Abstract
Amphibian metamorphosis is accompanied by extensive intestinal remodeling. This process, mediated by thyroid hormone (TH) and its nuclear receptors, affects every cell type. Gut remodeling in Xenopus laevis involves epithelial and mesenchymal proliferation, smooth muscle thickening, neuronal aggregation, formation of intestinal folds, and shortening of its length by 75%. Transgenic tadpoles expressing a dominant negative TH receptor (TRDN) controlled by epilthelial-, fibroblast-, and muscle-specific gene promoters were studied. TRDN expression in the epithelium caused abnormal development of virtually all cell types, with froglet guts displaying reduced intestinal folds, thin muscle and mesenchyme, absence of neurons, and reduced cell proliferation. TRDN expression in fibroblasts caused abnormal epithelia and mesenchyme development, and expression in muscle produced fewer enteric neurons and a reduced inter-muscular space. Gut shortening was inhibited only when TRDN was expressed in fibroblasts. Gut remodeling results from both cell-autonomous and cell-cell interactions.
Keywords: Metamorphosis, remodeling, intestine, thyroid hormone, transgenesis, thyroid hormone receptors, cell-cell interaction
INTRODUCTION
The metamorphosis of a tadpole into a frog is mediated entirely by thyroid hormone (TH) (Dodd and Dodd, 1976) and its receptors that function as nuclear transcription factors (Schreiber et al., 2001). The transformation of the herbivorous tadpole into a carnivorous frog is accompanied by the remodeling of virtually every cell type that composes the intestinal tract. The morphological changes have been described in detail for Xenopus laevis (Ishizuya-Oka and Shi, 2007; Schreiber et al., 2005). Remodeling occurs over the eight day period referred to as the climax of metamorphosis when endogenous TH concentration is highest. The tadpole gut is a simple tube lined with a single cell-thick epithelium (Fig 1A, B). There are few if any glands and only one involution in the duodenum of the small intestine called the typhlosole (Marshall and Dixon, 1978). Most of the mesenchymal cells (fibroblasts) are located under this fold. The outer longitudinal and inner circular muscle layers are one cell thick with no obvious space between them. A few single enteric neurons are present between the muscle layers. Increased DNA replication, especially in epithelial cells, initiates the TH-induced changes of metamorphic climax. Within just a few days the intestine begins to shorten so that by the end of climax, when the froglet begins to feed again, it is only 25% of its original length. The circular and longitudinal muscle fibers thicken during climax and are separated by a larger space containing mesenchyme and enteric neurons (Fig 1E). In addition, fibroblasts are more abundant between the epithelium and muscle. The tadpole single cell epithelium becomes temporarily heaped into many layers by the shortening of the intestine and constriction of intestinal diameter (Schreiber et al., 2005). By the end of climax, the intestine is configured once again as a single cell-thick epithelium, but it is now highly folded into ridges and troughs that more closely resemble the anatomy of a typical adult vertebrate intestine (Fig 1G).
Fig 1.
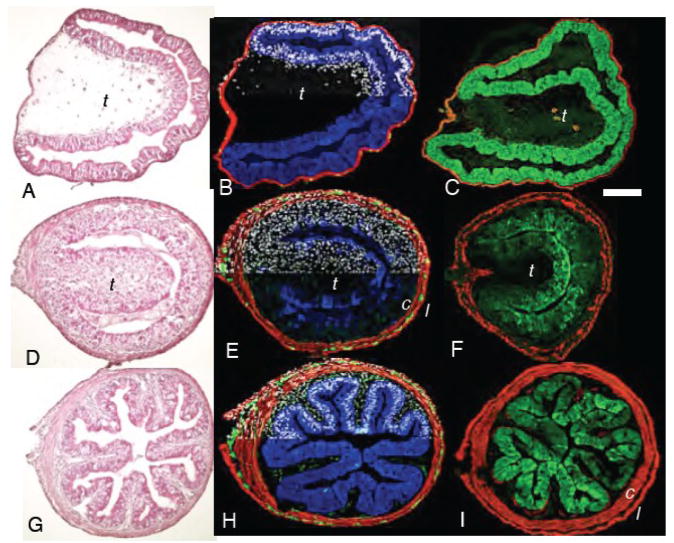
Virtually every tissue is affected during spontaneous metamorphic remodeling of the duodenum. Cross-sections of the duodenum from A–C) wild-type prometamorphic tadpoles NF57; D–F), metamorphic climax NF61; G–I), and the end of metamorphosis NF66. C,F, and I) Cross-sections of the duodenum from tadpoles transgenic for IFABP-GFP. The GFP antibody reaction is green; smooth muscle actin antibody is red. A,D, and G) hematoxylin and eosin. B,E and H) immunoreactivity against endogenous intestinal fatty acid binding protein (IFABP; blue), muscle-specific smooth muscle actin (red), enteric neuron-specific neural beta-tubulin (green); and a nuclear counter-stain (dapi; white) is shown for half of each section. t=typhlosole, c=circular muscle, l=longitudinal muscle. Scale bar in C denotes 0.2 mm length.
The cellular mechanisms responsible for this remodeling have been studied extensively, and tissue-tissue interactions are thought to play important roles in intestinal morphogenesis during embryogenesis (Chalmers and Slack, 1998) and at metamorphosis (Dauca et al., 1990; Hourdry and Dauca, 1977). In particular, in in vitro experiments the mesenchyme influences the transition from a larval to an adult epithelium (Ishizuya-Oka and Shimozawa, 1992). Epithelial cell death and proliferation increase transiently during metamorphic climax as part of the remodeling, but it is disputed whether the larval epithelium as a whole (Schreiber et al., 2005) or a subpopulation of adult stem cells (Ishizuya-Oka and Shi, 2005) are the progenitors of the adult epithelium. It has been suggested that matrix metalloproteinase 11 (stromelysin-3), a direct response gene of TH that is up-regulated in mesenchymal fibroblasts at metamorphic climax, modifies the basal lamina and facilitates larval epithelial apoptosis (Fu et al., 2005; Ishizuya-Oka et al., 2000; Patterton et al., 1995). Expression of sonic hedgehog in the epithelium is proposed to induce adult epithelial cell differentiation by activating BMP-4 in fibroblasts underlying the adult epithelial precursors (Ishizuya-Oka et al., 2006). Sonic hedgehog expression has also been shown to correlate with epithelial proliferation (Ishizuya-Oka et al., 2001).
By preparing transgenic X. laevis in which a variety of cell-specific promoters regulate the expression of a dominant negative form of the thyroid hormone receptor fused to GFP (TRDN-GFP) we have already demonstrated that tail resorption (Das et al., 2002), limb development (Brown et al., 2005) and remodeling of the larval skin (Schreiber and Brown, 2003) consist of multiple cell autonomous TH-controlled programs. An example of cell-cell interaction in metamorphosis is the control of β-cell aggregation in the pancreas by the remodeling exocrine cells (Mukhi et al., 2009) In this paper we apply this strategy to various cell types of the intestine to present clear evidence of the importance of both cell autonomous and cell-cell interaction in the remodeling of the intestine.
METHODS
Transgenesis, plasmids, and thyroid hormone treatment
Transgenic X. laevis were prepared by the sperm-mediated method that employs the restriction enzyme NotI (Amaya and Kroll, 1999; Kroll and Amaya, 1996). Five different promoters were used to control the expression of a dominant negative thyroid hormone receptor transgene fused to GFP (TRDN-GFP). The TRDN is a deletion of 12 amino acids from the carboxy terminus of X. laevis thyroid hormone receptor α (Marsh-Armstrong et al., 1999). The mutated receptor cannot bind TH and it lacks a coactivator binding site.
The constitutively-expressed cytomegalovirus (CMV) promoter (Turner and Weintraub, 1994) and mouse minimal collagen (Col) promoter (Bou-Gharios et al., 1996) in the plasmid pCS2+ construct with GFP fused to the N terminus of the TRDN have been described (Marsh-Armstrong et al., 2004; Schreiber et al., 2001). Experiments using the CMV promoter for transgenesis were carried out on F0 animals. The promoter is very strong and ubiquitous in embryos and early tadpoles, but weakens significantly by the start of metamorphic climax. The rat intestinal fatty acid binding protein (IFABP) promoter (a gift from Professor J.M.W. Slack), which has been previously shown to drive GFP expression specifically in epithelial cells of the intestine of X. laevis (Beck and Slack, 1999), was cloned into the pCS2+ plasmid driving either GFP alone or the TRDN-GFP construct. The X. laevis stromelysin-3 (ST3) promoter was cloned from X. laevis genomic DNA using the primers 5′-CGATGCGTCGACGCTTAAAGTTATTAGTGATCCAGG-3′ (forward) and 5′-GCGCGAATTCCTCACAAGCTGTAGGGTGAGTG-3′ (reverse) based on GenBank accession number AF019253.1(Li et al., 1998), and cloned into pCS2+ driving either GFP alone or the TRDN-GFP construct. Experiments using IFABP and ST3 promoters for transgenesis were carried out on F0 animals.
The X. laevis “cardiac actin” (pCar) promoter was placed under control of the tetracycline-inducible system (Urlinger et al., 2000). A description of the plasmid has been reported (Das and Brown, 2004). After 1 week of embryogenesis, 20 feeding F1 tadpoles were raised in 4 liters of 0.1 MMR (10 mM NaCl/0.2 mM KCl/0.1 mM MgCl2/0.2 mM CaCl2/0.5 mM Hepes, pH 7.5). The transgene was induced with 50 μg/ml doxycycline (Sigma) added to the rearing water. The medium was changed twice a week. The cell expression pattern of each transgenic experiment was verified by fluorescence and also by immunostaining of gut sections with an antibody to GFP (Torrey Pines Biolabs, San Diego) (Schreiber and Brown, 2003).
We have verified that IFABP, CMV, pCar, and Col promoters express GFP and TRDN-GFP in the same tissue-specific manner. Though we show that the ST3 promoter drives GFP specifically in the mesenchyme, the specificity of ST3-TRDN-GFP has not been verified.
Tadpoles were either allowed to undergo spontaneous metamorphosis, or premetamorphic tadpoles (NF48 to 54) were induced to metamorphose by treating their water with 5 or 10nM 3,5,3′triiodothyronine (T3) for 1–7 days.
BrdU labeling, immunohistochemistry, and in situ hybridization
To identify proliferating cells, live tadpoles (NF54) were injected i.p. with 10 μl of BrdU (10 mM) 12 h before fixation with 2% trichloroacetic acid for 2 h at room temperature, embedded in OCT compound, sectioned at 10 μm thickness with a cryostat, and labeled with Alexa Fluor 488-conjugated-anti-BrdU (Molecular Probes) using previously described methods (Schreiber et al., 2005). For immunohistochemisry and in situ hybridization tadpoles at different stages were fixed in 4% paraformaldehyde, embedded in OCT compound, and cryosectioned as previously described (Berry et al., 1998a; Berry et al., 1998b). Sections were processed for in situ hybridization by using digoxigenin-labeled antisense probes against the thyroid hormone up-regulated mRNAs bZip (U41859), sonic hedgehog (U26314), and stromelysin-3 (Z27093) (Berry et al. 1998a and b). Sections were also processed immunohistochemically with monoclonal antibodies against smooth muscle actin (Sigma) diluted 1/400, epithelial cadherin (product number 5D3, University of Iowa Developmental Studies Hybridoma Bank, Iowa City) diluted 1/1000, β-tubulin (Sigma) diluted 1/1000, and polyclonal antibodies against IFABP (a gift of Yun-Bo Shi, National Institute of Child Health and Human Development, Bethesda) diluted 1/1000 and GFP (Torrey Pines Biolabs, San Diego) diluted 1/1000. Primary antibodies were detected by using Alexa Fluor 488- and 568-conjugated secondary antibodies diluted 1/400 (Molecular Probes). Intestines of some NF57 tadpoles were processed in whole-mount by immunohistochemistry as described (Schreiber and Brown, 2003; Schreiber et al., 2005). Two regions of the intestine were sectioned: 1) the most anterior portion of the duodenum (approximately within the first 10 mm posterior to the junction of the bile duct and the small intestine), and 2) the posterior portion of the ileum (within the first 1 cm anterior to the ‘switchback’ point) (Schreiber et al., 2005).
Statistics
To measure intestine shortening in stage NF48 tadpoles after exogenous T3 treatment, the entire gastrointestinal tracts were excised, and lengths were measured from the stomach to the rectum. Differences in mean length within a group were assessed statistically by one-factor ANOVA (SuperANOVA, Abacus Concepts, Berkeley, CA), followed by Fisher’s pairwise comparisons. P values of ≤0.05 were considered significant.
RESULTS
Remodeling of the intestine during metamorphosis
A cross-section of an NF57 tadpole duodenum reveals several one cell-thick layers identifiable from the lumen outwards as the epithelium, sub-epithelial fibroblasts (mesenchyme), circular and longitudinal muscle fibers with an occasional enteric neuron located between the muscle fiber layers (Fig 1A, B). The typhlosole, a single large involution that is located in the duodenum and the anterior part of the small intestine, contains the largest source of fibroblasts loosely dispersed in an extensive extracellular matrix between the epithelium and muscle layers. During metamorphic climax the circular muscle layer begins to thicken (Fig 1E,F) and separates from the longitudinal layer by a widening space that becomes occupied by fibroblasts, extracellular matrix, and increasing numbers of enteric neurons (Fig 1E). These neurons aggregate into clusters within the inter-muscular space. The epithelium and sub-epithelial mesenchyme thicken. The typhlosole becomes smaller and its fibroblast density becomes very high as it is resorbed and replaced by multiple involutions of the epithelium and the mesenchyme. By the end of metamorphosis (NF66), the epithelium and sub-epithelial mesenchyme have folded into multiple ridges that weeks later will form the crypts and villi typical of an adult vertebrate intestine. The enteric neurons project into the newly formed intestinal folds (Fig. 1H).
During premetamorphosis DNA replication is low in the epithelium and the mesenchyme. At the onset of climax it becomes extremely active in both cell layers. BrdU incorporation remains high and uniform throughout the epithelium even after the intestinal folds have formed. It is not until about 2 weeks after metamorphosis that replication becomes restricted to the base of the intestinal folds (Schreiber et al., 2005)
Among the specialized genes that are expressed exclusively by the tadpole epithelium before and after metamorphosis is the intestinal fatty acid binding protein (IFABP) (Fig 1B and H). The expression of endogenous IFABP mRNA is extinguished completely at climax and then expressed again at the end of metamorphosis (NF66) (Ishizuya-Oka et al., 1997; Schreiber et al., 2005; Shi and Hayes, 1994). The IFABP protein drops at climax (NF62) (Fig. 1E) and is re-synthesized by the end of climax (Fig 1H). Tadpoles transgenic for IFABP-GFP express GFP exclusively in the epithelial layer of the entire small intestine (Fig 1C, F and I), including the ileum but not in the stomach or colon (data not shown). Like endogenous IFABP, protein expression of the GFP transgene is reduced by NF62 climax (Fig 1F), and is re-synthesized at NF66 (Fig. 1I).
The changes in the cross section of the intestine that occur during spontaneous metamorphosis are accompanied by a 75% shortening of gut length (Schreiber et al., 2005; Schreiber et al., 2001). The intestines of 2 week-old tadpoles following 7 days of treatment with exogenous T3 (10 nM) undergo changes similar to those of spontaneously metamorphosing tadpoles, but their guts shorten in length by only 36% (Table 1). Their sub-epithelial mesenchyme thickens dramatically and their intestinal cross-section constricts (Schreiber et al., 2005; Schreiber et al., 2001). Tadpoles transgenic for CMV-TRDN-GFP express the transgene in all cells of the intestine and both the shortening of the intestine and the cross-sectional changes are inhibited in these tadpoles (Table 1; (Schreiber et al., 2001)).
Table 1.
Gastrointestinal lengths of NF48 tadpoles after 7 days of 10 nMT3. Wild type (WT) are compared with transgenic animals having 4 different promoters driving TRDN-GFP.
| Experiment | Wild-type, or Promoter driving TRDN-GFP | Treatment Duration (days) | T3 | Gut length, mm, (±standard deviation) | n |
|---|---|---|---|---|---|
| I. | WT | 0 | − | 8.4 (0.5) a | 9 |
| WT | 2 | + | 5.4 (0.6) b | 9 | |
| WT | 3 | + | 4.8 (0.4) b | 9 | |
| WT | 4 | + | 4.3 (0.3) bc | 10 | |
| WT | 5 | + | 4.1 (0.5) c | 9 | |
| WT | 7 | + | 3.0 (0.4) d | 8 | |
| WT | 7 | − | 8.6 (0.7) a | 9 | |
| II. | CMV | 0 | − | 8.2 (1.0) a | 3 |
| CMV | 6 | + | 6.2 (0.6) b | 10 | |
| WT | 6 | + | 3.8 (0.5) c | 10 | |
| WT | 6 | − | 7.8 (1.0) a | 10 | |
| III. | Collagen | 0 | − | 8.0 (1.1) a | 3 |
| Collagen | 4 | + | 5.8 (0.9) b | 7 | |
| Collagen | 7 | + | 5.0 (0) bc | 2 | |
| WT | 4 | + | 4.6 (0.4) c | 6 | |
| WT | 7 | + | 3.3 (0.4) d | 6 | |
| WT | 7 | − | 8.3 (1.3) a | 7 | |
| IV. | pCar | 0 | − | 8.5 (1.3) a | 4 |
| pCar | 6 | + | 4.4 (0.7) b | 6 | |
| WT | 6 | + | 4.8 (0.6) b | 10 | |
| WT | 6 | − | 9.2 (0.6) a | 10 | |
| V. | IFABP | 0 | − | 7.9 (0.8) a | 5 |
| IFABP | 6 | + | 4.4 (0.03) b | 6 | |
| WT | 6 | + | 4.3 (0.3) b | 10 | |
| WT | 6 | − | 8.1 (0.9) a | 10 |
Mean gut lengths with different superscripted letters are significantly different from each other (p < 0.05) within the same experiment. WT denotes non-transgenic tadpoles.
Influence of the epithelium on intestinal remodeling
Even though its expression is restricted to the epithelium, the IFABP-TRDN-GFP transgene blocks TH-induced thickening of the mesenchyme in both the duodenum (not shown) and ileum (Figs 2, 5, 6). Constriction of the ileum that is normally seen after several days of hormone exposure in control pre-metamorphic tadpoles is also inhibited in transgenic animals (Fig 2C). Though expression is epithelial specific, the IFABP-TRDN-GFP transgene inhibits epithelial and sub-epithelial cell proliferation in both the duodenum (Fig 3) and the ileum (not shown). Compared with wild-type siblings, the IFABP-TRDN-GFP transgene also reduces the separation of the circular from longitudinal muscle fibers and formation of an intramuscular mesenchymal space in premetamorphic tadpoles induced to metamorphose for 5 days (data not shown).
Fig 2.
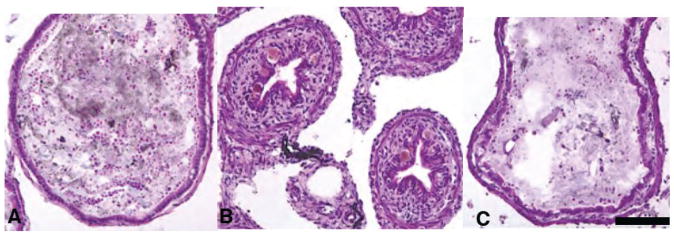
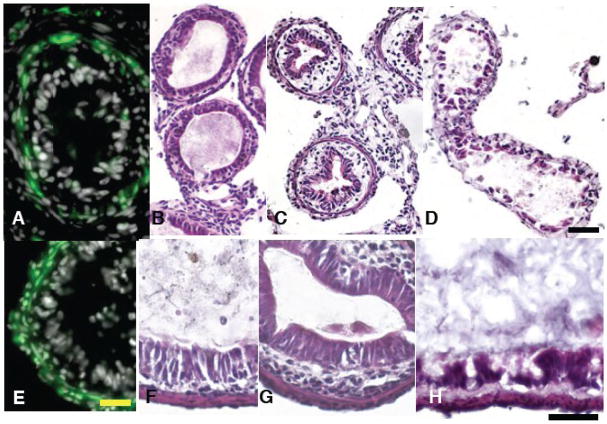
The IFABP-TRDN-GFP transgene inhibits thickening of the intestinal sub-epithelial mesenchyme and constriction of the ileum’s diameter in tadpoles precociously induced to metamorphose with exogenous thyroid hormone treatment. Cross sections of intestines from wild-type NF54 tadpoles treated for 5d with either A) 0 nM T3, or B) 5 nM T3. Cross sections of intestines from siblings transgenic for C) IFABP-TRDN-GFP treated with 5 nM T3 (5d) (C). Tissues are stained with hematoxylin and eosin. Scale bar in C denotes 0.2 mm length.
Fig 5.
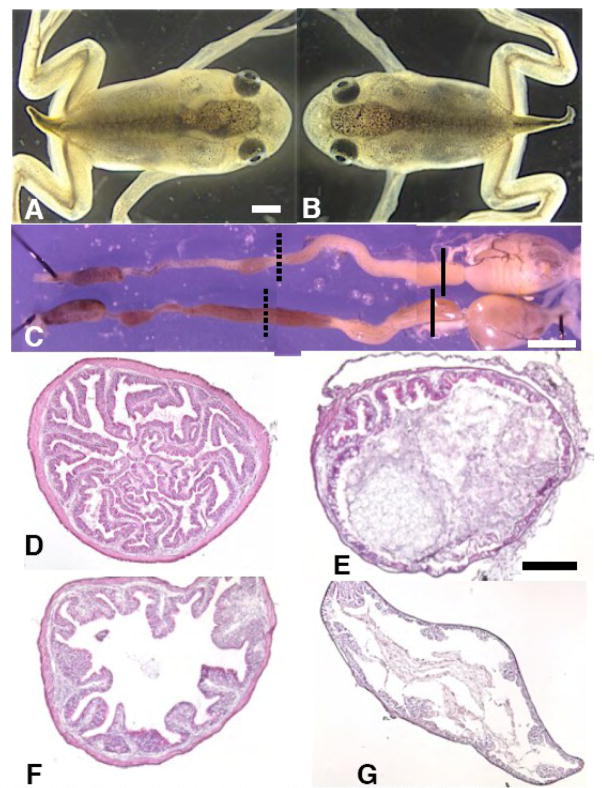
The IFABP-TRDN-GFP transgene dramatically inhibits remodeling of multiple intestine cell types during spontaneous metamorphosis. A) Wild-type NF65 froglet; B), a sibling transgenic for IFABP-TRDN-GFP; C) entire gastro-intestinal tracts from the froglets pictured in A and B removed two weeks after the onset of feeding and completion of metamorphosis (NF66+); wild-type (upper), transgenic (lower); D,E) cross-sections from the duodenums and F,G) from the illeums of the D,F) wild-type and E,G) transgenic froglet guts in C. Solid black lines in C denote cross-section regions of the duodenums shown in D and E, and dashed lines denote cross-section regions of the ileums shown in F and G. Tissues are stained with hematoxylin and eosin. Scale bar in A denotes 1.0 mm length; scale bar in C denotes 3.0 mm length. Scale bar in E denotes 0.2 mm length.
Fig 6.
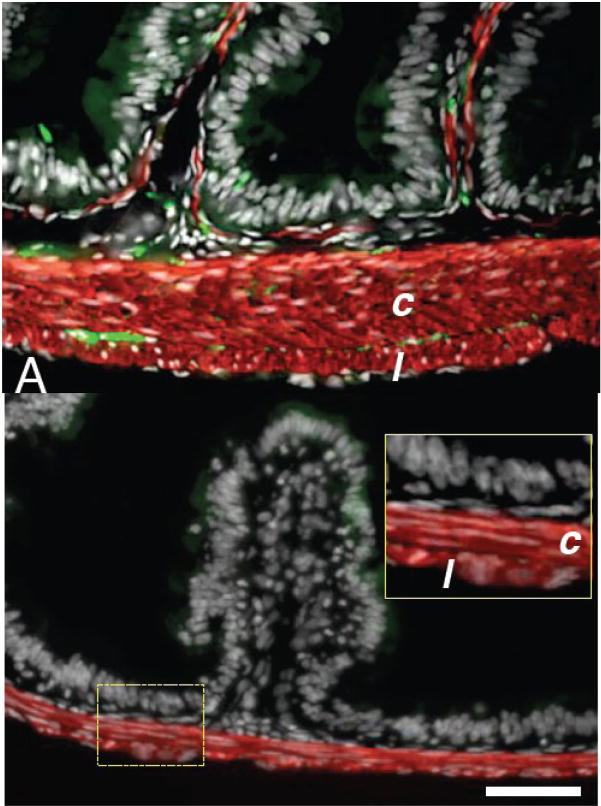
The IFABP-TRDN-GFP transgene inhibits circular muscle thickening and aggregation of enteric neurons. A) Duodenal cross-sections from a wild-type NF66+ froglet; B), from a transgenic froglet. Tissues were stained for immunoreactivity against smooth muscle actin (red) and neural beta-tubulin (green). Sections are counterstained for nuclei with dapi (white). Circular muscle fibers (c); longitudinal muscle fibers (l). Inset in B is a magnification of the region encompassed by the dashed yellow rectangle. Scale bar denotes 0.1 mm length.
Fig 3.
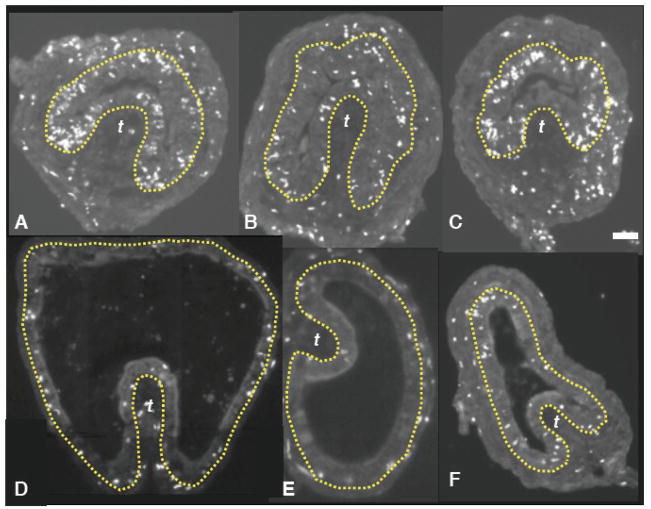
The IFABP-TRDN-GFP transgene inhibits cell proliferation in both the epithelium and mesenchme of the intestine. Cross sections of duodenums from NF 54 tadpoles after 72 hours of treatment with T3 (5 nM) and following a 12 hour pulse of BrdU injected interperitoneally. Cross-sections from A–C) three different wildtype tadpoles and from D–F) three tadpoles transgenic for IFABP-TRDN-GFP. BrdU immunoreactivity (white nuclear label). The epithelial-mesenchyme boundary is depicted by the yellow lines. Scale bar in C denotes 40 μm length.
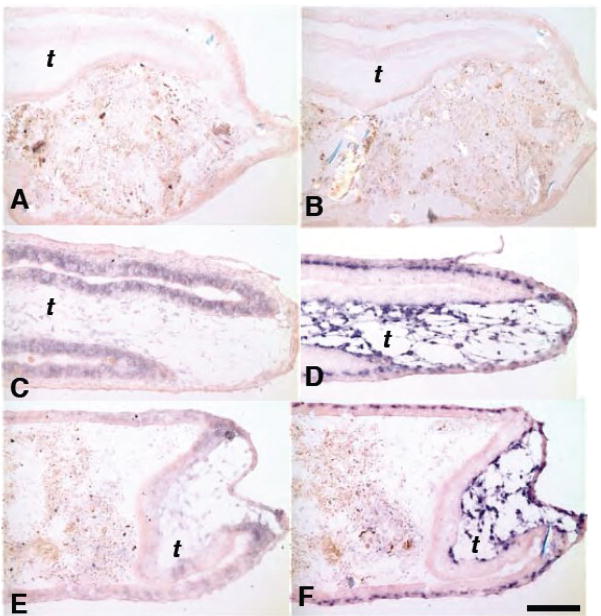
The direct response gene stromelysin-3 (ST3) is up-regulated in the subepithelial and intra-muscular mesenchymes of the transgenic tadpole (Fig 4F) just as it is in control tadpoles (Fig 4D), whereas bZip, a direct response gene activated by TH strongly in the epithelium is inhibited in these transgenic tadpoles (Fig 4E). The activation of Sonic hedgehog, another direct response gene of TH expressed in the epithelium (Stolow and Shi, 1995), is also inhibited by the transgene (data not shown). Therefore, inhibiting the TH-induction of the epithelium does not alter the induction of a direct response gene in the adjacent mesenchyme.
Fig 4.
The IFABP-TRDN-GFP transgene specifically inhibits TH direct-response genes in the epithelium, but not in the mesenchyme. Cross sections of duodenums from A,B) wild-type NF54 tadpoles treated with either 0 nM T3; C,D) 5nM T3 for 48 hrs. Cross-sections of duodenums from E,F) sibling tadpoles transgenic for IFABP-TRDN-GFP treated with 5nM T3 for 48 hrs. A,C,E) Expression of the TH direct-response gene, bZip, mRNA in the epithelium. B,D,F) Expression of the TH direct-response gene, stromelysin-3 (ST3), mRNA in the mesenchyme. mRNA localized by in situ hybridization (purple stain). Scale bar in F denotes 0.2 mm length.
There were no obvious gross morphological differences or developmental time disparities between control and transgenic tadpoles all the way through metamorphosis (Fig 5). NF65 froglets transgenic for IFABP-TRDN-GFP were indistinguishable from their non-transgenic siblings (Fig 5A–B). Transgenic and non-transgenic froglets changed their diet to worms and gained weight equally for two weeks post-metamorphosis (not shown). At this time their intestinal tracts were removed and compared (Fig. 5C). They have similar lengths and external appearance. However, the cross-sectional morphology of the duodenum and ileum from transgenic froglets was profoundly different from that of non-transgenic siblings. Transgenic frogs had extremely thin intestinal walls virtually devoid of intestinal folds (Fig 5E and G). The sub-epithelial fibroblasts of controls are several layers thick and surrounded by a prominent extracellular matrix (Fig 5D,F), while those of transgenic siblings have a sparse mesenchyme consisting of only one layer of fibroblasts (Fig 5E,G). The circular muscle layer of control duodenums is eight-ten cells thick, and longitudinal fibers were 1–2 cell layers thick (Fig 6A). However, in transgenic frogs the duodenal circular muscle layers were only 2–3 cells thick, and the longitudinal fibers 1 cell thick (Fig 6B). In the ileums of transgenic frogs an inter-muscular mesenchyme space was not clearly visible; the total muscle layers were approximately 2 cell layers thick, though longitudinal muscle fibers were not distinguishable from circular muscle based on morphology alone (not shown). The enteric neuronal cell bodies that are localized in the intramuscular space are absent in both the transgenic duodenum (Fig 6B) and ileum (not shown).
Similar to the above spontaneously metamorphosed froglets transgenic for IFABP-TRDN-GFP, the total gut lengths of NF48 transgenic tadpoles induced with TH for one week shortened normally compared with wild-type controls (Table 1), despite the dramatic accompanying cross-sectional differences.
Influence of fibroblasts on intestinal remodeling
Stromelysin-3 is known to be a direct response gene of TH that is expressed exclusively in the mesenchyme of the intestine (Patterton et al., 1995). The stromelysin-3 promoter (ST3) faithfully reproduces this cell-specific expression and retains its control by TH. Untreated tadpoles transgenic for ST3-GFP did not express GFP at NF48 (data not shown), but NF48 animals following 48 hrs of TH treatment expressed GFP specifically in the intestinal mesenchyme (Fig 7A). GFP was also strongly expressed specifically in intestinal fibroblasts during spontaneous metamorphosis (data not shown). In contrast with wild-type tadpoles of the same stage, the intestines of NF48 tadpoles transgenic for ST3-TRDN-GFP failed to constrict their cross-sectional diameter or thicken their sub-epithelial mesenchyme (Fig 7D). Many epithelial cells of TH-treated transgenic tadpoles appeared to be detached from the mesenchyme, forming gaps between cells.
Fig 7.
Both The ST3-TRDN-GFP and Col-TRDN-GFP transgenes inhibit epithelial development and thickening of the sub-epithelial mesenchyme. A) GFP is expressed specifically in the intestinal mesenchyme of tadpoles transgenic for ST3-GFP in an NF48 tadpole induced with T3 (10 nM) for 48 hours. B) Cross sections of intestine from an untreated NF48 wild-type tadpole; C) an NF48 wild-type tadpole treated for 7 days with 5 nM T; D) an NF48 tadpole transgenic for ST3-TRDN-GFP treated for 7d with 5 nM T3. E) GFP is expressed in the mesenchyme and smooth muscle layers (but not in the epithelium) of an NF48 tadpole transgenic for Col-GFP. Cross sections of intestine from F) an untreated NF54 wild-type tadpole; G) an NF54 wild-type tadpole treated for 7 days with 5 nM T3; H) an NF54 tadpole transgenic for Col-TRDN-GFP treated for 7d with 5 nM T3. Immunoreactivity against GFP in A and E is green, and nuclei are counterstained white with dapi. Tissues in B–D and F–H are stained with hematoxylin and eosin. Scale bar in E denotes 20 μm length; scale bars in D and H denote 50 μm length.
In contrast with ST3-GFP, which only expresses GFP in response to TH, the Col-GFP expresses GFP constitutively in both the mesenchyme and smooth muscle layers but never in the epithelium (Fig 7E). Similar to tadpoles transgenic for ST3-TRDN-GFP, in NF54 Col-TRDN-GFP tadpoles induced with TH for 4 days the intestines failed to constrict their cross-sectional diameter or thicken their sub-epithelial mesenchyme; the epithelial cells of these transgenic tadpoles also appeared highly necrotic, with large spaces between adjacent epithelial cells (Fig 7H). In NF48 Col-TRDN-GFP tadpoles induced with TH for 7 days all tissues of the gut appeared highly necrotic, with the epithelium virtually absent and many cells present in the gut lumen; cross-sectional diameter was also inhibited from constricting, and the mesenchyme failed to thicken (data not shown). Interestingly, NF48 tadpoles transgenic for Col-TRDN-GFP did show significantly less gut shortening in response to TH treatment compared with controls (Table 1). The effect of ST3-TRDN-GFP on gut shortening was not measured in this study.
Influence of smooth muscle on intestinal remodeling
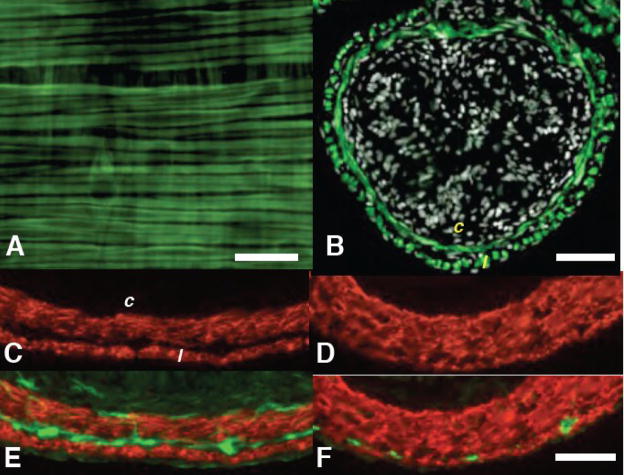
The intestines of tadpoles transgenic for pCar-GFP expressed GFP specifically in the circular and longitudinal smooth muscle fibers during all larval stages and throughout the entire length of the gut including the duodenum (Fig 8A–B). Although animals transgenic for pCar-TRDN-GFP die at NF63 (Das et al., 2002), the intestinal muscles have begun to thicken and separate significantly by that stage (Fig 8C), allowing us to study this aspect of gut development. The separation of longitudinal from circular muscle is already completed by NF61 (Schreiber et al., 2005), and separation is clearly visible by NF60 (data not shown). Compared with spontaneously metamorphosing wild-type tadpoles at NF63 (Fig 8C) the separation of the longitudinal and circular muscle fibers of these transgenic tadpoles is dramatically reduced (Fig 8D), the inter-muscular mesenchyme is virtually absent, and enteric neuronal aggregation was inhibited in the inter-muscular region (Fig 8E–F). Their intestinal folds developed normally (not shown), and total gut lengths of transgenic tadpoles precociously induced with TH shortened the same extent as controls (Table 1). Transgenic tadpoles showing this intestinal phenotype also exhibit inhibited leg skeletal muscle development and tail muscle that is resistant to TH (Das et al., 2002).
Fig 8.
The pCar-TRDN-GFP transgene inhibits smooth muscle separation, thickening of the inter-muscular mesenchyme, and aggregation of enteric neurons. A) The whole-mounted duodenum of an NF57 tadpole transgenic for pCar-GFP expresses GFP (green) specifically in the longitudinal (oriented left-right) and circular (oriented top-bottom) muscle fibers. Cross sections from the duodenum of B) NF63 tadpole transgenic for pCar-GFP stained for immunoreactivity against GFP (green) and counterstained for nuclei with dapi (white); C,D) stained with smooth muscle actin antibody; E,F) stained with smooth muscle actin and neural beta tubulin antibodies. (E). C,E) control NF63 tadpole; D,F) NF63 tadpole transgenic for pCar-TRDN-GFP. Circular (c) and longitudinal (l) smooth muscle fibers. Scale bar in A denotes 20 μm length; scale bar in B denotes 100 μm length; scale bar in F denotes 40 μm length.
DISCUSSION
The tadpole stage is free living with an herbivorous diet. Although remodeling can be induced in pre-metamorphic tadpoles with very low levels of exogenous T3 the intestine does not change normally until climax, the final 8 days of metamorphosis when the endogenous TH is at its highest concentration. There is a behavioral change that accompanies intestinal remodeling so that at the end of metamorphosis the frog has become a carnivore. An explanation for the timing of intestinal remodeling is the up-regulation of deiodinase 2 (D2) at climax (Cai and Brown, 2004) in the mesenchyme. D2 is the enzyme that synthesizes the more active hormone T3 from the circulating precursor T4. This expression of D2 raises the local level of T3 just at the time of remodeling. This timing sequence suggests that TH-induced remodeling of the intestine begins in the mesenchyme.
The role of cell-cell interaction in vertebrate intestine formation during embryogenesis has been well documented. Epithelial-mesenchyme signaling establishes the anteroposterior axis (Roberts et al., 1998). Smooth muscle layers are derived from intestinal fibroblasts that have been exposed to intestinal epithelium (Kedinger et al., 1990; McHugh, 1995). Genes such as sonic hedgehog and BMP4 have been implicated in mammalian (Bitgood and McMahon, 1995; Ramalho-Santos et al., 2000), chicken (Roberts et al., 1995; Sukegawa et al., 2000) intestinal morphogenesis and in the amphibian intestine at metamorphosis (Ishizuya-Oka et al., 2006; Stolow and Shi, 1995). Evidence for the influence of the mesenchyme over the development of the epithelium at metamorphosis has been shown by tissue recombination studies in culture (Ishizuya-Oka and Shimozawa, 1994). Our studies show that this example of cell-cell interaction is just one of several that are involved in intestinal remodeling at metamorphosis.
We have assessed the influence of inhibiting the TH-response of single cell types of several different tissues on intestinal remodeling in vivo. Expression of the TRDN-GFP specifically in the epithelium affects cross-sectional (radial) remodeling, but not intestinal shortening. The IFABP and stromelysin promoters are cell specific in their expression (Figs 1 and 7). The former inhibits the development of the epithelium (Figs 3, 4, 5), thickening of the mesenchyme (Figs 2, 5), smooth muscle layers (Fig 6), and enteric neuron development (Fig 6). At the end of metamorphosis even though the external morphology of the intestine appears the same as controls (Fig 5), the intestinal wall is as thin as the original tadpole intestine, a single cell cuboidal epithelium with little mesenchyme and a single cell thick circular and longitudinal muscle. Whereas the control intestine has developed complex involutions at the end of climax, the TRDN-GFP transgenic has only rudimentary intestinal folds (Figs 5, 6). The transgenic frog pictured in Fig 5 grew at the same rate as the control for two months. No detrimental phenotype was observed in any of the several frogs that we raised to this stage. We did not raise any to sexual maturity.
The response to TH of the ST3-driven TRDN-GFP transgenic resembles that of the IFABP-TRDN-GFP phenotype, but in addition the epithelium appears highly fragmented and pycnotic (Fig 7). Inhibiting TH-induced change in the mesenchyme may cause death of the epithelial cells. Stromelysin-3 is a TH direct response gene. Its expression change induced by TH correlates with remodeling at metamorphosis. However, the phenotype caused by the IFABP-TRDN-GFP transgene must be due to other genes within the mesenchyme, because endogenous stromelysin up regulation is not affected by inhibitory levels of TRDN-GFP in the epithelium (Fig 4).
The two phenotypes seen in muscle-specific TH-inhibition were the absence of a mesenchymal space between the circular and longitudinal muscle layers, and a dramatic reduction of enteric neuronal cell bodies that normally aggregate in this intra-muscular space (Fig 8). Considering that human intestinal smooth muscle cells are known to synthesize collagens that are thought to play roles in intestinal morphoghenesis (Graham et al., 1987), and that thyroid hormone has been shown to induce type I collagen production by intestinal smooth muscle during bullfrog (Ranacatesbeiana) metamorphosis (Asahina et al., 1999), the inhibition of the inter-muscular extracellular matrix space by the TRDN in this study may result from the failure of smooth muscle to produce a critical component of the extracellular matrix, such as collagen (Ishizuya-Oka and Shimozawa, 1994; Kordylewski, 1983). Extracellular matrix proteins such as tenascin, fibronectin, and collagen are known to influence enteric neuronal development in Xenopus laevis (Epperlein et al., 1990); the reduced presence of intramuscular neurons in the transgenic tadpoles may result from the minimized extracellular matrix between the muscle layers. Muscle expression of the TRDN-GFP transgene did not influence TH-induced shortening. Exploration of this phenotype is incomplete because these animals die at about NF63 from other causes (Brown et al., 2005; Das et al., 2002; Marsh-Armstrong et al., 2004).
Like other complex tissues and organs in amphibians, the various cell types that compose the intestine respond directly to TH. We have shown a cell autonomous TH-response in the epithelium (Figs 3, 4, 5), the mesenchyme (Fig 7), and the muscle (Fig 8). However, in addition blocking the epithelium and mesenchyme’s response to TH drastically interferes with remodeling changes in other cell types (Figs 2, 3, 5, 6, 7). TH induces extensive DNA replication in the epithelium and the mesenchyme (Fig 3). Blocking the epithelial response also inhibits DNA replication in the mesenchyme and thickening of the muscle layers. Since the intestinal mesenchyme has been shown to have the potential to differentiate into smooth muscle (Sukegawa et al., 2000), we propose that reduced proliferation of the mesenchyme in frogs transgenic for IFABP-TRDN-GFP may result in reduced development of smooth muscle.
One thyroid hormone responsive gene of the tadpole intestinal epithelium that is inhibited by the IFABP-TRDN-GFP construct is sonic hedgehog. The hedgehog family of genes is expressed in the embryonic gut endoderm in all vertebrates studied (Bitgood and McMahon, 1995; Echelard et al., 1993; Ekker et al., 1995; Krauss et al., 1993; Roberts et al., 1995; Stolow and Shi, 1995). Interestingly, embryos of mouse genetic knock-outs for Indian hedgehog (Ihh) are accompanied by dramatically reduced villi, smooth muscle thickness, enteric enervation, and mesenchymal proliferation in the duadenum, as well a generally very thin gut wall and increased gut diameter (Ramalho-Santos et al., 2000). These intestinal phenotypes in Ihh knock-out mice are remarkably similar to the phenotypes we report here in metamorphosing tadpoles and froglets transgenic for the IFABP-TRDN-GFP construct, leaving open the possibility that the frog phenotype ultimately results from inhibition of a member of the hedgehog family. In future experiments we will try to rescue the intestinal phenotypes of tadpoles transgenic for the IFABP promoter driving TRDN-GFP with hedgehog constructs driven by the same epithelial promoter.
Multiple TH-controlled cell autonomous and cell-cell interaction programs also occur in remodeling of the pancreas. TH-induces de-differentiation of the tadpole exocrine pancreas at metamorphic climax (Mukhi et al., 2008). At the same time the small β-cell islets aggregate (Mukhi and Brown, 2009, in press). This clustering of insulin-producing cells in the pancreas depends upon exocrine remodeling. The dedifferentiation of the exocrine pancreas is characterized by cessation of the synthesis of the pancreatic enzymes that are products of the acinar cells (Shi and Brown, 1990), just as the intestinal epithelium cells stop synthesizing IFABP. By analogy it would appear that the tadpole epithelium is dedifferentiating to a progenitor state at climax. However, to date the only genes shown to be up regulated in the intestinal epithelial cells at climax are direct response genes of TH (Shi and Brown, 1993).
Acknowledgments
We thank Rejeanne Juste for her excellent technical assistance. The comments of three anonymous reviewers significantly enhanced the quality of the manucript. This research was supported by grants from the National Institutes of Health and the G. Harold and Leila Mathers Charitable Trust (to D.D.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Amaya E, Kroll KL. A method for generating transgenic frog embryos. Methods Mol Biol. 1999;97:393–414. doi: 10.1385/1-59259-270-8:393. [DOI] [PubMed] [Google Scholar]
- Asahina K, Utoh R, Obara M, Yoshizato K. Cell-type specific and thyroid hormone-dependent expression of genes of alpha1(I) and alpha2(I) collagen in intestine during amphibian metamorphosis. Matrix Biol. 1999;18:89–103. doi: 10.1016/s0945-053x(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JM. Gut specific expression using mammalian promoters in transgenic Xenopus laevis. Mech Dev. 1999;88:221–227. doi: 10.1016/s0925-4773(99)00217-8. [DOI] [PubMed] [Google Scholar]
- Berry DL, Rose CS, Remo BF, Brown DD. The expression pattern of thyroid hormone response genes in remodeling tadpole tissues defines distinct growth and resorption gene expression programs. Dev Biol. 1998a;203:24–35. doi: 10.1006/dbio.1998.8975. [DOI] [PubMed] [Google Scholar]
- Berry DL, Schwartzman RA, Brown DD. The expression pattern of thyroid hormone response genes in the tadpole tail identifies multiple resorption programs. Dev Biol. 1998b;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bou-Gharios G, Garrett LA, Rossert J, Niederreither K, Eberspaecher H, Smith C, Black C, Crombrugghe B. A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol. 1996;134:1333–1344. doi: 10.1083/jcb.134.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Cai L, Das B, Marsh-Armstrong N, Schreiber AM, Juste R. Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2005;102:12455–12458. doi: 10.1073/pnas.0505989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Slack JM. Development of the gut in Xenopus laevis. Dev Dyn. 1998;212:509–521. doi: 10.1002/(SICI)1097-0177(199808)212:4<509::AID-AJA4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Das B, Brown DD. Controlling transgene expression to study Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101:4839–4842. doi: 10.1073/pnas.0401011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H, Brown DD. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc Natl Acad Sci U S A. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauca M, Bouziges F, Colin S, Kedinger M, Keller MK, Schilt J, Simon-Assmann P, Haffen K. Development of the vertebrate small intestine and mechanisms of cell differentiation. Int J Dev Biol. 1990;34:205–218. [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM. In: Physiology of the Amphibia. Lofts, editor. New York: Academic; 1976. pp. 467–599. [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ekker SC, McGrew LL, Lai CJ, Lee JJ, von Kessler DP, Moon RT, Beachy PA. Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis. Development. 1995;121:2337–2347. doi: 10.1242/dev.121.8.2337. [DOI] [PubMed] [Google Scholar]
- Epperlein HH, Krotoski D, Halfter W, Frey A. Origin and distribution of enteric neurones in Xenopus. Anat Embryol (Berl) 1990;182:53–67. doi: 10.1007/BF00187527. [DOI] [PubMed] [Google Scholar]
- Fu L, Ishizuya-Oka A, Buchholz DR, Amano T, Matsuda H, Shi YB. A causative role of stromelysin-3 in extracellular matrix remodeling and epithelial apoptosis during intestinal metamorphosis in Xenopus laevis. J Biol Chem. 2005;280:27856–27865. doi: 10.1074/jbc.M413275200. [DOI] [PubMed] [Google Scholar]
- Graham MF, Drucker DE, Diegelmann RF, Elson CO. Collagen synthesis by human intestinal smooth muscle cells in culture. Gastroenterology. 1987;92:400–405. doi: 10.1016/0016-5085(87)90134-x. [DOI] [PubMed] [Google Scholar]
- Hourdry J, Dauca M. Cytological and cytochemicaI changes in the intestinal epithelium during anuran metamorphosis. Int Rev Cytol. 1977;5:337–385. [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Shimizu K, Suzuki K, Ueda S. Shh/BMP-4 signaling pathway is essential for intestinal epithelial development during Xenopus larval-to-adult remodeling. Dev Dyn. 2006;235:3240–3249. doi: 10.1002/dvdy.20969. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Li Q, Amano T, Damjanovski S, Ueda S, Shi YB. Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis. J Cell Biol. 2000;150:1177–1188. doi: 10.1083/jcb.150.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shi YB. Molecular mechanisms for thyroid hormone-induced remodeling in the amphibian digestive tract: a model for studying organ regeneration. Dev Growth Differ. 2005;47:601–607. doi: 10.1111/j.1440-169X.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shi YB. Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev Dyn. 2007;236:3358–3368. doi: 10.1002/dvdy.21291. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux’s Archiv Dev Biol. 1992;201:322–329. doi: 10.1007/BF00592113. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277:427–436. doi: 10.1007/BF00300215. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Damjanovski S, Li Q, Liang VC, Shi YB. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: immunohistochemical detection of intestinal fatty acid-binding protein. Dev Biol. 1997;192:149–161. doi: 10.1006/dbio.1997.8749. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Inokuchi T, Amano T, Damjanovski S, Stolow M, Shi YB. Thyroid hormone-induced expression of sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation. 2001;69:27–37. doi: 10.1046/j.1432-0436.2001.690103.x. [DOI] [PubMed] [Google Scholar]
- Kedinger M, Simon-Assmann P, Bouziges F, Arnold C, Alexandre E, Haffen K. Smooth muscle actin expression during rat gut development and induction in fetal skin fibroblastic cells associated with intestinal embryonic epithelium. Differentiation. 1990;43:87–97. doi: 10.1111/j.1432-0436.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kordylewski L. Light and electron microscopic observations of the development of intestinal musculature in Xenopus. Z Mikrosk Anat Forsch. 1983;97:719–734. [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Li J, Liang VC, Sedgwick T, Wong J, Shi YB. Unique organization and involvement of GAGA factors in transcriptional regulation of the Xenopus stromelysin-3 gene. Nucleic Acids Res. 1998;26:3018–3025. doi: 10.1093/nar/26.12.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Cai L, Brown DD. Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101:165–170. doi: 10.1073/pnas.2136755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron. 1999;24:871–878. doi: 10.1016/s0896-6273(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Marshall JA, Dixon KE. Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis tadpoles. J Anat. 1978;126:133–144. [PMC free article] [PubMed] [Google Scholar]
- McHugh KM. Molecular analysis of smooth muscle development in the mouse. Dev Dyn. 1995;204:278–290. doi: 10.1002/aja.1002040306. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Horb ME, Brown DD. Remodeling of insulin producing β-cells during Xenopus laevis metamorphosis. Developmental Biology. 2009;328:384–391. doi: 10.1016/j.ydbio.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhi S, Mao J, Brown DD. Remodeling the exocrine pancreas at metamorphosis in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105:8962–8967. doi: 10.1073/pnas.0803569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterton D, Hayes WP, Shi YB. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev Biol. 1995;167:252–262. doi: 10.1006/dbio.1995.1021. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Schreiber AM, Brown DD. Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. Proc Natl Acad Sci U S A. 2003;100:1769–1774. doi: 10.1073/pnas.252774999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci U S A. 2005;102:3720–3725. doi: 10.1073/pnas.0409868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci U S A. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB, Brown DD. Developmental and thyroid hormone-dependent regulation of pancreatic genes in Xenopus laevis. Genes Dev. 1990;4:1107–1113. doi: 10.1101/gad.4.7.1107. [DOI] [PubMed] [Google Scholar]
- Shi YB, Brown DD. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J Biol Chem. 1993;268:20312–20317. [PubMed] [Google Scholar]
- Shi YB, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- Stolow MA, Shi YB. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 1995;23:2555–2562. doi: 10.1093/nar/23.13.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]