Abstract
Pavlovian conditioning research has shown that unconditioned responses (UCR) to aversive unconditioned stimuli (UCS) are reduced when a UCS is predictable. This effect is known as UCR diminution. In the present study, we examined UCR diminution in the functional magnetic resonance imaging (fMRI) signal by varying the rate at which a neutral conditioned stimulus (CS) was paired with an aversive UCS. UCR diminution was observed within several brain regions associated with fear learning, including the amygdala, anterior cingulate, auditory cortex, and dorsolateral prefrontal cortex when a CS continuously relative to intermittently predicted the UCS. In addition, an inverse relationship between UCS expectancy and UCR magnitude was observed within the amygdala, anterior cingulate, and dorsolateral prefrontal cortex, such that as UCS expectancy increased the UCR decreased. These findings demonstrate UCR diminution within the fMRI signal, and suggest that UCS expectancies modulate UCR magnitude.
Keywords: UCR diminution, fear, conditioning, fMRI, learning, amygdala
Introduction
During Pavlovian fear conditioning a neutral conditioned stimulus (CS) is repeatedly paired with an aversive unconditioned stimulus (UCS). Once the CS-UCS association has been formed, the CS produces a conditioned fear response (CR) in anticipation of the UCS. Unlike CRs, the unconditioned response (UCR) produced by the aversive UCS is typically characterized as an unlearned response. However, learning-related changes in UCRs have been observed in prior Pavlovian conditioning research (Domjan, 2005). Specifically, CS-UCS pairings attenuate UCR amplitude relative to a UCS presented alone (Kimble and Ost, 1961, and Kimmel, 1966). This attenuation of the UCR is known as UCR diminution and has been observed in several fear conditioning studies (Baltissen and Boucsein, 1986, Grings and Schell, 1971, and Pendergrass and Kimmel, 1968). Previous behavioral investigations of UCR diminution in humans have shown that UCRs are affected by manipulations of CSs that precede UCS presentations. These studies demonstrated diminution of the unconditioned skin conductance response (SCR) when a UCS was preceded by a CS+ relative to a CS− (Marcos and Redondo, 1999a, and 2002), the CS-UCS time interval was held constant rather than varied (Kimble and Ost, 1961, Hymowitz, 1973, Marcos and Redondo, 1999b, and Peeke and Grings, 1968), and the CS was fear-relevant as opposed to fear-irrelevant (Merckelbach and van den Hout, 1991).
Prior studies of UCR diminution indicate that reductions in the UCR develop as the CS-UCS relationship is established (Kimble and Ost, 1961, and Donegan and Wagner, 1987). This effect appears to be related to the predictability of the UCS (Grings, 1973) and may be an active process mediated by conscious UCS expectancies (Lykken and Tellegen, 1974). For example, a predictable UCS is perceived as less aversive (Schell and Grings, 1971) and produces an attenuated UCR (Grings, 1973, and Lykken and Tellegen, 1974).
Although UCR diminution has not been reported in prior functional magnetic resonance imaging (fMRI) investigations of Pavlovian fear conditioning, activity associated with the acquisition and expression of conditional fear have been observed in several brain regions. The hippocampus, thalamus, cingulate, insula, orbitofrontal, and sensory cortex respond to CSs paired with an aversive stimulus (Büchel et al., 1998, Büchel et al., 1999, Dunsmoor et al., 2007, Knight et al., 1999, Knight et al., 2004, LaBar et al., 1998, and Phelps et al., 2004). Further, the amygdala appears to be involved in forming CS-UCS associations and producing CRs (Büchel et al., 1998; Cheng et al., 2003, Cheng et al., 2006, Knight et al., 2005, and LaBar et al., 1998). Neuroimaging research of fear conditioning also indicates that the magnitude of the CR is influenced by the CS-UCS pairing rate (Dunsmoor et al., 2007). The insula and dorsolateral prefrontal cortex (dlPFC) appear to respond to the uncertainty of receiving an aversive UCS, whereas amygdala and anterior cingulate cortex (ACC) responses increase linearly with the frequency at which the CS and UCS are paired (Dunsmoor et al., 2007). These results suggest that learning-related changes in fMRI signal UCRs may also be observable within many of these regions during human Pavlovian fear conditioning.
In the present study we investigated the influence of the CS-UCS pairing rate and UCS expectancy on the magnitude of unconditioned fMRI signal responses. Prior behavioral research indicates that when a UCS is predictable the UCR is reduced (Lykken et al., 1972, and Peeke and Grings, 1968), and knowledge of the CS-UCS relationship diminishes autonomic responses to aversive stimuli (Grings, 1973). Additionally, previous fMRI studies suggest that the predictability of stimulus intensity modulates the UCR produced by a painful UCS (Seymour et al., 2004). Therefore we hypothesized that greater UCR diminution would be observed to a UCS that is continuously relative to intermittently paired with a CS, and that expectations of UCS presentation would further diminish UCR amplitude.
Materials and Methods
Participants
Eighteen healthy right-handed volunteers [11 female and 7 male; age (mean ± SEM): 30.17 ± 1.63 years; age range: 23 to 47 years] participated in this study. All subjects provided written informed consent in compliance with the National Institute of Mental Health Institutional Review Board.
Conditioning Procedures
Participants received presentations of three pure tones (700, 1000, and 1300 Hz) that served as the CSs (10 s duration). One tone co-terminated with a 500 ms loud white noise (100db) UCS (9.5 s inter-stimulus interval) on 100% of trials (CS100). A second tone (CS50) was paired with the UCS on 50% of trials (CS50+), and presented alone on 50% of trials (CS50−). A third tone (CS−) was never paired with the UCS. Forty trials of each CS were presented over four 920 s conditioning blocks (10 trials of each CS were presented in all blocks), and each CS was separated by a 20 s inter-trial interval. CSs were counterbalanced and presented in a pseudorandom order such that no more than two trials of the same CS were consecutively presented.
UCS expectancy
During the conditioning session participants continuously rated their expectancy of receiving the UCS using an MRI compatible joystick to control a rating bar presented on a visual display. Participants were instructed to update their ratings on a continuous scale from 0 to 100 (0 = certain that the UCS will not be presented, 50 = uncertain whether the UCS will be presented, 100 = certain that the UCS will be presented) and to continuously update (sampled at 10 Hz) their rating to reflect their current UCS expectancy. UCS expectancy was calculated as the average response during the last 1 s of the CS presentation.
SCR data acquisition
A Contact Precision Instruments, skin conductance monitoring system was used to monitor skin conductance response (SCR) throughout the conditioning session. SCR was sampled (80 Hz) with a pair of surface gel cup electrodes (silver/silver chloride, 6 mm diameter, Biopac model TSD203) attached to the distal phalanx of the middle and ring fingers of the left hand. The SCR signal was digitized at the electrodes and a 10 Hz filter was applied. Unconditioned SCRs were calculated by subtracting the average skin conductance measurement 1 second prior to the UCS from the maximum SCR during the 5 seconds following the UCS presentation.
Functional image acquisition and analysis
Structural and functional imaging was completed on a 3-T General Electric Signa scanner using an 8-channel RF head coil. Functional imaging of the entire brain was conducted using a gradient-echo echo-planar pulse sequence (TR = 2000 ms, TE = 30 ms, FOV = 240 mm, matrix = 64 × 64, slice thickness = 4 mm) during each of four 920 s blocks of stimulus presentations. High-resolution anatomical images (MPRAGE) were obtained to serve as an anatomical reference. Image processing was performed with the AFNI software package (Cox, 1996, and Cox and Hyde, 1997). Echo-planar time series data were motion corrected and reregistered to the fifth volume of the first imaging block (Cox and Jesmanowicz, 1999). Head motion parameters and joystick movement regressors were included in the analysis to account for brain activity unrelated to CS and UCS presentation. Hemodynamic response functions were obtained by deconvolving the input for the UCS on trials paired with the CS100 and CS50+ from the fMRI time series using a least-squares procedure. The percent area under the second through fourth images of the hemodynamic response curve (AUC), which occur following the UCS, was used as an index of the unconditioned fMRI signal produced by the UCS. Functional maps reflecting the AUC were converted to a standard stereotaxic coordinate system (Talairach and Tournoux, 1988) and spatially blurred using a 2-mm full-width-at-half-maximum isotropic Gaussian filter. Brain activity associated with the UCS was compared to a resting baseline. Further analysis was restricted to areas of activation that were larger than 250 mm3 in volume and that showed a significant increase in activity during the UCS relative to baseline (t > 3.96, p < 0.0005). No region showed decreased activity relative to baseline. A t-test contrast of fMRI data from regions meeting these criteria was then performed with a corrected p < 0.05 significance threshold (see Table 1)
Results
Behavioral data
Participants demonstrated their knowledge of the CS-UCS relationships with UCS expectancy ratings that varied with the rate at which the CS and UCS were paired (F=16.74, p < 0.05). The lowest ratings were observed during the CS− (all values reflect the mean ± SEM: 43.02 ± 5.21), intermediate ratings during the CS50 (57.71 ± 3.02), and the highest ratings to CS100 presentations (73.04 ± 4.36). Ratings during the CS50+ (54.70 ± 3.22) were significantly lower than during CS100 trials (73.04 ± 4.36) (t [17] = 3.04 p < 0.05) (Figure 1a). To ensure that UCS presentations were inherently less predictable on CS50 versus CS100 trials, five paired and five unpaired CS50 trials were presented within each of the four conditioning blocks. Analysis of UCS expectancy on these trials demonstrated a small, but significantly larger UCS expectancy ratings (t [17] = 2.53 p > 0.05) to the CS50− (60.75 ± 3.27) despite not being paired with the UCS. These data demonstrate that participants were unable to develop a successful strategy to predict the UCS on these intermittently paired trials. The larger UCS expectancies on CS50− trials appears to reflect a gamblers fallacy (Perruchet, 1985) in which participants’ expectancy ratings were influenced by whether the UCS was paired with the CS50 on the previous trial. During training, differential UCS expectancies to CS presentations developed relatively quickly. However, the learning-related changes observed in the current study did not reach the magnitude observed in prior fear conditioning studies (Knight et al., 2004; Cheng et al., 2007). This may be due to the use of auditory tones in the present study, which appear to be more difficult to discriminate than the visual stimuli used in previous studies, because of the loud gradient noise produced during scan sessions. Additionally, fear conditioning studies often incorporate a single CS+ and CS−, and discrimination between these two CSs generally occurs rapidly (Knight et al., 2004; Cheng et al., 2007). Including three distinct CSs may have made it more difficult for some participants to learn all CS-UCS relationships, particularly during early trials.
Figure 1.
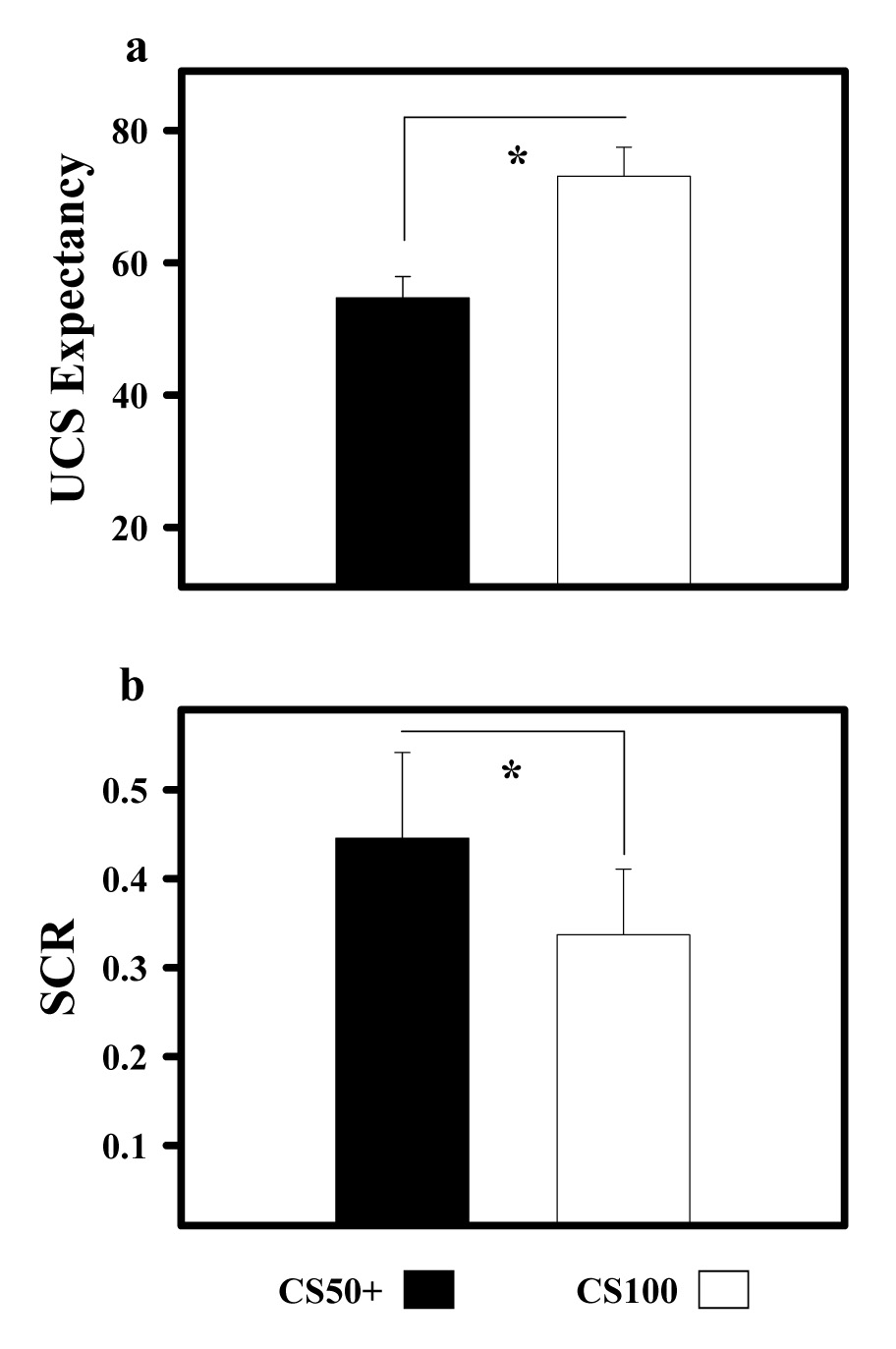
UCS expectancy and SCR data. a) UCS expectancy ratings indicate that participants expected the UCS on CS100 trials and were uncertain whether the UCS would be presented on CS50+ trials. b) Unconditioned SCR data reveal significant UCR diminution on CS100 trials relative to CS50+ trials. Asterisk (*) indicates significant differences.
Significant unconditioned SCR diminution was observed on CS100 relative to CS50+ trials (Figure 1b and Figure 2). Unconditioned SCRs following the CS100 (0.33 ± 0.07) were significantly smaller than the UCRs that followed CS50+ (0.44 ± 0.09) trials (t [17] = 2.63 p < 0.05). No significant correlation was found between UCS expectancy and unconditioned SCR production on CS100 (r = 0.019) or CS50+ (r = 0.033) trials.
Figure 2.
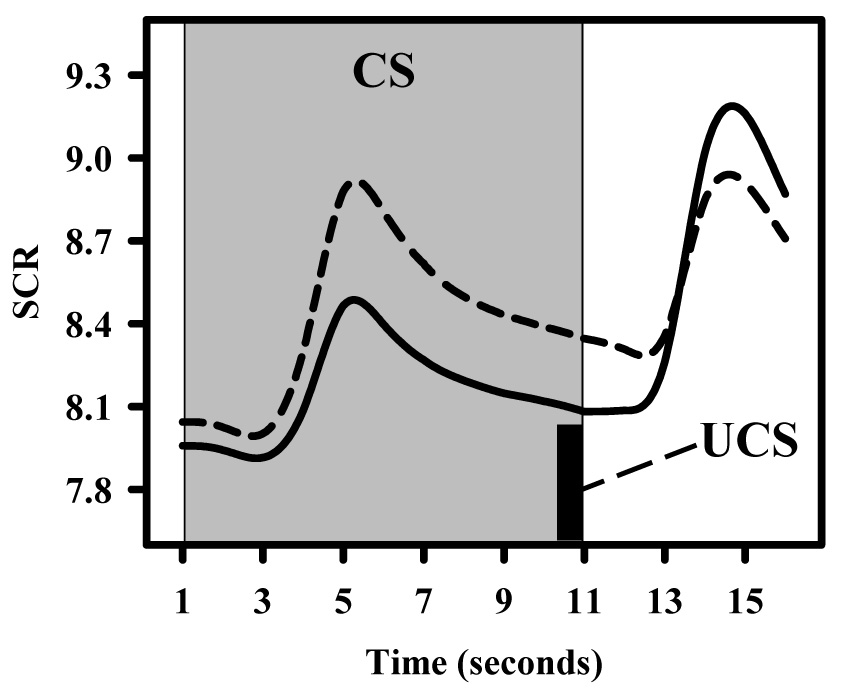
SCR time course from CS100 (dashed line) and CS50+ (solid line) trials for a representative subject. SCRs were larger to CS100 than to CS50+ trials showing the typical learning-related change observed in the conditioned response during most fear conditioning studies. In addition, a learning-related change in the UCR can also be observed with a smaller SCR following the UCS on CS100 than on CS50+ trials. This reduction in the unconditioned SCR on CS100 relative to CS50+ trials is known as UCR diminution.
fMRI
Several brain regions showed significant responses to the UCS including the thalamus, cerebellum, anterior cingulate, posterior cingulate, left dlPFC, bilateral inferior parietal lobe, and bilateral auditory cortex. UCR diminution was observed within a subset of these regions, including thalamus, cerebellum, anterior cingulate, left dlPFC, bilateral inferior parietal lobe, and left auditory cortex (Table 1 and Figure 3 and Figure 4). In each of these regions the unconditioned fMRI signal response was significantly greater to the UCS on CS50+ relative to CS100 trials. No areas showed significantly greater responses to the UCS during CS100 relative to CS50+ trials.
Figure 3.

UCR diminution within dorsolateral Prefrontal cortex (dlPFC) and anterior cingulate cortex. Significant UCR diminution was observed within a) left dlPFC and b) anterior cingulate on CS100 relative to CS50+ trials. Additionally, UCS expectancy was negatively correlated with unconditioned fMRI signal responses on CS50+ trials, such that as expectancy increased UCR magnitude decreased within the a) left dlPFC and b) anterior cingulate. Asterisk (*) indicates significant difference.
Figure 4.
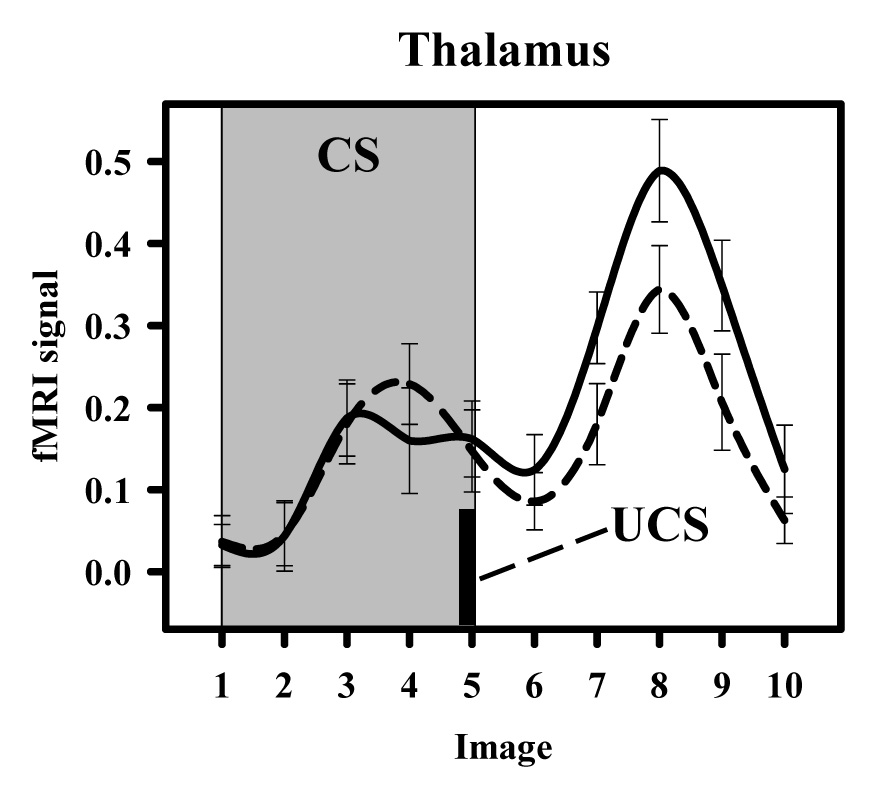
Functional MRI time course from the thalamus. Graph depicts time course data during the conditioned stimulus (CS; grey background) and following unconditioned stimulus (UCS; black bar) presentation. Activity within this region was similar during the 10 s CS period of CS100 (dashed line) and CS50+ (solid line) presentations. However, the unconditioned response produced by the UCS was diminished on CS100 relative to CS50+ trials.
Correlation analyses revealed a significant inverse relationship between UCS expectancy and the unconditioned fMRI signal such that as UCS expectancy increased the magnitude of the UCR decreased within the dlPFC (r = −0.503, p < 0.05) and anterior cingulate (r = −0.585, p < 0.05) on CS50+ trials (Figure 3). No relationship between UCS expectancy and UCR amplitude was observed on CS100 trials, which may be related to the relatively high UCS expectancy ratings observed on these trials.
Because amygdala activity did not meet the cluster size and significance threshold for the whole brain analysis, an additional region of interest (ROI) analysis was conducted using the built-in AFNI Talairach-Tournoux neuroanatomical atlas (Cox, 1996). Three participants were excluded from this analysis because they did not show a sufficient temporal signal-to-noise ratio (TSNR > 30) within this ROI. UCR magnitude within the right amygdala (Figure 5) was significantly diminished to UCSs following the CS100 (0.08 ± 0.05) relative to CS50+ (0.16 ± 0.05) trials (t [14] = 2.54 p < 0.05). In addition, we observed an inverse relationship between UCS expectancy and UCR amplitude within the right amygdala on CS50+ trials, such that as UCS expectancy increased the magnitude of the unconditioned fMRI signal decreased (r = −0.514, p < 0.05).
Figure 5.
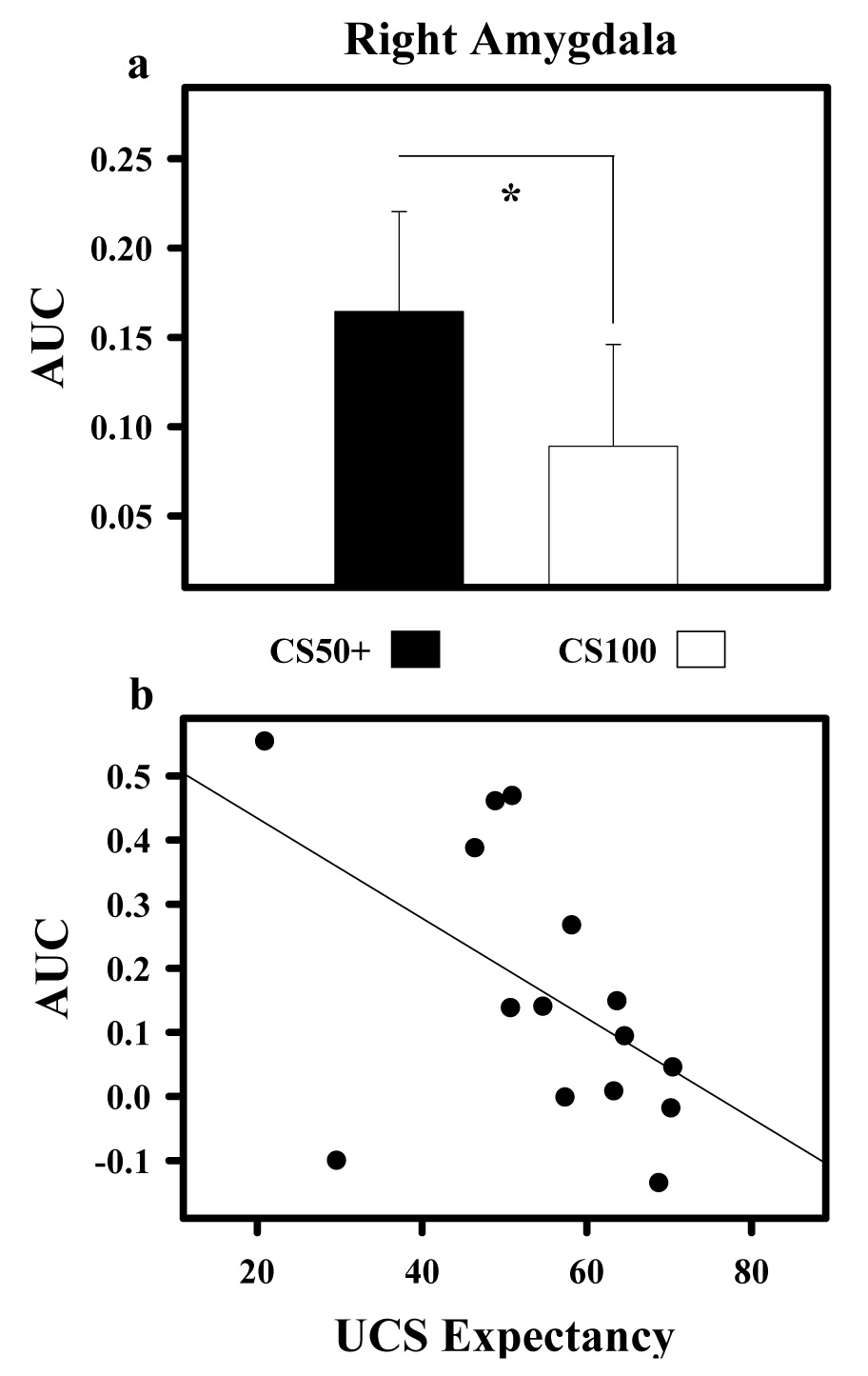
UCR diminution within right amygdala. a) Area under the hemodynamic response curve (AUC) shows unconditioned fMRI signal responses were significantly reduced on CS100 relative to CS50+ trials within the right amygdala. b) An inverse relationship between UCS expectancy and UCR amplitude was observed, such that as UCS expectancy increased, the magnitude of the unconditioned fMRI signal decreased on CS50+ trials. Asterisk (*) indicates significant difference.
Discussion
The present study investigated the effect of the CS-UCS pairing rate and UCS expectancy on UCR diminution. Although prior fMRI research has investigated the neural substrates of Pavlovian fear conditioning, limited attention has been given to the study of learning-related changes in fMRI signal UCRs. Consistent with prior behavioral studies in humans (Baltissen and Boucsein, 1986, Marcos and Redondo, 1999, and Rust, 1976), diminution of the unconditioned SCR was observed to continuously signaled UCSs. In addition, UCR diminution was observed within several brain regions including the right amygdala, thalamus, anterior cingulate, cerebellum, bilateral inferior parietal lobe, and left dlPFC. Finally, UCS expectancy appeared to modulate the magnitude of the unconditioned fMRI signal response within several of these regions, such that as UCS expectancy increased, UCR amplitude within the amygdala, anterior cingulate, and dlPFC decreased.
Although UCR diminution has not been systematically investigated in previous fMRI research, brain imaging studies of temporal difference learning indicate that prediction errors may alter the fMRI signal produced by aversive stimuli (Jensen et al., 2007; Ploghaus et al., 2000; Seymour et al., 2004). For example, activity within the ventral striatum, insula, anterior cingulate, lingual gyrus, and orbital frontal cortex appear to correlate with UCS prediction errors (Jensen et al., 2007). The present UCR diminution findings are generally consistent with this prior work, showing an inverse relationship between UCS expectancy and UCR amplitude within the amygdala, anterior cingulate, and dlPFC. In addition, many of the regions showing UCR diminution in the present study have been implicated in prior fear learning and memory research. For example, the amygdala is a principal site of fear learning that appears to be involved in forming CS-UCS associations and producing CRs (Cheng et al., 2006, Helmstetter, 1992, Knight et al., 2005, and LeDoux, 2000). Further, amygdala activity is often observed in fMRI studies of Pavlovian fear conditioning, showing larger responses to CS+ than CS− presentations (Büchel et al., 1998, Dunsmoor et al., 2007, Labar et al., 1998, and Tabbert et al., 2005). In the present study, unconditioned fMRI signal responses within the right amygdala showed significant UCR diminution, suggesting this region may modulate learning-related behavioral changes in UCRs. However, we did not find a significant relationship between the magnitude of amygdala activity and the unconditioned SCRs produced. Although, prior fMRI studies have demonstrated the amygdala modulates the production of conditioned SCRs (Cheng et al., 2003, 2006, 2007, and Knight et al., 2005), future work will need to further investigate this region’s role in the production of learning-related changes in UCRs.
The anterior cingulate cortex has also been implicated in prior fMRI studies of fear conditioning (Knight et al., 1999, 2004, and LaBar et al., 1998). This region appears to be involved in attending to feared stimuli (Han et al., 2003), and activity within this area parallels the CS-UCS pairing rate during conditioning (Dunsmoor et al., 2007). The differential activity observed within the anterior cingulate in the present study may reflect changes in orienting to an aversive UCS. Previous investigations suggest that unpredictable stimuli elicit larger behavioral responses due to their novelty, while the response to a reliably signaled UCS tends to habituate (Baltissen and Boucsein, 1986, and Furedy and Klajner and 1974). In the present study, the predictability of the UCS on CS100 trials may have reduced UCR amplitude. In contrast, UCRs may have been better maintained on CS50+ trials, as UCS presentation was less certain on these intermittently paired trials. Therefore, the UCR diminution observed within the anterior cingulate may reflect decreased orienting to the continuously signaled UCS, while the intermittently signaled UCS continued to elicit a relatively larger orienting response. If the UCR diminution observed in this study reflects differences in orienting to the UCS, then UCRs to intermittently and continuously signaled UCSs should be diminished relative to an unsignaled UCS because both are better predictors of UCS presentation than an UCS presented alone. However, it is possible that the inherent uncertainty associated with intermittently signaled UCS presentations may have a unique effect on UCR magnitude that could delay UCR habituation relative to both continuously and unsignaled UCSs. For example, the conditioning context may have a larger influence on UCR diminution to an unsignaled compared to intermittently signaled UCS. In this case, the UCR to an intermittently signaled UCS would be larger than UCRs to both continuously and unsignaled UCSs.
The dlPFC may also mediate attentional processes similar to those of the anterior cingulate (MacDonald et al., 2000). Prior work has shown the dlPFC supports trial by trial fear learning during the acquisition of CS-UCS contingency awareness (Carter et al., 2006). Our current findings demonstrate UCR diminution within the dlPFC that may support this type of top down attentional process during fear conditioning. Further, prior work suggests that UCRs may be inhibited when the UCS is consciously expected (Lykken et al., 1972, and Lykken and Tellegen, 1974). In the present study, UCR diminution within the dlPFC varied with UCS expectancies. We observed an inverse relationship between UCS expectancy and UCR magnitude within this region, such that as UCS expectancy increased, the unconditioned fMRI signal response decreased. In addition to the dlPFC, UCS expectancy was also negatively correlated with the unconditioned fMRI signal responses within the right amygdala and anterior cingulated cortex. As UCS expectancy increased, UCRs within these brain regions decreased. This inverse relationship was observed during intermittently paired CS-UCS trials, when UCS expectancy ratings indicated participants’ were uncertain of UCS presentations. The current results suggest that UCS expectancies may modulate UCR magnitude within the right amygdala, anterior cingulate, and left dlPFC.
Functional MRI studies in which stimuli have a consistent relationship and are closely presented in time can have difficulty dissociating brain activity produced by the stimuli presented. In the present study, the CS and UCS coterminated on every CS100 trial. Therefore, we examined the fMRI time course from regions showing UCR diminution to ensure that the diminution observed was not due to differences in brain activity produced prior to UCS presentation. These data indicate that the differential UCRs observed following UCS presentation are not simply due to greater CR activity to the CS50 than to the CS100. In many cases, the fMRI signal was similar during the 10 s CS period of CS100 and CS50 and only differed following UCS presentation (see Figure 4). In other cases, as previously reported for the amygdala (Dunsmoor et al., 2007), CS elicited activity was slightly larger during the CS period of the CS100 relative to the CS50 and had returned to similar levels prior to the production of the differential UCRs observed in this study. In addition, two discrete responses that reflect the CR and UCR can be observed in the fMRI time course (see Figure 3). Taken together, these data suggest that the UCR diminution observed in this study is not merely due to a misattribution of the differential CRs produced by CS presentations.
In conclusion, we observed learning-related changes in the unconditioned fMRI signal response during Pavlovian fear conditioning. Although learning-related changes in the CR have been extensively reported in prior neuroimaging studies of Pavlovian conditioning, the current results are the first demonstration of UCR diminution using functional neuroimaging during Pavlovian fear conditioning. These results demonstrate that UCRs, which are typically considered unlearned responses, can show learning-related changes (i.e. UCR diminution) within several brain regions. In addition, UCR magnitude within a subset of these regions was negatively correlated with UCS expectancies. This finding indicates that conscious expectations may influence unconditioned fMRI responses to aversive stimuli. Taken together, these results suggest that the predictive quality of a CS and the resultant expectations of a UCS may modulate the brain activity elicited by aversive stimuli.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
Abbreviations
- CR
conditioned response
- UCR
unconditioned response
- UCS
unconditioned stimulus
- CS
conditioned stimulus
- CS100
CS paired with the UCS on every trial
- CS50
CS paired with the UCS on half the trials (includes CS50+ and CS50−)
- CS50+
CS50 trials in which the UCS was paired with the UCS
- CS50−
CS50 trials in which the UCS was not paired with the UCS
- SCR
skin conductance response
- dlPFC
dorsolateral prefrontal cortex
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltissen R, Boucsein W. Effects of a warning signal on reactions to aversive white noise stimulation: does warning "short-circuit" habituation? Psychophysiology. 1986;23:224–231. doi: 10.1111/j.1469-8986.1986.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;26:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral Neuroscience. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdale corresponds to early rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for the analysis and visualization of FMRI data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: a functional perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Wagner AR. Conditioned diminution and facilitation of the UR. A sometimes opponent-process interpretation. In: Gormezano I, Prokasy WF, Thompson RF, editors. Classical Conditioning III. Hillsdale, NJ: Erlbaum; 1987. pp. 339–369. [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral Neuroscience. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Furedy JJ, Klajner F. On evaluating autonomic and verbal indices of negative perception. Psychophysiology. 1974;11:121–124. doi: 10.1111/j.1469-8986.1974.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Grings WW. Cognitive factors in electrodermal conditioning. Psychological Bulletin. 1973;79:200–210. doi: 10.1037/h0033883. [DOI] [PubMed] [Google Scholar]
- Grings WW, Schell AM. Effects of trace versus delay conditioning, interstimulus interval variability, and instructions on UCR diminution. Journal of Experimental Psychology. 1971;90:136–140. doi: 10.1037/h0031343. [DOI] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiology and Behavior. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Hymowitz N. Comparisons between variable-interval and fixed-interval schedules of electric shock delivery. Journal of the Experimental Analysis of Behavior. 1973;19:101–111. doi: 10.1901/jeab.1973.19-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble GA, Ost JWP. A conditioned inhibitory process in eyelid conditioning. Journal of Experimental Psychology. 1961;61:150–156. doi: 10.1037/h0044932. [DOI] [PubMed] [Google Scholar]
- Kimmel HD. Inhibition of the unconditioned response in classical conditioning. Psychological Review. 1966;73:232–241. doi: 10.1037/h0023270. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–2000. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Macindoe Y, Tellegen A. Perception: autonomic responses to shock as a function of predictability in time and locus. Psychophysiology. 1972;9:318–333. doi: 10.1111/j.1469-8986.1972.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Tellegen A. On the validity of the perception hypothesis. Psychophysiology. 1974;11:125–132. doi: 10.1111/j.1469-8986.1974.tb00833.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marcos JL, Redondo J. Differential effects of expectancy and associative mechanisms on diminution of unconditioned response in electrodermal classical conditioning. Psicothema. 2002;14:375–381. [Google Scholar]
- Marcos JL, Redondo J. Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological Psychology. 1999a;50:89–102. doi: 10.1016/s0301-0511(99)00007-1. [DOI] [PubMed] [Google Scholar]
- Marcos JL, Redondo J. Effects of CS-US interval modification on diminution of the unconditioned response in electrodermal classical conditioning. Biological Psychology. 1999b;50:191–201. doi: 10.1016/s0301-0511(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Merckelbach H, van den Hout MA. Fear relevance and diminution of unconditioned skin conductance responses. Z. Psychol. 1991;199:267–277. [PubMed] [Google Scholar]
- Peeke SC, Grings WW. Magnitude of UCR as a function of variability in the CS-UCS relationship. Journal of Experimental Psychology. 1968;77:64–69. doi: 10.1037/h0025769. [DOI] [PubMed] [Google Scholar]
- Pendergrass VE, Kimmel HD. UCR diminution in temporal conditioning and habituation. Journal of Experimental Psychology. 1968;77:1–6. doi: 10.1037/h0025815. [DOI] [PubMed] [Google Scholar]
- Perruchet P. A pitfall for the expectancy theory of human eyelid conditioning. Pavlovian Journal of Biological Science. 1985;20:163–170. doi: 10.1007/BF03003653. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins NP, Matthews PM. Learning about pain: The neural substrates of the prediction error for aversive events. Proceedings of the National Academy of Sciences. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust J. Unconditioned response diminution in the skin resistance response. Journal of General Psychology. 1976;95:77–84. doi: 10.1080/00221309.1976.9710867. [DOI] [PubMed] [Google Scholar]
- Schell A, Grings WW. Judgments of UCS intensity and diminution of the unconditioned GSR. Psychophysiology. 1971;8:427–432. doi: 10.1111/j.1469-8986.1971.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowlak RS. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Hemodynamic responses of the amygdala, the orbitofrontol cortex and the visual cortex during a fear conditioning paradigm. International Journal of Psychophysiology. 2005;57:15–23. doi: 10.1016/j.ijpsycho.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


