Abstract
AIM: To investigate the functional significance of insulin-like growth factor binding protein-5 (IGFBP-5) overexpression in pancreatic cancer (PaC).
METHODS: The effects of IGFBP-5 on cell growth were assessed by stable transfection of BxPC-3 and PANC-1 cell lines and measuring cell number and DNA synthesis. Alterations in the cell cycle were assessed by flow cytometry and immunoblot analyses. Changes in cell survival and signal transduction were evaluated after mitogen activated protein kinase and phosphatidylinositol 3-kinase (PI3K) inhibitor treatment.
RESULTS: After serum deprivation, IGFBP-5 expression increased both cell number and DNA synthesis in BxPC-3 cells, but reduced cell number in PANC-1 cells. Consistent with this observation, cell cycle analysis of IGFBP-5-expressing cells revealed accelerated cell cycle progression in BxPC-3 and G2/M arrest of PANC-1 cells. Signal transduction analysis revealed that Akt activation was increased in BxPC-3, but reduced in PANC-1 cells that express IGFBP-5. Inhibition of PI3K with LY294002 suppressed extracellular signal-regulated kinase-1 and -2 (ERK1/2) activation in BxPC-3, but enhanced ERK1/2 activation in PANC-1 cells that express IGFBP-5. When MEK1/2 was blocked, Akt activation remained elevated in IGFBP-5 expressing PaC cells; however, inhibition of PI3K or MEK1/2 abrogated IGFBP-5-mediated cell survival.
CONCLUSION: These results indicate that IGFBP-5 expression affects the cell cycle and survival signal pathways and thus it may be an important mediator of PaC cell growth.
Keywords: Insulin-like growth factor-binding protein 5, Extracellular signal-regulated mitogen activated protein kinases, Cyclin-dependent kinase inhibitor p27, Pancreatic neoplasms
INTRODUCTION
Pancreatic cancer (PaC) is the fourth leading cause of cancer deaths in the United States. Annually, the incidence of PaC almost equals the number of deaths related to this disease, and these numbers have begun to increase[1]. Pancreatic tumors are highly chemoresistant, so chemotherapy rarely leads to eradication of the malignancy. Even with chemo- and radiation therapy, median survival for patients who have undergone resection is less than two years[1]. Therefore, a better understanding of aberrant signaling pathways that promote tumor growth is necessary to identify inhibitors to potentially enhance the response of current chemotherapeutics.
Insulin-like growth factors (IGFs) regulate cellular growth, differentiation, and apoptosis[2]. In non-malignant cells, IGF-binding proteins (IGFBPs) modulate the activity of IGF-I and -II, and their affinity for IGFs can be altered by proteolysis, phosphorylation, and adherence to cell-surface or extracellular matrix proteins[3]. Insulin-like growth factor binding proteins (IGFBPs) can also act independently of IGF to either stimulate or inhibit growth of various cell types. Both IGF-I and the IGF-I receptor (IGF-IR) are expressed in pancreatic tumors and cell lines[4–6], and IGFBP-1, -3, and -5 have been associated with pancreatic cancer[7–9].
We found that IGFBP-5 is expressed at higher levels in pancreatic tumors than in non-malignant pancreatic tissues[9]. The role of IGFBP-5 in cancer cell growth and proliferation, however, is not fully understood and has not been studied in pancreatic cancer. In this study, therefore, we investigated the functional significance of IGFBP-5 overexpression in pancreatic cancer by stable transfection of the IGFBP-5 cDNA into two pancreatic cancer cell lines to better represent the heterogeneous genetic background of pancreatic tumors. We examined the effects of IGFBP-5 on cell proliferation and on cell cycle distribution and the status of key cell cycle regulators. We also investigated the mechanism of IGFBP-5-mediated growth effects by assessing the activation status of Akt and extracellular signal-regulated kinase-1 and -2 (ERK1/2) and the effects of inhibition of the phosphatidylinositol 3-kinase (PI3K) and mitogen activated protein kinase (MAPK) pathways after serum deprivation. These studies show that IGFBP-5 can enhance pancreatic cancer cell growth by altering the expression and activity of cell cycle regulators and the activation of key signaling intermediates.
MATERIALS AND METHODS
Cell lines, cloning, and stable transfection
Human pancreatic cancer cell lines BxPC-3 and PANC-1 were obtained from the American Type Culture Collection (Manassas, VA). PANC-1 cells were grown in DMEM and BxPC-3 cells were grown in RPMI 1640, both media were supplemented with 100 mL/L fetal bovine serum.
The full-length cDNA encoding human IGFBP-5 was synthesized by reverse transcription polymerase chain reaction from pancreatic tumor cDNA. The amplified product spanned 822 bp (nt 749-1570) of the published human IGFBP-5 mRNA, covering the start (752) and stop codons (1568) (GenBank, NM_000599). The primers used were as follows: 5'-CACCAAGATGGTGTTGCTC-3' (sense) and 5'-TCACTCAACGTTGCTGCTGTCGAA-3' (antisense). The sense primer included sequences to facilitate TOPO cloning (underlined). The amplified product was cloned into the pENTR/SD TOPO vector (Invitrogen, Carlsbad, CA) and the sequence of the IGFBP-5 insert was confirmed by sequencing.
The full-length human IGFBP-5 cDNA was transferred into the expression vector pIRESpuro3GW[10] using Invitrogen’s Gateway cloning technology and cells were stably transfected using LipofectAMINE (Invitrogen). IGFBP-5 transfectants (/IGFBP-5) and vector controls (/Vec) were selected in medium containing puromycin (2 μg/mL PANC-1 and 1.5 μg/mL BxPC-3). Individual clones were expanded and successful transfection was confirmed by immunoblot analysis of conditioned medium concentrated using Microcon YM 10 filter devices after 24 h growth in serum-free medium (SFM) and detected with α-IGFBP-5 antibodies (R&D Systems, Minneapolis, MN). Two clones were selected per cell line, one that expressed low levels of IGFBP-5 (IGFBP-5L) and one that expressed high levels (IGFBP-5H).
Growth studies
Stable transfectants were seeded (3.5 × 104 cells/well) in 24-well plates in the appropriate growth medium for 24 h. The medium was then removed, cells were washed with phosphate-buffered saline (PBS), and fresh growth medium or SFM was added to the cells. Cells were either cultured continuously in the same medium or SFM changed every 24 h. Growth was assessed based on cell number and [3H]-thymidine incorporation at various times in the above culture conditions.
Cell number
The number of cells in each well was determined by harvesting the cells with trypsin-EDTA solution and counting cells in an aliquot using a Z1 Particle Counter (Beckman-Coulter) in duplicate.
[3H]-thymidine incorporation
At the end of incubations, medium was removed, cells were washed with PBS, and then 2 μCi/mL [3H-methyl]-thymidine in SFM was added for the last 4 h of each growth period at 37°C. After the labeling period, cells were precipitated with ice-cold methanol: acetic acid (3:1, v/v) at 4°C overnight. The cells were washed with a solution of 800 mL/L methanol in water, and DNA was dissolved by adding 0.5 mol/L NaOH (300 μL) for 30 min. Radioactivity contained in 100 μL aliquots was determined by scintillation counting.
Cell cycle analysis
Cells were seeded (3.5 × 105 PANC-1 cells/well or 4 × 105 BxPC-3 cells/well) in 6-well plates in the appropriate growth medium for 24 h. The medium was removed, cells were washed with PBS, and either fresh growth medium or SFM was added. After 48 h, cells were trypsinized and aliquots of 1 × 106 cells were fixed overnight in 700 mL/L ethanol at 4°C. Fixed cells were washed and suspended in 1 mL of 50 μg/mL propidium iodide, 1 mg/mL RNase A, and 1 g/L BSA in PBS for 30 min in the dark at room temperature. Using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA), cell cycle analysis was performed on 10 000 cells for each sample. Quantitation of cell cycle distribution was performed using CellQuest software (Becton Dickinson).
Immunoblot analysis
For immunoblots, equivalent amounts of protein were resolved by SDS-PAGE and blotted onto PVDF membrane (Invitrogen), followed by blocking with 50 g/L nonfat milk powder in 10 mmol/L Tris, pH 7.6, 100 mmol/L NaCl, and 1 mL/L Tween 20. Immunoblots were incubated with the following primary antibodies: α-IGFBP-5 (R&D Systems); α-phospho-ERK1/2, α-ERK1/2, α-phospho-Akt (Ser 473), α-Akt (Cell Signaling Technology, Danvers, MA); α-GAPDH (Ambion, Austin, TX); and α-cyclin D1, α-CDK4, α-cyclin B1, α-cdc2, α-cyclin E, α-CDK2, α-p21, α-p27, α-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Antigen-antibody complexes were detected using the appropriate secondary antibody linked to horseradish peroxidase and visualized using ECL Plus chemiluminescent reagent (Amersham Biosciences, Piscataway, NJ).
Cyclin-dependent kinase assays
CDK activity assays were performed essentially as described previously[11] using histone H1 and Rb773-928 (Upstate Biotechnology) as substrates for CDK2 and CDK4, respectively. Reaction products were resolved by SDS-PAGE and visualized by autoradiography.
Cell viability and proliferation
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Roche, Indianapolis, IN) were performed to evaluate cell growth and viability of IGFBP-5 expressing cell lines after treatment with LY294002 or U0126 (Calbiochem, San Diego, CA). Cells were seeded (0.9 × 104 cells/well PANC-1 and 1 × 104 cells/well BxPC-3) in 96-well plates in the appropriate growth medium. After 24 h the medium was removed, cells were washed with PBS, SFM was added, cells were incubated for 24 h, and inhibitors (LY294002, U0126, or vehicle) were then added to the medium. After 48 h, MTT assays were performed according to the manufacturer’s protocol. The viable cells were expressed as a percentage of the optical absorbance relative to the optical absorbance of the corresponding control or untreated cells.
Analysis of effect of IGFBP-5-conditioned medium on signaling
IGFBP-5 transfected or vector transfected PANC-1 and BxPC-3 cells were seeded in 10 cm plates in the appropriate growth medium. After 24 h the medium was removed, cells were washed with PBS, SFM was added, and cells were incubated for 48 h. Conditioned medium was then collected and centrifuged at 1500 r/min for 10 min. PANC-1 and BxPC-3 cells were seeded in 6-well plates in the appropriate growth medium. After 24 h the medium was removed, cells were washed with PBS, SFM was added, and the cells were incubated for 24 h. PANC-1 and BxPC-3 cells were then treated for 15 min, 24, 48 and 72 h with conditioned medium from IGFBP-5 or vector-transfected controls. Cells were lysed and samples were prepared for immunoblot analysis.
Statistical analysis
Data are expressed as the mean ± SE or a percentage. Statistical analyses of data were performed using a two-way analysis of variance (ANOVA) and Bonferroni post-test (GraphPad Software; San Diego, CA). For data normalized to the vector transfected control, variables were log transformed prior to statistical analysis. P < 0.05 was considered significant.
RESULTS
IGFBP-5 overexpression promotes BxPC-3 cell growth after serum deprivation
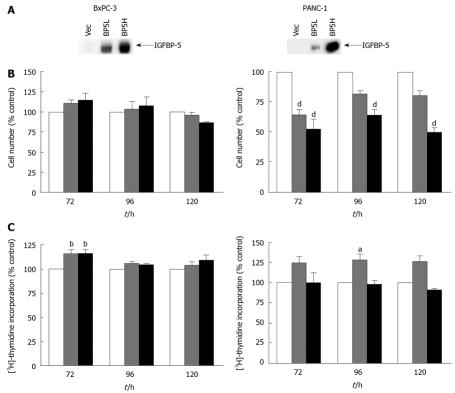
The stable expression of IGFBP-5 in transfected PaC cells was verified by immunoblot analysis after 24 h growth in serum-free conditions and concentration of conditioned medium (Figure 1A). To examine dose-dependent effects and to obviate insertion effects resulting from the generation of the stable transfectants, growth effects were assessed by analyzing cell number and thymidine incorporation using cell lines expressing different levels of IGFBP-5 designated as low (IGFBP-5L) and high (IGFBP-5H). In serum-containing medium, cell numbers were significantly lower in PANC-1 cells expressing IGFBP-5 than in vector transfected control cells (Figure 1B). Although the decrease in PANC-1 cell number corresponded to the increase in IGFBP-5 expression, a similar association in DNA synthesis and IGFBP-5 expression was not observed (Figure 1C). These results suggest that IGFBP-5 inhibits growth of PANC-1 cells cultured in the presence of serum. In contrast, no growth effects were observed in BxPC-3 cells grown in serum-containing medium.
Figure 1.
Effect of IGFBP-5 expression on PaC cells grown in the presence of serum. Stable transfectants (empty vector /Vec-white bar, low /IGFBP-5L-gray bar, high/IGFBP-5H-black bar) were seeded in growth media. (A) IGFBP-5 expression was verified by immunoblot analysis of secreted IGFBP-5 after 24 h growth in SFM and concentration of conditioned medium. Cell counts (B) and thymidine incorporation (C) were determined at 72, 96 and 120 h post-seeding. Data shown represent the mean ± SE of IGFBP-5-transfected cell growth relative to vector-transfected controls (100%) from 3-5 separate experiments performed in duplicate. aP < 0.05, bP < 0.01, dP < 0.001.
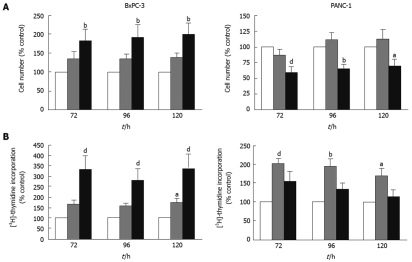
IGFBP-5 has been shown to have both growth factor-dependent and -independent growth and survival effects in both normal and cancer cells[3]. Therefore, we hypothesized that IGFBP-5 may aid growth of PaC cells when the bioavailability of growth factors is low. To investigate this, we examined IGFBP-5-mediated growth effects in the absence of exogenous growth factors. As IGFs are present in FBS, we performed these studies in the absence of serum as has been done previously to elucidate IGF-dependent and -independent effects[5,12–14]. Consistent with our hypothesis, both cell number and DNA synthesis increased significantly in BxPC-3 cells expressing high levels of IGFBP-5 in SFM (Figure 2A and B, respectively). In contrast, high levels of IGFBP-5 significantly reduced the number of PANC-1 cells (Figure 2A). Interestingly, low levels of IGFBP-5 significantly elevated thymidine incorporation in PANC-1 cells, similar to the effects observed in cells grown in serum-containing medium, though the increase was less dramatic in the presence of high levels of IGFBP-5 (Figure 2B). These data suggest that, in the absence of exogenous growth factors, IGFBP-5 promotes growth of BxPC-3 cells but inhibits growth of PANC-1 cells.
Figure 2.
Effect of IGFBP-5 expression on PaC cells grown in the absence of serum. Stable transfectants (empty vector/Vec-white bar, low/IGFBP-5L-gray bar, high/IGFBP-5H-black bar) were seeded in growth media for 24 h then switched to serum-free medium (SFM) for the remainder of the growth period. Cell counts (A) or thymidine incorporation (B) were determined at 72, 96 and 120 h post-seeding. Data shown represent the mean ± SE of IGFBP-5-transfected cell growth relative to vector-transfected controls (100%) from 3-5 separate experiments performed in duplicate. aP < 0.05, bP < 0.01, dP < 0.001.
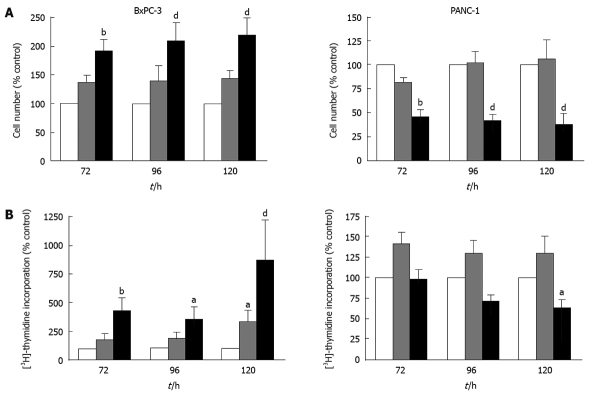
Growth factors and their receptors are upregulated and associated with increased tumorigenicity in pancreatic cancer[15]. Additionally, in PaC cells the overexpression of autocrine growth factors has been shown to allow cell proliferation in the absence of serum[16,17]. In the previous experiments, PaC cells were grown without replenishing SFM for 120 h. Thus, to determine whether IGFBP-5 confers a growth advantage in the absence of endogenously produced growth factors, cells were grown with SFM changed daily. In BxPC-3 cells cultured with frequent medium changes, IGFBP-5 expression was associated with significantly elevated thymidine incorporation and cell number (Figure 3). In contrast, significantly reduced cell numbers were observed in PANC-1 cells expressing high levels of IGFBP-5 grown with frequent changes of SFM (Figure 3A). Therefore, even in the absence of growth factors, IGFBP-5 enhanced BxPC-3 cell proliferation but inhibited PANC-1 cell growth, and these effects were exacerbated by frequent changes of SFM.
Figure 3.
Effect of IGFBP-5 expression on PaC cells grown with daily changes of SFM. Stable transfectants (empty vector/Vec-white bar, low/IGFBP-5L-gray bar, high/IGFBP-5H-black bar) were seeded in growth media for 24 h and switched to SFM, which was replaced every 24 h for the remainder of the growth period. Cell counts (A) and thymidine incorporation (B) were determined at 72, 96 and 120 h post-seeding. Data shown represent the mean ± SE of IGFBP-5-transfected cell growth relative to vector-transfected controls (100%) from 3-5 separate experiments performed in duplicate. aP < 0.05, bP < 0.01, dP < 0.001.
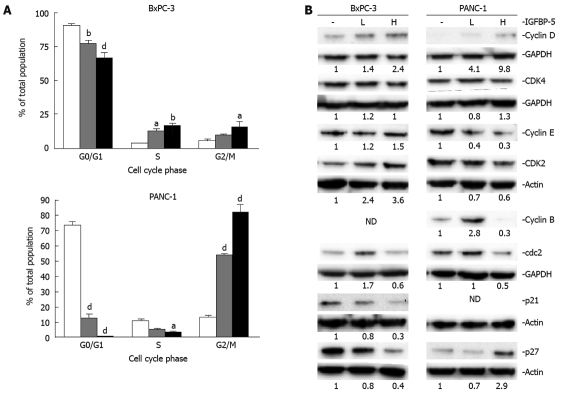
IGFBP-5 expression is associated with cell cycle dysregulation in serum-containing growth conditions
In the growth studies, PANC-1 cells that overexpress IGFBP-5 had lower cell numbers but more DNA synthesis than control cells. These data suggest a possible block in the cell cycle at the G2/M transition. In contrast, BxPC-3 cells expressing high levels of IGFBP-5 exhibited higher cell numbers and thymidine incorporation, indicating accelerated progression through the cell cycle. While IGFBP-5 expression has different effects on growth of the two cell lines, these data indicate that the effects might result from a common overall process-cell cycle dysregulation.
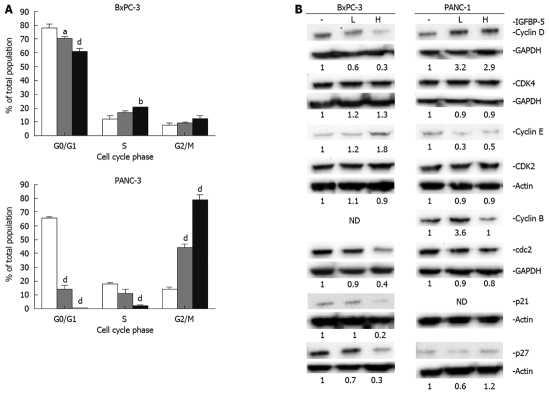
Therefore, we examined the cell cycle distribution and status of key cell cycle regulators of G1/S (cyclin D/CDK4, cyclin E/CDK2, p21Cip1, and p27Kip1) and G2/M (cyclin B/cdc2) transitions in transfected cell lines in the presence (Figure 4) and absence (Figure 5) of serum. The examination of cell cycle distribution by propidium iodide staining and FACS analysis revealed that IGFBP-5 expression in BxPC-3 cells grown in the presence of serum resulted in significantly fewer cells in G1 phase and an increased percentage of cells in S phase (Figure 4A). In contrast, in PANC-1 cells grown in the presence of serum, IGFBP-5 expression was associated with significantly fewer cells in G1 and S phases and an accumulation of cells in G2/M phase (Figure 4A). To identify the cell cycle components responsible for these effects, the expression of key cell cycle regulators was determined in the same growth conditions used for analyzing cell cycle distribution. In BxPC-3 cells, IGFBP-5 overexpression was associated with markedly lower levels of cyclin D, cdc2, p21Cip1, and p27Kip1 but elevated levels of cyclin E (Figure 4B). In contrast, in PANC-1 cells overexpression of IGFBP-5 resulted in elevated levels of cyclin D but reduced levels of cyclin E (Figure 4B). Expression of cyclin B in BxPC-3 and p21 in PANC-1 cells was not detected.
Figure 4.
Cell cycle distribution of PaC cells that overexpress IGFBP-5 grown in the presence of serum. Stable transfectants (empty vector/Vec-white bar, low/IGFBP-5L-gray bar, high /IGFBP-5H-black bar) were seeded in growth media. After 24 h, the medium was removed and replaced with fresh growth media for 48 h. (A) Cell cycle distribution was determined by propidium iodide staining and FACS analysis. Data shown represent the mean ± SE from 3 experiments performed in duplicate. (B) Cell lysates were prepared for immunoblot analysis of cell cycle regulators and their expression was detected using the appropriate antisera. Membranes were subsequently stripped and reprobed for actin or GAPDH as a load control. Analyses of cyclin E/cdk2 and cyclin B/cdc2 expression were performed simultaneously, therefore, only a single load control, actin or GAPDH, respectively, is shown for each pair. Quantitation of band density represents the level of IGFBP-5-associated expression relative to vector-transfected controls (1.0) normalized to the expression level of actin. ND: Not detected. The results are representative of 3 experiments. aP < 0.05, bP < 0.01, dP < 0.001.
Figure 5.
Cell cycle distribution of PaC cells that overexpress IGFBP-5 grown in the absence of serum. Stable transfectants (empty vector/Vec-white bar, low/IGFBP-5L-gray bar, high /IGFBP-5H-black bar) were seeded in growth media. After 24 h, the medium was changed to SFM for 48 h; A: Cell cycle distribution was determined by propidium iodide staining and FACS analysis. Data shown represent the mean ± SE from 3 separate experiments performed in duplicate. B: Cell lysates were prepared for immunoblot analysis of cell cycle regulators and their expression was detected using the appropriate antisera. Membranes were subsequently stripped and reprobed for actin or GAPDH as a load control. Analyses of cyclin E/cdk2 and cyclin B/cdc2 expression were performed simultaneously, therefore, only a single load control, actin or GAPDH, respectively, is shown for each pair. Quantitation of band density represents the level of IGFBP-5 associated expression relative to vector-transfected controls (1.0) normalized to the expression level of actin. ND: Not detected. The results are representative of 3 experiments. aP < 0.05, bP < 0.01, dP < 0.001.
IGFBP-5 expression is associated with cell cycle dysregulation during serum starvation
In the absence of serum, IGFBP-5 expression resulted in marked alterations in cell proliferation, particularly in BxPC-3 cells; therefore, the cell cycle was analyzed in serum-starved cells. Serum deprivation exacerbated the effects of IGFBP-5 expression on cell cycle distribution that were observed in serum-containing growth conditions. In BxPC-3 cells, the percentage of IGFBP-5-expressing cells was reduced in G1 phase but elevated in both S and G2/M phases (Figure 5A). In PANC-1 cells, IGFBP-5 expression was associated with a reduced percentage of cells in G1 and S phases and an increased percentage of cells in G2/M phase (Figure 5A).
To determine whether serum starvation changes expression profiles of cell cycle regulators in cells that express IGFBP-5, we assessed the expression in cells grown in SFM. With IGFBP-5 expression, BxPC-3 cells showed prominent increases in the levels of cyclin D and CDK2, and to a lesser extent, cyclin E, which is consistent with enhanced cell proliferation. However, the levels of the G1/S transition inhibitors p21Cip1 and p27Kip1 were reduced (Figure 5B). IGFBP-5 expression in PANC-1 cells in the absence of serum produced increased levels of p27Kip1 and cyclin D and reduced levels of cyclin E and CDK2 (Figure 5B), which reflects the observed growth arrest and accompanying enhanced thymidine incorporation (Figure 2). Similar to PaC cells grown in the presence of serum, cyclin B expression in BxPC-3 and p21 expression in PANC-1 cells was not detected.
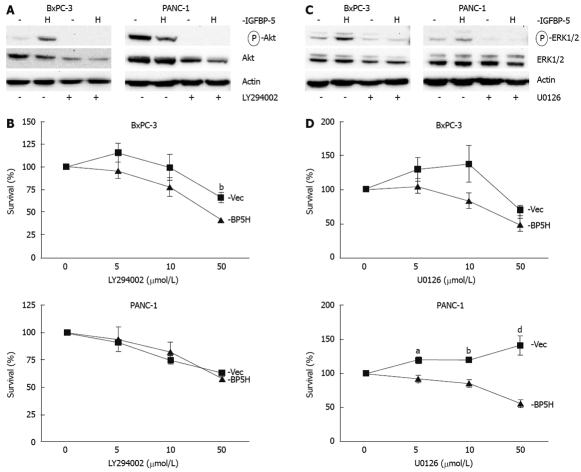
PI3K signaling is required for IGFBP-5-enhanced growth of BxPC-3 cells
To investigate the mechanism of IGFBP-5-enhanced cell growth after serum deprivation, we examined the PI3K and MAPK pathways, which have been shown to enhance proliferation and survival of pancreatic cancer cells[18–21]. Immunoblot analysis of the PI3K signaling intermediate Akt revealed a 3.5-fold increase in the level of phosphorylated Akt in BxPC-3 cells expressing IGFBP-5, but Akt phosphorylation was reduced in PANC-1 cells expressing IGFBP-5 (Figure 6A). When cells were treated with a PI3K inhibitor, LY294002, IGFBP-5 no longer conferred a growth advantage in BxPC-3 cells (Figure 6B). These results indicate that PI3K signaling is necessary for IGFBP-5-enhanced growth of BxPC-3 cells.
Figure 6.
PI3K or MEK1/2 inhibition decreases IGFBP-5-enhanced growth of serum-starved PaC cells. Stable transfectants (empty vector/Vec-closed square or high /BP5H-closed triangle) were seeded in growth media. The serum-containing medium was changed to SFM 24 h post-seeding. After 24 h in serum-free conditions, a PI3K inhibitor (LY294002, A and B) or MEK1/2 inhibitor (U0126, C and D) was added to the medium. After 48 h, an MTT assay (B and D) was performed to assess cell viability. Cell lysates were prepared at this time for immunoblot analysis of Akt or ERK1/2 activation (A and C, respectively) using phospho-specific antibodies. For immunoblots, cells were treated with 25 μmol/L LY294002 or 10 μmol/L U0126, as indicated. Membranes were subsequently stripped and reprobed for total Akt or ERK1/2 (A and C, respectively). Actin level was monitored to normalize protein loading. Data shown represent the mean ± SE from 4 separate experiments performed in triplicate. The percentage of viable cells was determined relative to control-treated cells (100%). aP < 0.05, bP < 0.01, dP < 0.001.
MAPK signaling is required for IGFBP-5-enhanced growth of pancreatic cancer cells
To determine whether IGFBP-5 mediates cell growth via MAPK signaling in PaC cells, the survival studies were repeated with U0126, a MEK1/2 inhibitor. Immunoblot analysis revealed that ERK1/2 activation (phosphorylation) is 2.6-fold greater in BxPC-3 cells expressing IGFBP-5 (Figure 6C) and IGFBP-5-mediated growth is abrogated when the MAPK pathway is blocked with U0126 treatment (Figure 6D). Similarly, ERK1/2 activation is 2-fold greater in PANC-1 cells that express IGFBP-5 (Figure 6C) and significantly fewer IGFBP-5-expressing cells survive when the MAPK pathway is blocked (Figure 6D). These studies suggest that the MAPK pathway is necessary for IGFBP-5-enhanced cell growth after serum deprivation.
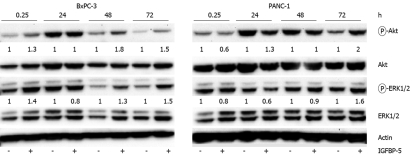
Effect of IGFBP-5-conditioned medium on signaling in PaC cells
To determine whether IGFBP-5 induced changes in Akt and ERK1/2 activation through an autocrine pathway, we applied conditioned medium from IGFBP-5-transfected cells to PaC cells and examined the effect on Akt and ERK1/2 activation. Consistent with our expression studies, BxPC-3 cells treated with IGFBP-5-conditioned medium increased activation of Akt and ERK1/2 compared with cells treated with conditioned medium from vector transfected cells (Figure 7). Similar levels of phosphorylated protein were observed in cells exposed to vector-transfected or IGFBP-5-conditioned medium at 24 h, however, only PaC cells exposed to IGFBP-5-conditioned medium had a prolonged induction of Akt and ERK1/2 activation (72 h).
Figure 7.
IGFBP-5-conditioned medium enhances activation of Akt and ERK1/2 in PaC cells. IGFBP-5- (+) or vector-transfected (-) control cells were grown for 48 h in SFM. Conditioned medium was collected and applied to BxPC-3 or PANC-1 cells for indicated times. Cell lysates were prepared and immunoblot analysis of phosphorylated Akt or ERK1/2 was performed. Membranes were subsequently stripped and reprobed for total Akt or ERK1/2. Actin level was monitored to normalize protein loading. Quantitation of band density represents the level of IGFBP-5-associated expression relative to vector-transfected controls (1.0) normalized to the expression level of total Akt or ERK1/2. Results are representative of three independent experiments.
The effect of IGFBP-5 conditioned medium on ERK1/2 activation in PANC-1 cells was similar to the results observed in cells expressing IGFBP-5. After exposure of PANC-1 cells to IGFBP-5-conditioned medium, Akt and ERK1/2 activation levels were slightly reduced after 15 min, reached control levels by 48 h, and were elevated by 72 h (Figure 7). Interestingly, in PANC-1 cells that express IGFBP-5, activation of Akt was reduced whereas in cells exposed to IGFBP-5-conditioned medium Akt activation was elevated (2 fold). To determine if the IGFBP-5-mediated growth effects observed in PaC cells that express IGFBP-5 could be replicated by application of conditioned medium, PANC-1 and BxPC-3 cell growth was examined. The cell number and level of DNA synthesis observed in cells exposed to IGFBP-5-conditioned medium compared to controls in these growth conditions were not significantly different (data not shown). These results suggest that enhanced activation of Akt and ERK1/2 in IGFBP-5 expressing PaC cells is due to an autocrine pathway.
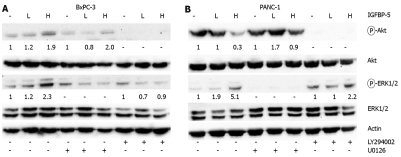
Effect of IGFBP-5 on Ras-mediated cross-talk
After serum deprivation of PANC-1 cells, Akt activation is reduced whereas ERK1/2 activation is elevated in cells expressing IGFBP-5. In BxPC-3 cells that express IGFBP-5, Akt and ERK1/2 phosphorylation is elevated. Previously, it has been shown that Ras- and PI3K-mediated pathways exhibit cross-talk in breast cancer cells[22]. Therefore, we sought to determine whether the differential effects observed between the PaC cells which express wild-type K-Ras (BxPC-3) or harbor mutated K-Ras (PANC-1) were a result of cross-talk between these pathways. Serum-deprived PaC cells were exposed to either U0126 or LY294002 and activation of ERK1/2 and Akt was examined by immunoblot analysis. After MEK1/2 inhibition in serum-starved BxPC-3 cells, Akt activation is decreased, however IGFBP-5 expressing cells maintain a level of Akt activation that is 2-fold greater than vector-transfected cells (Figure 8A). Inhibition of the PI3K pathway suppresses IGFBP-5-enhanced activation of ERK1/2 in serum-deprived BxPC-3 cells. These results suggest that Akt activation is necessary for enhanced activation of ERK1/2 associated with IGFBP-5. In contrast, inhibition of MEK1/2 does not completely block IGFBP-5-enhanced Akt activation, therefore, ERK1/2-independent pathways must contribute to increased Akt activation associated with IGFBP-5.
Figure 8.
Effect of IGFBP-5 on signaling in serum-starved PaC cells. BxPC-3 (A) and PANC-1 (B) stable transfectants [empty vector- (-), low IGFBP-5-L, or high IGFBP-5-H] were deprived of serum for 24 h then incubated in the presence of vehicle (DMSO), LY294002, or U0126 (10 μmol/L) for an additional 48 h. Cell lysates were prepared for immunoblot analysis of Akt or ERK1/2 activation using phospho-specific antibodies. Membranes were subsequently stripped and reprobed for total Akt or ERK1/2. Actin level was monitored to normalize protein loading. Quantitation of band density represents the level of IGFBP-5-associated expression relative to vector-transfected controls (1.0) normalized to the expression level of total Akt or ERK1/2. Results are representative of three independent experiments.
In PANC-1 cells, inhibition of PI3K signaling is associated with elevated levels of ERK1/2 activation in the presence of IGFBP-5 compared to vector controls (Figure 8B). Interestingly, IGFBP-5 is associated with an elevated level of Akt activation (1.7 to 3 fold) when MAPK signaling is blocked compared to that observed in the absence of MAPK inhibition (Figure 8). These results suggest that inhibition of either the MAPK or PI3K pathway in IGFBP-5 expressing PANC-1 cells may shift signaling to the other pathway.
DISCUSSION
Previously, we determined that IGFBP-5 was differentially expressed in pancreatic tumors compared to non-malignant pancreatic tissue[9]. IGFBP-5 has been implicated in cell survival in muscle, prostate cancer, and breast cancer cells[23–25], however, the role of IGFBP-5 in pancreatic cancer has not been investigated. In this study we have examined the effects of IGFBP-5 expression on two cell lines, BxPC-3 and PANC-1, both of which express the IGF-1 receptor and are responsive to IGF-1[26]. Growth of the cell lines in the presence of serum, used as a surrogate for IGF-1 and other growth factors[12–14], revealed that IGFBP-5 reduced PANC-1 cell growth thus opposing the cell proliferative effects of IGF-1 and other growth factors. This result is similar to the growth inhibitory effects of IGFBP-5 observed in breast cancer cells[27].
When tumors reach a certain size, their growth is limited by a lack of available growth factors; therefore, mechanisms that circumvent growth factor deprivation and allow tumor growth are utilized. Consistent with this notion, we found that IGFBP-5 promotes cell growth after serum starvation. In the presence of IGFBP-5 after serum starvation, cell number and DNA synthesis are significantly elevated in BxPC-3 cells, with a clear dose-dependency reflected by increased cell growth with increased IGFBP-5 expression. In contrast, high levels of IGFBP-5 in PANC-1 cells inhibited growth. Interestingly, low levels of IGFBP-5 appeared to enhance DNA synthesis as measured by thymidine incorporation, but this effect was lost with higher IGFBP-5 expression, though the reason for these differences remains unclear. Intriguingly, the growth promoting effects of IGFBP-5 on BxPC-3 cells parallel those observed in prostate cells[24].
The upregulation of oncogenes and downregulation of tumor suppressor genes contributes to the uncontrolled growth and survival of pancreatic cancer cells[28]. To determine if IGFBP-5 overexpression contributes to the dysregulation of pancreatic cancer cell growth, we analyzed cell cycle distribution and found that IGFBP-5 promotes progression through the cell cycle in BxPC-3 cells but leads to a G2/M cell cycle arrest in PANC-1 cells. These results are consistent with observed changes in cell number and DNA synthesis associated with IGFBP-5 expression. Other studies have shown that serum-starved BxPC-3 cells undergo cellular arrest and increased apoptosis. However, after serum stimulation cellular proliferation proceeds and apoptosis is reduced; both responses are dependent on PI3K activation[29]. Our study shows that serum-deprived BxPC-3 cells that express IGFBP-5 continue to proliferate and thus IGFBP-5 may mimic the effect of serum exposure and provide the mitogenic stimulus necessary for tumor growth when the bioavailability of growth factors is low. Thus, in this context IGFBP-5 can act similarly to, and in the absence of, IGF-I to enhance proliferation of pancreatic cancer cells.
Neuroblastoma cells exposed to increasing doses of IGFBP-5 dramatically increased proliferation in the presence of low levels of IGFBP-5 but returned to control levels at a higher dose[30]. For this study, we chose clones which express IGFBP-5 at either a low or high level to determine if IGFBP-5 affects pancreatic cancer cell growth in a dose-dependent manner. Indeed, the effects of IGFBP-5 on the growth of BxPC-3 and PANC-1 cells appeared to be dose-dependent; however, the effect was additive rather than inverse as observed in neuroblastoma cells. Low levels of IGFBP-5 promote progression of BxPC-3 cells through the cell cycle and this effect is exacerbated by high levels of IGFBP-5. PANC-1 cells that expressed low levels of IGFBP-5 had cell numbers similar to the control but two-fold increases in DNA synthesis; whereas cells with high levels of IGFBP-5 had reduced cell numbers but maintained or slightly exceeded control levels of DNA synthesis which may be due to elevated Cyclin D1 levels observed in these conditions (Figure 4). Both low and high levels of IGFBP-5 in PANC-1 cells led to cell cycle arrest at G2/M; however, when cells expressed high levels of IGFBP-5 a greater percentage accumulated in G2/M phase. Overexpression of IGFBP-5 in breast cancer cells also resulted in G2/M cell cycle arrest as well as induction of apoptosis[27], though we did not observe an increase in the percentage of cells with subG1 DNA content.
Our studies show that changes resulting in IGFBP-5-enhanced BxPC-3 cell growth are reflected by elevations in cyclins D and E and accompanying reductions in p21Cip1 and p27Kip1 CDK inhibitors, which may allow BxPC-3 cells that express IGFBP-5 to rapidly progress through the G1/S transition and result in enhanced cell growth observed in cell growth studies. In a survey of pancreatic tumors, CDK2 and CDK4 upregulation was observed to directly correlate with progression of pancreatic cancer; in contrast cyclin E upregulation was observed in 25% of high-grade intraepithelial neoplasia and 75% of ductal carcinoma and cyclin D1 had the lowest frequency of upregulation in late stage disease further demonstrating the deregulation of the G1/S transition in the early stages of PaC[31]. The progressive loss of p27Kip1 expression has been correlated with tumor grade and clinical stage (i.e. positive immunohistochemical staining in well-differentiated, early stage tumors) and thus may be involved in the malignant progression of PaC[32,33]. In contrast to the growth effects of IGFBP-5 observed in BxPC-3 cells, PANC-1 cells expressing IGFBP-5 exhibited reduced levels of cyclin E and CDK2 and an elevated level of p27Kip1, which may explain the distinct G2/M arrest observed for this cell line. This likely protects cells from unfavorable conditions and allows cells to rapidly re-enter the cell cycle when conditions are favorable.
IGFBP-5-mediated growth effects are often associated with activation of the PI3K and MAPK pathways[24,30]. In PaC cells, it has been observed that Akt is constitutively activated at a high level in PANC-1 cells but not in BxPC-3 cells[34], which our study confirms; whereas MAPK activity is not constitutively active in PANC-1 or BxPC-3 cells[5,12]. In this study, overexpression of IGFBP-5 elicited increased phosphorylation of Akt in BxPC-3 cells but reduced phosphorylation in PANC-1. Both cell lines, however, exhibited a modest increase in ERK1/2 phosphorylation with IGFBP-5 expression. Upon inhibition of either the PI3K or MAPK pathway, IGFBP-5 no longer enhanced growth of BxPC-3 or PANC-1 cells after serum deprivation. These data suggest, therefore, that IGFBP-5 promotes cell growth in BxPC-3 cells by enhancing PI3K-Akt signaling. Consistent with our findings, a conditionally active form of Akt has been shown to transform cells by promoting progression through S and G2/M phases of the cell cycle, decreasing expression of p27Kip1, and increasing activity of CDK2[35], similar to our observations in BxPC-3 cells overexpressing IGFBP-5.
In this study, we have demonstrated that in BxPC-3 cells IGFBP-5 promotes proliferation via activation of the PI3K pathway; whereas it promotes growth arrest via activation of the MAPK pathway in PANC-1 cells. Although both cell lines employed in these studies were derived from pancreatic adenocarcinomas, they represent different genetic backgrounds and reflect differences observed in human tumors. For example, both cell lines express the IGF-1R and are responsive to IGF-1[26], however, BxPC-3 cells have wild-type K-ras and express lower levels of PTEN mRNA while PANC-1 cells express higher levels of PTEN mRNA and possess an activating K-ras mutation. Thus, while the opposing effects of IGFBP-5 on these two cell lines may not be satisfying to those expecting a uniform response from cells originating from the same tumor type, these results highlight the clinical challenge of treating a disease that does not actually represent a single entity. Tumors arising from the same organ may exhibit a great diversity in the signal transduction pathways they utilize and treatments will continue to fail if they do not account for these differences. In both cell lines employed in this study the predominant pathway stimulated by IGFBP-5 (i.e. Akt in BxPC-3 and ERK1/2 in PANC-1) is maintained, although at a lower level, in the presence of pharmacological inhibition of the other pathway, which may represent a novel mechanism by which IGFBP-5 promotes PaC cell growth and survival.
Pancreatic tumors are highly chemoresistant, thus treatment rarely leads to eradication of the malignancy. Since tumors are comprised of cells with heterogeneous genetic backgrounds they often do not respond uniformly to any specific treatment. This cellular complexity is underscored by the divergent characteristics of the two PaC cell lines used in our study. Our results, therefore, highlight the need to identify multiple chemotherapeutic targets for treating pancreatic cancer. Further studies aimed at identifying signaling intermediates involved in IGFBP-5-enhanced cell growth and survival within the diverse cellular population of pancreatic tumors may therefore elucidate potentially important therapeutic targets.
COMMENTS
Background
Pancreatic cancer (PaC) is the fourth leading cause of cancer deaths in the United States. Annually, the incidence of PaC almost equals the number of deaths related to this disease, and these numbers have begun to increase. Previously, insulin-like growth factor binding protein-5 (IGFBP-5) was demonstrated to be differentially expressed in pancreatic tumors compared to non-malignant pancreatic tissue. IGFBP-5 has been implicated in cell survival in muscle, prostate cancer, and breast cancer cells; however, the role of IGFBP-5 in pancreatic cancer has not been investigated.
Research frontiers
IGFPB-5 is expressed at higher levels in pancreatic tumors than in non-malignant pancreatic tissues. The role of IGFBP-5 in cancer cell growth and proliferation, however, is not fully understood and has not been studied in pancreatic cancer. In this study, the authors demonstrate that IGFBP-5 can enhance pancreatic cancer cell growth by altering the expression and activity of cell cycle regulators and the activation of key signaling intermediates.
Innovations and breakthroughs
In this study, the effects of IGFBP-5 expression were examined on two cell lines, BxPC-3 and PANC-1, both of which express the IGF-I receptor and are responsive to IGF-I. In BxPC-3 cells IGFBP-5 promotes proliferation via activation of the PI3K pathway; whereas it promotes growth arrest via activation of the MAPK pathway in PANC-1 cells. The opposing effects of IGFBP-5 on these two cell lines highlight the clinical challenge of treating a disease that does not actually represent a single entity. Tumors arising from the same organ may exhibit a great diversity in the signal transduction pathways they utilize and treatments will continue to fail if they do not account for these differences.
Applications
Pancreatic tumors are highly chemoresistant, thus treatment rarely leads to eradication of the malignancy. Since tumors are comprised of cells with heterogeneous genetic backgrounds they often do not respond uniformly to any specific treatment. The results of this study highlight the need to identify multiple chemotherapeutic targets for treating pancreatic cancer. Further studies aimed at identifying signaling intermediates involved in IGFBP-5-enhanced cell growth and survival within the diverse cellular population of pancreatic tumors may therefore elucidate potentially important therapeutic targets.
Terminology
Insulin-like growth factors (IGFs) regulate cellular growth, differentiation, and apoptosis and IGF-binding proteins (IGFBPs) modulate the activity of IGFs. IGFBPs can also act independently of IGF to either stimulate or inhibit growth of various cell types. Both IGF-I and the IGF-I receptor (IGF-IR) are expressed in pancreatic tumors and cell lines, and IGFBP-1, -3, and -5 have been associated with pancreatic cancer.
Peer review
The authors investigate the functional significance of IGFBP-5 overexpression in pancreatic cancer. The manuscript is interesting, with some novel aspects of the IGFBP-5 biology in pancreatic cancer cells disclosed.
Acknowledgments
We thank Eric Siegel for helpful discussions regarding the statistical analyses.
Supported by A grant from the Arkansas Master Tobacco Settlement and Arkansas Biosciences Institute
Peer reviewer: Carlos J Pirola, PhD, FAHA, Instituto de Investigaciones Medicas A Lanari, Combatientes de Malvinas 3150, Buenos Aires-1427, Argentina
S- Editor Li LF L- Editor O'Neill M E- Editor Yin DH
References
- 1.Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642–1654. doi: 10.1053/j.gastro.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–2011. [PubMed] [Google Scholar]
- 5.Nair PN, De Armond DT, Adamo ML, Strodel WE, Freeman JW. Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2. Oncogene. 2001;20:8203–8214. doi: 10.1038/sj.onc.1205044. [DOI] [PubMed] [Google Scholar]
- 6.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–1011. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karna E, Surazynski A, Orłowski K, Łaszkiewicz J, Puchalski Z, Nawrat P, Pałka J. Serum and tissue level of insulin-like growth factor-I (IGF-I) and IGF-I binding proteins as an index of pancreatitis and pancreatic cancer. Int J Exp Pathol. 2002;83:239–245. doi: 10.1046/j.1365-2613.2002.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, Kawamura T, Inaba Y, Kurosawa M, Motohashi Y, et al. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer. 2004;110:584–588. doi: 10.1002/ijc.20147. [DOI] [PubMed] [Google Scholar]
- 9.Johnson SK, Dennis RA, Barone GW, Lamps LW, Haun RS. Differential expression of insulin-like growth factor binding protein-5 in pancreatic adenocarcinomas: identification using DNA microarray. Mol Carcinog. 2006;45:814–827. doi: 10.1002/mc.20203. [DOI] [PubMed] [Google Scholar]
- 10.Herzog C, Kaushal GP, Haun RS. Generation of biologically active interleukin-1beta by meprin B. Cytokine. 2005;31:394–403. doi: 10.1016/j.cyto.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian S, Chandraratna RA, Eckert RL. A novel retinoid-related molecule inhibits pancreatic cancer cell proliferation by a retinoid receptor independent mechanism via suppression of cell cycle regulatory protein function and induction of caspase-associated apoptosis. Oncogene. 2005;24:4257–4270. doi: 10.1038/sj.onc.1208586. [DOI] [PubMed] [Google Scholar]
- 12.Giehl K, Skripczynski B, Mansard A, Menke A, Gierschik P. Growth factor-dependent activation of the Ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene. 2000;19:2930–2942. doi: 10.1038/sj.onc.1203612. [DOI] [PubMed] [Google Scholar]
- 13.Levitt RJ, Pollak M. Insulin-like growth factor-I antagonizes the antiproliferative effects of cyclooxygenase-2 inhibitors on BxPC-3 pancreatic cancer cells. Cancer Res. 2002;62:7372–7376. [PubMed] [Google Scholar]
- 14.Murphy LO, Cluck MW, Lovas S, Otvös F, Murphy RF, Schally AV, Permert J, Larsson J, Knezetic JA, Adrian TE. Pancreatic cancer cells require an EGF receptor-mediated autocrine pathway for proliferation in serum-free conditions. Br J Cancer. 2001;84:926–935. doi: 10.1054/bjoc.2001.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansel DE, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Annu Rev Genomics Hum Genet. 2003;4:237–256. doi: 10.1146/annurev.genom.4.070802.110341. [DOI] [PubMed] [Google Scholar]
- 16.Murphy LO, Abdel-Wahab YH, Wang QJ, Knezetic JA, Permnert J, Larsson J, Hollingsworth AM, Adrian TE. Receptors and ligands for autocrine growth pathways are up-regulated when pancreatic cancer cells are adapted to serum-free culture. Pancreas. 2001;22:293–298. doi: 10.1097/00006676-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 17.DeArmond D, Brattain MG, Jessup JM, Kreisberg J, Malik S, Zhao S, Freeman JW. Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene. 2003;22:7781–7795. doi: 10.1038/sj.onc.1206966. [DOI] [PubMed] [Google Scholar]
- 18.Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3'-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28:353–358. doi: 10.1097/00006676-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- 20.Gysin S, Lee SH, Dean NM, McMahon M. Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 2005;65:4870–4880. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]
- 21.Yip-Schneider MT, Schmidt CM. MEK inhibition of pancreatic carcinoma cells by U0126 and its effect in combination with sulindac. Pancreas. 2003;27:337–344. doi: 10.1097/00006676-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 23.Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J Cell Physiol. 2000;183:330–337. doi: 10.1002/(SICI)1097-4652(200006)183:3<330::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Miyake H, Nelson C, Rennie PS, Gleave ME. Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3'-kinase pathway. Endocrinology. 2000;141:2257–2265. doi: 10.1210/endo.141.6.7520. [DOI] [PubMed] [Google Scholar]
- 25.Perks CM, McCaig C, Holly JM. Differential insulin-like growth factor (IGF)-independent interactions of IGF binding protein-3 and IGF binding protein-5 on apoptosis in human breast cancer cells. Involvement of the mitochondria. J Cell Biochem. 2000;80:248–258. doi: 10.1002/1097-4644(20010201)80:2<248::aid-jcb140>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Takeyama H. IGF-1 Mediates PTEN Suppression and Enhances Cell Invasion and Proliferation via Activation of the IGF-1/PI3K/Akt Signaling Pathway in Pancreatic Cancer Cells. J Surg Res. 2008;80:Epub ahead of print. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Butt AJ, Dickson KA, McDougall F, Baxter RC. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278:29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 28.Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 29.Perugini RA, McDade TP, Vittimberga FJ Jr, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 30.Tanno B, Negroni A, Vitali R, Pirozzoli MC, Cesi V, Mancini C, Calabretta B, Raschellà G. Expression of insulin-like growth factor-binding protein 5 in neuroblastoma cells is regulated at the transcriptional level by c-Myb and B-Myb via direct and indirect mechanisms. J Biol Chem. 2002;277:23172–23180. doi: 10.1074/jbc.M200141200. [DOI] [PubMed] [Google Scholar]
- 31.Al-Aynati MM, Radulovich N, Ho J, Tsao MS. Overexpression of G1-S cyclins and cyclin-dependent kinases during multistage human pancreatic duct cell carcinogenesis. Clin Cancer Res. 2004;10:6598–6605. doi: 10.1158/1078-0432.CCR-04-0524. [DOI] [PubMed] [Google Scholar]
- 32.Hu YX, Watanabe H, Li P, Wang Y, Ohtsubo K, Yamaguchi Y, Sawabu N. An immunohistochemical analysis of p27 expression in human pancreatic carcinomas. Pancreas. 2000;21:226–230. doi: 10.1097/00006676-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Culhaci N, Sagol O, Karademir S, Astarcioglu H, Astarcioglu I, Soyturk M, Oztop I, Obuz F. Expression of transforming growth factor-beta-1 and p27Kip1 in pancreatic adenocarcinomas: relation with cell-cycle-associated proteins and clinicopathologic characteristics. BMC Cancer. 2005;5:98. doi: 10.1186/1471-2407-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–997. [PubMed] [Google Scholar]
- 35.Mirza AM, Kohn AD, Roth RA, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]