It is possible that the increase in cortisol levels associated with valproate treatment contributed to enhanced cue-induced desire for cocaine in a similar manner.
Weaknesses in the current study must be acknowledged. Though cue reactivity was consistent across two testing sessions and no order effects with cue type and valproate treatment were found, the cocaine cue exposure response in this study was relatively weak. This shortcoming limited our ability to interpret the effects of valproate on the physiological response to cocaine cue exposure. The study population was limited to crack cocaine abusers whose preferred route of administration was matched with the cocaine cues employed, and there were few females included, which limits the generalizability of the findings. This was not a treatment study, and as such an interest in quitting was not a requirement for study participation. Results from the 7 patients that had not undergone prior cocaine abuse treatment could potentially be qualitatively different from the 13 that had (see Perkins et al., 2006). Despite these shortcomings the current study found clear evidence that valproate treatment produced an increase in cue-induced cocaine craving. This was further substantiated by the elevated levels of spontaneous cocaine craving reported by patients over the 24 hr period preceding cue tests. Overall, these findings do not support further investigations of valproate as a treatment for cocaine dependence.
Acknowledgments
Funding Source
This study was supported by NIDA grants DA12277 and DA017556 (M Reid). NIDA had no further role on this study in terms of study design, implementation, data analysis and manuscript preparation.
We wish to thank Tom Cooper, Deborah Guzman, Sumithra Raghavan, Pooja Paunikar, and Erin Weinstein for their support in this study.
Footnotes
Contributors:
Study design, implementation, data analysis and manuscript preparation were contributed by Malcolm Reid. Study implementation, patient care, and manuscript preparation were contributed by Vatsal Thakkar.
Conflict of Interest
None of the authors have any financial interest with the study sponsor or the study medication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aydin K, Seraroglu A, Okuyaz C, Bideci A, Gucuyener K. Serum insulin, leptin, and neuropeptide levels in epileptic children treated with valproate. J Child Neurol. 2005;20:848–851. doi: 10.1177/08830738050200101501. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Psychological Corporation. Harcourt Brace & Company; San Antonio: 1978. Beck Depression Inventory. [Google Scholar]
- Beck AT. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall SM, Mickalian JD, Reid MS, Crawford CA, Delucchi KL, Carr K, Hall S. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ration 1 and progressive-ratio schedules. Psychopharmacology. 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse. 2003;50:261–265. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–125. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Implications of recent research for program quality in cocaine dependence treatment. Subst Use Misuse. 2000;35:2011–2030. doi: 10.3109/10826080009148248. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Recent advances in the psychotherapy of addictive disorders. Curr Psychiatry Res. 2005;7:329–336. doi: 10.1007/s11920-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Chiodera P, Gnudi A, Volpi R, Marchesi C, Marchesi M, Davoli D, Capretti L, Coiro V. Effects of the GABAergic agent sodium valproate on the arginine vasopressin in responses to hypertonic stimulation and upright posture in man. Clin Endocrinol. 1989;30:389–395. doi: 10.1111/j.1365-2265.1989.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Chong SA, Mythily M, Mahendran R. Cardiac effects of psychotropic drugs. Ann Acad Med Singapore. 2001;30:625–631. [PubMed] [Google Scholar]
- Derlet RW, Albertson TE. Anitconvulsant modification of cocaine-induced toxicity in the rat. Neuropharmacology. 1990;29:255–259. doi: 10.1016/0028-3908(90)90010-o. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW, Childress AR, O’Brien CP. Failure of ritanserin to block cocaine cue reactivity in humans. Drug Alcohol Depend. 1996;42:167–174. doi: 10.1016/s0376-8716(96)01278-1. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Schiffer WK, Brodie JD, Lennon IC, Taylor SJ, Dewey SL. Gama-aminobutyric acid mimetic druges differentially inhibit the dopaminergic response to cocaine. European Journal of Pharmacology. 2000;395:129–135. doi: 10.1016/s0014-2999(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, Kosten TR. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, George TP, Kosten TR. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–1632. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: Randomized, double-blind trial. J Clin Psychopharmacology. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Center BA, Pearson VL, Carlson GA, Crea F. A pilot, open clinical study of depakote in the treatment of cocaine abuse. Human Psyhoparmacol Clin Exp. 2001;16:257–264. doi: 10.1002/hup.252. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Jensen M, Johnson M, White HS. Distinct features of seizures induced by cocaine and amphetamine analogs. European Journal of Psychopharmacology. 1999;377:167–173. doi: 10.1016/s0014-2999(99)00419-7. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug and Alcohol Dependence. 2004;73:279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Hersh D, Bauer LO, Kranzler HR. Carbamazepine and cocaine-cue reactivity. Drug Alcohol Depend. 1995;39:213–221. doi: 10.1016/0376-8716(95)01165-3. [DOI] [PubMed] [Google Scholar]
- Isojarvi JI, Ansakorpi H, Suominen K, Tolonen U, Repo M, Myllyla VV. Interictal cardiovascular autonomic responses in patients with epilepsy. Epilepsia. 1998;39:420–426. doi: 10.1111/j.1528-1157.1998.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang XQ. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. International J of Neuropsychopharmacology. 2007;10:85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- Jung BJ, Dawson R, Jr, Sealy SA, Peris J. Endogeneous GABA release is reduced in the striatum of cocaine-sensitized rats. Synapse. 1999;34:103–110. doi: 10.1002/(SICI)1098-2396(199911)34:2<103::AID-SYN3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the cocaine selective severity assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Bauer LO. Bromocriptine and cocaine cue reactivity in cocaine-dependent patients. Brit J Addict. 1992;87:1537–48. doi: 10.1111/j.1360-0443.1992.tb02661.x. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PE, Hays LR, Rush CR. Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology. 2004;171:441–449. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- Loscher W. Cardiovascular effects of GABA, GABA-aminotransferase inhibitors and valproic acid following systemic administration in rats, cats and dogs: pharmacological approach to localize the site of action. Arch Int Pharmacodyn Ther. 1982;257:32–58. [PubMed] [Google Scholar]
- Loscher W. In vivo administration of valproate reduces the nerve terminal (synaptosomal) activity of GABA aminotransferase in discrete brain areas of rats. Neuroscience Letters. 1993;160:177–180. doi: 10.1016/0304-3940(93)90407-c. [DOI] [PubMed] [Google Scholar]
- Mezinskis J, Dyrenforth S, Goldsmithm RJ, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatric Annals. 1998;28:577–583. [Google Scholar]
- Myrick H, Brady K. Divalproex treatment of cocaine dependence. Psychopharmacology Bulletin. 2003;37(Suppl 2):89–97. [PubMed] [Google Scholar]
- Myrick H, Henderson S, Brady KT, Malcom R, Measom M. Divalproex loading in the treatment of cocaine dependence. J Psychoactive Drugs. 2001;33:283–287. doi: 10.1080/02791072.2001.10400575. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behav. 1990;15:55–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Piazza PV, LeMoal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–51. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Phillips NI, Fowler LJ. The effects of sodium valproate on gamma-aminobutyrate metabolism and behavior in naïve and ehtanolamine-O-sulphate pretreated rats and mice. Biochemical Pharmacology. 1982;31:2257–2261. doi: 10.1016/0006-2952(82)90111-3. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychophamrcology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug and Alcohol Dependence. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Casadonte P, Montgomery A, Majewska D, Sanfilipo M, Baker S, Braunstein D, Robinson J, Rotrosen J. A controlled trial of olanzapine, valproate or co-enzyme Q10/L-carnitine versus placebo for the treatment of cocaine dependence. Addiction. 2005;100(Suppl 1):43–57. doi: 10.1111/j.1360-0443.2005.00990.x. [DOI] [PubMed] [Google Scholar]
- Reid MS, Angrist B, Montgomery A, Majewska D, Baker S, O’Leary S, Stone J, Robinson J, Rotrosen J. A clinical trial of mecamylamine versus placebo for the treatment of cocaine dependence. Substance Abuse. 2006;26:5–14. doi: 10.1300/j465v26n02_02. [DOI] [PubMed] [Google Scholar]
- Reid MS, Palamar J, Rhagavan S, Flammino F. Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology. 2007;192:147–158. doi: 10.1007/s00213-007-0755-6. [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Howard B, Nilsen D, Prichep L. Cocaine cue versus cocaine dosing in humans: Evidence for distinct neurophysiological response profiles. Pharmacology Biochemistry and Behavior. 2008;9:155–164. doi: 10.1016/j.pbb.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Designing studies of drug conditioning in humans. Psychopharmacology. 1992;106:143–153. doi: 10.1007/BF02801965. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Using cue reactivity to screen medications for cocaine abuse: a test of amantadine hydrochloride. Addict Behav. 1992;17:491–499. doi: 10.1016/0306-4603(92)90009-k. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Dependency. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Douaihy A, Cornelius JR, Kirisci L, Kelly TM, Hayes J. Divalproex utility in bipolar disorder with co-occurring cocaine dependence: a pilot study. Addict Behav. 2007;32:410–415. doi: 10.1016/j.addbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Satel SL, Krystal JH, Delgado PL, Kosten TR, Charney DS. Tryptophan depletion and attenuation of cue-induced craving for cocaine. Am J Psychiatry. 1995;152:778–83. doi: 10.1176/ajp.152.5.778. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charvuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Renee-Aubin L, O’Malley SS. Psychological stress, drug cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mitchell E, Kosten TR. Tiagabine affects subjective responses to cocaine in humans. Pharmacol Biochem Behav. 2005;82:569–573. doi: 10.1016/j.pbb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology. 2006;184:645–651. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Abe S, Yamaguchi M, Baba A, Hori T, Shiraishi H, Ito T. Effects of cocaine dministration on receptor binding and subunits mRNA of GABA(A)-benzodiazepine receptor complexes. Synapse. 2000;38:198–215. doi: 10.1002/1098-2396(200011)38:2<198::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fujita T, Tanaka S, Takano K, Yonemasu Y. Effect of anticonvulsants upon experimental limbic seizure status and regional blood flow in the hippocampus. No To Shinkei. 1992;44:234–240. [PubMed] [Google Scholar]
- Vlasov PN, Karlov VA, Kushlinski NE. The pharmacological and hormonal effects of carbamazepine and valproic acid in the treatment of reproductive age women with epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova. 2001;101:26–30. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Gately SJ, Dewey SS, Pappas N. Enhanced sensitivity to benzodiazapines in active cocaine-abusing subjects: a PET study. American Journal of Psychiatry. 1998;155:200–6. doi: 10.1176/ajp.155.2.200. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: Successes and challenges. Pharmacology and Therapeutics. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Personality and Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, Coleman FS, Mindrum G, Kahn R, Osman S, Mezinskis J, Li SH, Lewis D, Horn P, Montgomery MA, Elkashef A. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Dependency. 2007;91:141–148. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, Berger P, Elkashef A. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- Yang P, Beasley A, Swann A, Dafny N. Valproate modulates the expression of methylphenidate (Ritalin) sensitization. Brain Res. 2000;874:216–220. doi: 10.1016/s0006-8993(00)02500-2. [DOI] [PubMed] [Google Scholar]
- Zullino DF, Khazaal Y, Hattenschwiler J, Borgeat F, Besson J. Anticonvulsant drugs in the treatment of substance withdrawal. Drugs Today (Barc) 2004;40:603–19. doi: 10.1358/dot.2004.40.7.850478. [DOI] [PubMed] [Google Scholar]