Abstract
The intestinal tract is in intimate contact with the commensal microflora. Nevertheless, how commensals communicate with cells to ensure immune homeostasis is still unclear. In this study, we found that gut flora DNA (gfDNA) plays a major role in intestinal homeostasis through toll like receptor 9 engagement. TLR9 deficient mice displayed increased frequencies of CD4+Foxp3+ regulatory T cells (Treg) within intestinal effector sites, and reduced constitutive IL-17 and IFN-γ producing effector T cells (Teff). Complementing this, gfDNA limited lamina propria dendritic cells induced Treg conversion in vitro. Further, Treg/Teff disequilibrium in TLR9−/− mice led to impaired immune responses to oral infection and to oral vaccination, which were rescued through neutralization of Treg. Impaired intestinal immune responses were recapitulated in mice treated with antibiotics and were reversible following reconstitution with gfDNA. Together these data point to gfDNA as a natural adjuvant for priming intestinal responses via modulation of Treg/Teff equilibrium.
Immune reactivity against intestinal flora or dietary antigen poses a significant risk to the host and can lead to severe tissue damage (Izcue et al., 2006). To avoid this consequence, the host has a multitude of complementary regulatory mechanisms in place. Nevertheless, inability to overcome these regulatory mechanisms in the face of an invasive pathogen may compromise effective immunity. Lending weight to this paradox is the fact that commensal and pathogenic microbes interact with the host immune system through similar conserved ligands that are cardinal features of microorganisms (Sansonetti and Di Santo, 2007). Many of these ligands signal through the toll like family of receptors (TLR) (Sansonetti and Di Santo, 2007). TLRs are widely expressed by cells of hematopoietic origin, as well as non-hematopoietic cells, including the epithelial cells lining the intestinal tract (Takeda et al., 2003). The initial identification of TLRs in the splenic and peripheral blood leukocyte compartments paved our understanding of how engagement of these molecules by invasive pathogens can activate inflammatory cascades that initiate adaptive immunity (Medzhitov et al., 1997). However, mucosal tissues via their interaction with commensals are the only environments in constant contact with TLR ligands. Yet, the purpose of TLR signaling by the commensal flora has only recently begun to be elucidated. For example, it is now clear that TLR signaling in the intestinal epithelial compartment is crucially involved in the maintenance of intestinal homeostasis and tissue repair (Rakoff-Nahoum et al., 2004). Further, these signals also positively regulate the sampling of luminal contents by DC from the underlying lamina propria compartment (Chieppa et al., 2006). Commensal floral interactions with TLRs have also been shown to mediate tolerance to food antigens (Bashir et al., 2004).
CD4+ T cells of the Foxp3+ regulatory lineage (Treg) also mediate intestinal homeostasis. Their absence or failure to properly patrol the gut associated lymphoid tissues (GALT) leads to reactivity against the commensal flora and subsequent colitis (Izcue et al., 2006). We and others have shown that naïve T cells can become Foxp3+ Treg following oral exposure to antigen (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). While thymically derived Treg are essential for this protection, converted Treg formed in the GALT following naïve CD4+ T cell encounter with antigen may provide additional protection. This process was associated with the capacity of GALT APCs to generate Treg via a mechanism that, in addition to TGF-β, is dependent on the Vitamin A metabolite retinoic acid (Coombes et al., 2007; Denning et al., 2007; Sun et al., 2007).
Previous work has demonstrated that TLR signaling can influence both the function and expansion of Treg (Sutmuller et al., 2006b). In some instances this control is exerted via direct TLR engagement on Treg, while in others it occurs through Teff and/or APC stimulation. While some interactions have been proposed to increase Treg activity, including TLRs 4 and 5 (Caramalho et al., 2003; Crellin et al., 2005), others involving TLRs 2, 8 and 9 have been shown to limit Treg function (Liu et al., 2006; Pasare and Medzhitov, 2003; Peng et al., 2005; Sutmuller et al., 2006a). TLR stimulation of Treg may also influence their proliferation (Liu et al., 2006; Sutmuller et al., 2006a). Putting these elements together, the constant exposure of the intestinal immune system to the flora provides a rationale to consider the physiological impact of TLR signaling on the presence and function of Treg in the GALT. TLR9 recognizes unmethylated cytosine phosphate guanosine (CpG) dinucleotides, which are abundant in prokaryotic DNA found in intestinal flora. Using synthesized sequences containing CpG, previous studies have shown that engagement of TLR9 expressed on not just DCs, but also Treg and Teff can limit Treg suppressive function (Larosa et al., 2007; Pasare and Medzhitov, 2003). We have recently demonstrated that the presence of CpG at the time of Leishmania infection can limit accumulation of Treg in the infected dermis (Wu et al., 2006). Previous work identified an association between Crohn’s disease and a promotor polymorphism in the TLR9 gene in humans (Torok et al., 2004). Such association supports the idea of a role for gut floral DNA (gfDNA) sensing in the pathophysiology of IBD. However, whether host interaction between ligands expressed on gfDNA and TLR9 can influence Treg and subsequently local immune responses in the gut is unknown.
In the present study, we investigated how constitutive interaction between gfDNA and TLR9 in the gut would influence intestinal immune responses. This was addressed using a model of oral infection, microsporidia, and a model of oral vaccination, OVA, with the mutant form of Escherichia coli labile toxin (LT) as mucosal adjuvant (Chong et al., 1998). We found that in intestinal tissues, gfDNA acts as a natural immunological adjuvant and critically controls the balance between Treg and Teff cell frequency and function. This reveals a previously unidentified aspect of gut flora in the regulation of mucosal immunity. Such control appears to be essential for development of effective immune responses against invading microorganisms as well as mucosally administered vaccines and correlates with restricted peripheral Treg conversion.
RESULTS
TLR9 signaling regulates Treg/Teff ratio in intestinal tissues
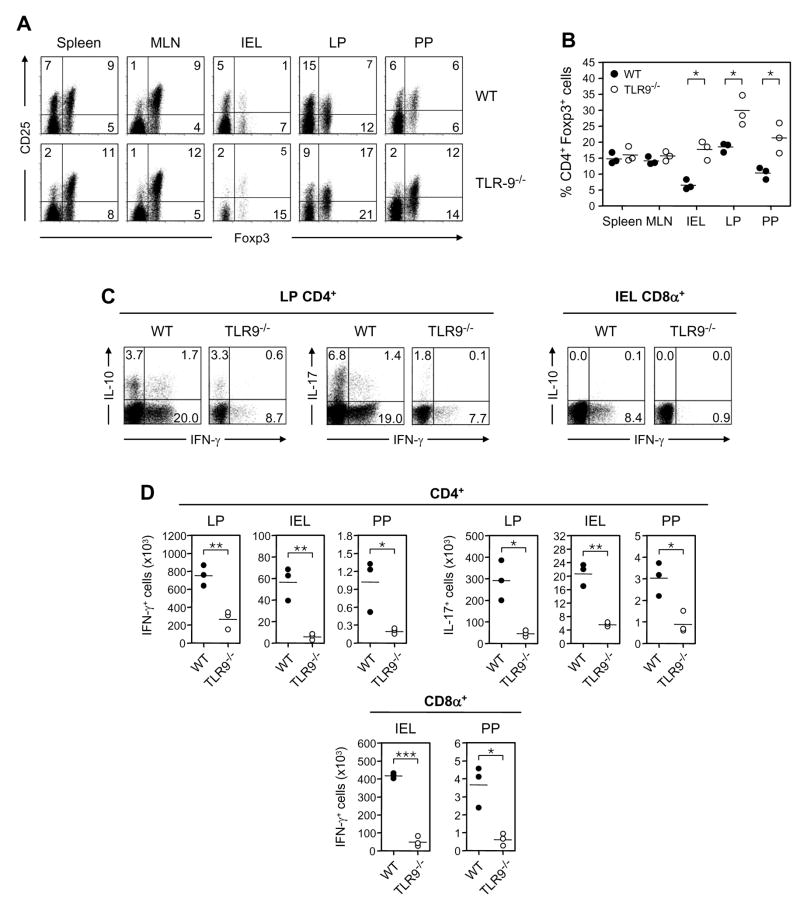
To address the role of TLR9 signaling in CD4+Foxp3+ (Treg) homeostasis in the GALT we first Treg populations in the peripheral tissues and the GI tract of TLR9−/− and WT mice raised compared in the same animal facilities. With the exception of the mesenteric lymph nodes (MLN), naïve TLR9−/− mice had higher percentages and absolute numbers of Treg than their WT counterparts in most of the GALT, including the intestinal epithelium lymphocyte (IEL), small intestinal lamina propria (LP), and Peyer’s patch (PP) compartments (Fig. 1, A and B and Fig S1A). These differences held in mice co-housed in the same cage; moreover, differences were restricted to the small intestine compartment as Treg frequencies were similar in the spleen, MLN and the large intestine lamina propria of WT and TLR9−/− mice (Fig 1A, B and Fig S1B). These elevated Treg frequencies did not appear to be caused by an ongoing reduction in effector T cell (Teff) proliferation compared to Treg proliferation in TLR9−/− mice as assessed by staining for the nuclear proliferation antigen, Ki-67 (Fig. S1C). We did not observe this alteration in Foxp3+ expression patterns in either TLR4−/− or TLR2−/− mice (data not shown).
Figure 1. TLR9 signaling regulates Treg frequency and steady-state Teff cytokine production in gut associated lymphoid tissue of uninfected mice.
(A)Comparative assessment of CD4+Foxp3+ Treg in spleen, mesenteric lymph node (MLN), intestinal epithelium lymphocyte (IEL), intestinal lamina propria (LP) and Peyer’s patch (PP) compartments in age-matched naïve WT and TLR9−/− mice. CD4+TCR-β+-gated cells were analyzed for expression of Foxp3 and CD25 by flow cytometry. Numbers in quadrants refer to the percentage of each subset. (B) Treg percentages in naïve WT (●) and TLR9−/− (○) mice. Each dot represents the results from one experiment (three mice pooled per group) and crossbars depict the mean of three independent experiments (*, p<0.05 compared with WT mice). (C) Loss of TLR9 reduces the basal frequency of IL-17 and IFN-γ producing CD4+ T cells in the LP and IFN-γ producing CD8α+ T cells in the IEL compartments. Numbers in quadrants refer to the percentage of each subset. (D) Absolute numbers of CD4+ and/or CD8α+ T lymphocytes producing IFN-γ and IL-17 in naïve WT (●) and TLR9−/− (○) mice. Each dot represents one mouse and each bar the mean of three mice analyzed (*, p<0.05; **, p<0.01; ***, p<0.001 compared with WT mice). For C and D, data shown are representative of two independent experiments with similar results.
The enhanced Treg frequencies found in the effector GALT sites of TLR9−/− mice indicated that basal cytokine production by Teff might also be affected in these tissues. Since most conventional αβ T cells are CD4+ in the LP and CD8+ in the IEL, we were specifically interested in cytokine production by these two subsets. TLR9 and WT mice have similar numbers of CD4 and CD8+T cells in these respective compartments (data not shown). Within the LP, we noted a significant reduction in the percentage and absolute number (Fig. 1C and D) of CD4+ T cells producing IFN-γ and/or IL-17 in the LP of TLR9−/− mice. IL-10 production was less affected (Fig 1C). Similarly, within the IEL, the proportion and absolute number of CD8+ T cells producing IFN-γ was dramatically reduced in TLR9−/− mice (Fig. 1C and D). Thus, in the absence of TLR9 signaling, a tissue specific disequilibrium of T cell subsets in the gut emerges, in which Treg frequencies are augmented and constitutive inflammatory cytokine production by Teff is reduced.
TLR9 signaling is requisite for optimal responses to oral infection and vaccination
We reasoned that the concomitant gain of Treg and loss of cytokine producing Teff in the gut tissues of TLR9−/− mice could influence their ability to mount protective immune responses during gastrointestinal infection. Microsporidia are obligate intracellular parasites capable of infecting a wide range of hosts through the alimentary tract (Didier, 2005). Infection with the species, Encephalitozoon cuniculi, (E. cuniculi), produces a robust IFN-γ response in mice that is required for protection against oral as well as intraperitoneal (IP) challenge (Khan and Moretto, 1999; Moretto et al., 2004). To address this possibility, we took advantage of E. cuniculi’s ability to induce productive immune responses through both i.p. and oral routes of infection. TLR9−/− mice infected i.p. with E. cuniculi mounted protective responses that were comparable to WT cohorts, based on ELISA measurement of IFN-γ secretion during antigen recall and parasite burden 11 days post infection (p.i.) (Fig 2A and B). Thus, recognition by TLR9 is not required for peripheral immunity to the parasite.
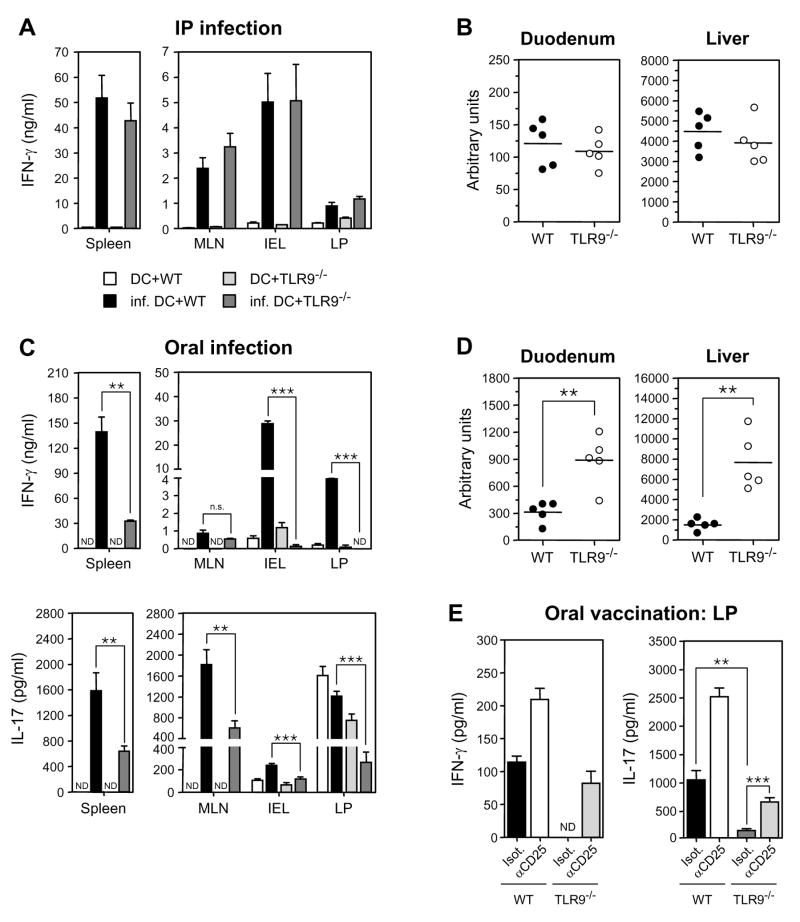
Figure 2. TLR9−/− mice mount impaired protective responses to E. cuniculi following oral infection and respond poorly to oral vaccination.
WT and TLR9−/− mice were infected with E. cuniculi spores i.p. (A–B) or through oral route (C–D). (A and C) ELISA of IFN-γ from i.p. infected and IFN-γ and IL-17 from orally infected mice in supernatants of bulk leukocyte preparations from 11 d post-infected WT and TLR9−/− mice restimulated with uninfected (DC) or E. cuniculi-infected BMDC (inf. DC). (B and D) Parasite loads are increased in orally infected TLR9−/− mice. Parasite loads were measured in the duodenum and liver of i.p. (B) or orally (D) infected mice 11 d post-infection by quantitative real-time PCR. Data shown are representative of three independent experiments with five mice per group (**, p<0.01). (E) WT and TLR9−/− mice were orally immunized with a mixture of OVA and the mutant E. coli LT(R129G) on d 0 and d 7, and treated with anti-CD25 mAb or isotype control antibody. On d 14, IFN-γ and IL-17 secretion by LP cells were evaluated by ELISA after in vitro restimulation with BMDC infected with recombinant vaccinia virus expressing OVA. For A, C and E, histograms represent the mean cytokine concentration of triplicate wells ± SD and are representative of at least two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001;.n.s., non significant; ND, not detected).
Conversely, immune responses in orally infected TLR9−/− mice were severely impaired when compared to WT mice (Fig 2C and D). Recall responses revealed that antigen specific IFN-γ production was severely impaired in the IEL and LP of orally infected TLR9−/− mice (Fig 2C). Moreover, whereas restimulation of splenocytes from WT mice resulted in large secretion of IFN-γ the amount of IFN-γ secreted by splenocytes from TLR9−/− mice was reduced 4-fold (Fig 2C). IL-17 secretion was also significantly impaired in all tested tissues of infected TLR9−/− mice (Fig 2C). In accord with a defect in pro-inflammatory cytokine secretion, parasite burdens were significantly higher in orally infected TLR9−/− than in WT cohorts, both in primary site (duodenum) and dissemination site (liver) (Fig 2D). This difference was associated with higher systemic levels of IL-10 and IL-4 (Fig S1D and S1E), though, we did not observe any bias towards a Th2 response in the GALT (Fig S1E). The increased Treg frequency within the IEL, LP and PP persisted in orally infected TLR9−/− mice when assessed on d 11 p.i. (Fig S1F). Thus, these data support the idea that augmented gut Treg/Teff ratios in TLR9−/− mice and/or defects in Teff priming may predispose them to impaired mucosal immune responses during oral challenges.
To further probe the impaired intestinal immune responses in TLR9−/− mice, we employed an oral vaccination model in which mice were gavaged with a mixture of OVA and the mucosal adjuvant, LT(R129G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli (Chong et al., 1998). Following immunization, single cell suspensions of tissues were incubated with DCs infected with vaccina virus expressing OVA or loaded with OVA peptide. As was the case with oral infection by E. cuniculi, markedly reduced levels of both IFN-γ and IL-17 were found in supernatants from the IEL and LP of TLR9−/− mice in comparison to WT animals (Fig 2E, Fig S2 and Fig S1G). To determine the contribution of Treg to this phenotype, mice were treated with anti-CD25 (α-CD25) prior to immunization. As expected, α-CD25 treatment in WT mice enhanced IFN-γ and IL-17 production (Fig 2E). Importantly, α-CD25 treatment in TLR9−/− mice restored IFN-γ secretion to WT levels, whereas treatment with isotype control yielded no increase. IL-17 secretion also rebounded to WT levels in the LP of α-CD25 treated TLR9−/− mice. Similar results were observed when TLR9−/− mice received α-CD25 prior to oral infection with E. cuniculi. Parasite control was also significantly increased in the duodenum and liver of TLR9−/− mice treated with α-CD25 compared to isotype control (Fig S3A, B). Though α-CD25 treatment increased cytokine production to WT levels, these levels were still reduced relative to WT mice treated with α-CD25. This could suggest that in addition to increased Treg frequencies, impaired effector responses may account for the effect observed in absence of TLR9 signaling. On the other hand, because a large fraction of Foxp3 positive cells do not express CD25, antibody treatment is only partially efficient at reducing Foxp3 numbers and/or function in the GI tract (Fig S4). Taken together, these data suggest that the elevated proportion of Treg in TLR9−/− mice contributes to their impaired ability to respond to both oral infection with E. cuniculi and mucosal vaccination.
TLR9 signaling in the hematopoietic compartment negatively regulates intestinal Treg frequencies and promotes mucosal effector responses
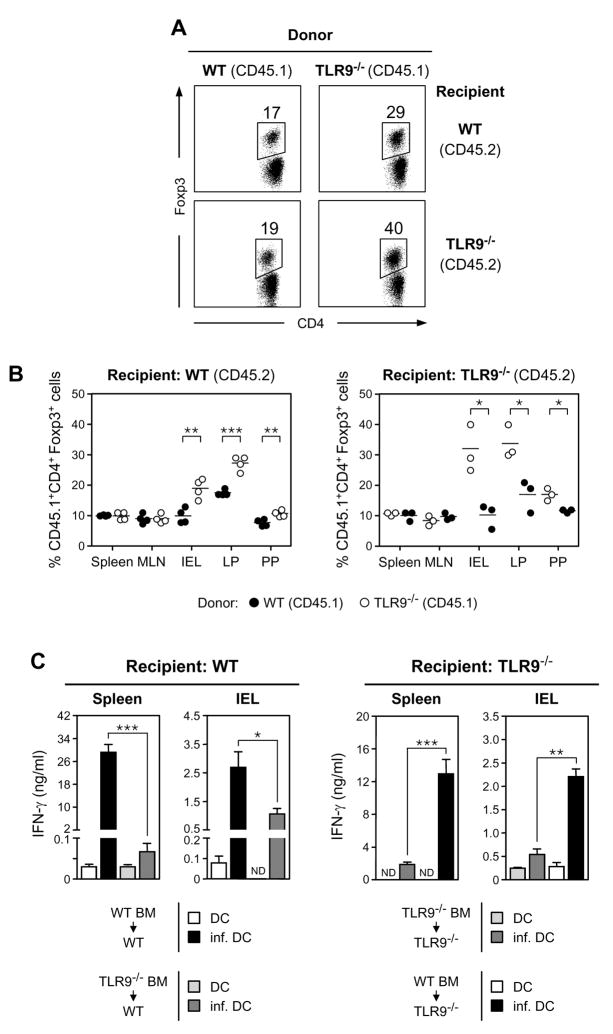
TLR9 is expressed in several radiosensitive hematopoietic lineages found within small intestinal tissue and heavily expressed on the surface of the intestinal epithelia derived from radioresistant stromal cells (Lee et al., 2006). Although our data demonstrate that TLR9 expression is required for intestinal mucosal responses, we wanted to better address the cellular compartment required for immune-sensitization. To pursue this question, bone marrow (BM) chimeric mice were generated so that TLR9 expression in the intestinal tract was predominantly restricted to the epithelia or to hematopoietic lineages and that the origin of both compartments can be tracked based on the expression of congenic markers. At 8 weeks post reconstitution, Treg frequencies were elevated in the TLR9−/− BM compartment relative to the WT BM compartment in reconstituted hosts (Fig 3A, B). Conversely, Treg frequencies were reduced in the WT BM compartment relative to the TLR9−/− BM compartment in reconstituted hosts. (Fig 3A, B). Importantly these changes were restricted to the gut compartment recapitulating the site-specific dysregulation of Treg frequencies in TLR9−/− mice. When irradiated WT congenic mice were reconstituted with TLR9−/− BM, resulting in the absence of TLR9 on most hematopoietic cells, the IFN-γ recall response to oral infection with E. cuniculi was severely impaired and parasite load increased in the duodenum and liver (Fig 3C, Fig S5). In contrast, when TLR9 deficiency was mostly restricted to the intestinal epithelia in TLR9−/− mice reconstituted with WT BM, the IFN-γ recall response improved significantly following oral infection with E. cuniculi and parasite control was enhanced (Fig 3C, Fig S5). Notably, the reciprocal chimeras (WT → TLR9−/− and TLR9−/− → WT) resulted in an intermediate phenotype, in terms of parasite burden. While we cannot rule out a role for non-hematopoietic cells in regulating effector priming, it is important to note that chimerism was less complete in GI tissues than peripheral lymph nodes (less than 40 % versus above 80% chimerism, data not shown). These findings indicate that TLR9 engagement by hematopoietic derived cells is sufficient for modulation of Treg frequencies and priming the gut for immune responsiveness to oral infection with E. cuniculi.
Figure 3. Expression of TLR9 by hematopoietic cells is sufficient to negatively modulate levels of Treg and positively favors immune responses in the GALT.
Levels of Foxp3+ Treg at homeostasis (A and B) and immune responses against E. cuniculi at 11 d after oral infection (C) were analyzed in BM chimeric mice, in which the hematopoietic or the nonhematopoietic compartment lacks TLR9 expression. (A) Comparative assessment of CD4+Foxp3+ Treg in the intestinal lamina propria (LP) of naïve irradiated WT and TLR9−/− mice reconstituted with BM cells from WT or TLR9−/− congenic mice. Percentages of CD4+TCR-β+Foxp3+ were evaluated in the donor (CD45.1+) compartment. (B) Foxp3+ Treg percentages in naive irradiated WT or TLR9−/− mice reconstituted with BM cells from WT (●) or TLR9−/− (○) congenic mice. Percentages of CD4+TCR-β+Foxp3+ were evaluated in the donor (CD45.1+) compartment. Each dot represents one mouse and each bar the mean of three mice analyzed. (C) ELISA measurement of IFN-γ production by spleen and IEL compartments after in vitro restimulation with uninfected (DC) or E. cuniculi-infected (inf. DC) BMDC. Histograms represent the mean cytokine concentration from triplicate wells ± SD. Data shown are representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001; ND, not detected).
TLR9 signaling limits Treg conversion in vitro through promotion of Teff cytokines
We and other groups recently demonstrated that oral exposure to antigen could induce conversion of naïve CD4+Foxp3− T cells into Treg in the GALT (Coombes et al., 2007; Mucida et al., 2005; Sun et al., 2007). Our findings indicated that a specialized population of DC originating from the small intestinal LP (LpDC) mediated this process through endogenous transforming growth factor-beta (TGF-β) and retinoic acid (RA) – where TGF-β was critical for Treg differentiation and RA enhanced conversion (Benson et al., 2007; Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007). The involvement of the commensal flora in Treg conversion was not addressed in these studies. Based on the increased proportion of intestinal Treg in TLR9−/− mice and in the TLR9−/− hematopoietic compartment of chimeric WT mice, we hypothesized that CpG-containing DNA motifs derived from the commensal flora (gfDNA) would restrict Treg conversion. To test this possibility, we first examined the effect of TLR9 engagement on in vitro conversion.
The synthetic TLR9 agonist ODN 1826 (CpG) was added to purified LpDCs and CD4+CD25− CD44loFoxp3− T cells cocultured in Treg polarizing conditions, namely TGF-β and α-CD3. As shown previously, after 5 days of coculture in the absence of CpG, we observed both a high proportion of Foxp3+ cells and induction of the intestinal homing integrin heterodimer, α4β7, owing to RA instructing signals (Fig 4A). Stimulation with CpG, however, led to a dose dependent decrease in the frequency of Foxp3+ cells, while expression of α4β7 was maintained on Foxp3 negative cells (Fig 4A, Fig S6A). This observation suggested a decoupling of the tolerogenic and homing effects of RA in the presence of a strong adjuvant such as CpG (Johansson-Lindbom et al., 2005). The dramatic impact of CpG on Treg conversion contrasted with other bacterially derived TLR ligands, including peptidoglycan (PGN), lipolysaccharide (LPS), and flagellin (Flgn), which engage TLRs 2, 4 and 5, respectively. Even at high concentrations, these ligands exerted very little effect on Treg conversion (Fig 4B). LpDCs were not refractory to PGN, LPS or Flgn stimulation as evidenced by cytokine production and or upregulation of CD40, CD80 and CD86 expression following overnight incubation (data not shown). To confirm that the effects observed on Treg conversion were the result of TLR engagement by LpDCs, we also performed this experiment with T cells that cannot respond to TLR9 (Myd88−/−) and found similar results (Fig S6B). For the remainder of these experiments, we used TLR9−/− or Myd88−/− T cells to focus our study on the effects of TLR engagement on LpDCs.
Figure 4. Engagement of TLR9 signaling potently limits Treg conversion.
FACS-sorted naïve CD4+CD25−CD44loFoxp3− T cells isolated from Foxp3eGFP mice were cultured in Treg polarizing conditions with WT LpDCs in the presence of the indicated TLR ligands. (A) Dot plots gated on viable CD4+7-AAD− T cells illustrate α4β7 versus Foxp3 expression after culture in presence or absence of CpG (10 μg/ml). (B) PGN (TLR2), LPS (TLR4), Flgn (TLR5) or CpG (TLR9) were added in the culture at starting concentrations of 2 μg/ml, 10 μg/ml, 1 μg/ml and 10 μg/ml, respectively. Two subsequent five-fold dilutions of ligand were tested (gray wedge). Results were normalized to conditions plated in the absence of TLR ligands with 100% equaling conversion in Treg polarizing conditions. Cross bars indicate the high and lows of duplicate cultures. (C) CFSE dilution analysis of naïve CD4+CD25−CD44lo T cells isolated from TLR9−/− mice, cultured as in (A) and analyzed for CD4 and Foxp3 expression at the indicated time points. Overlay of the histograms for the CFSE dilution profiles of the CD4+Foxp3− T cells, indicated in the boxed in regions (solid line: control; dotted line: in presence of CpG). Data shown are representative of three independent experiments with similar results.
Cells failing to up-regulate Foxp3 may have simply outgrown Treg in CpG stimulated cocultures. However, CFSE dilution analysis indicated that the Foxp3− cells proliferated equivalently in both CpG stimulated and non-stimulated cocultures (Fig 4C). Moreover, before the cells that had upregulated Foxp3 began to divide, the frequency of Foxp3+ cells was already 50% lower in cocultures containing CpG. Together these observations suggested that TLR9 signaling influenced Treg conversion at least in part through the inhibition of naïve T cell differentiation into Treg.
IL-6 and TGF-β in tandem can direct the production of IL-17 secreting T cells (Th17) over Treg conversion (Bettelli et al., 2006; Veldhoen et al., 2006). Importantly, LpDCs produce far more IL-6 than SpDCs in response to CpG stimulation (Fig 5A). Thus, in CpG stimulated cocultures, in lieu of Treg we observed T cells producing IL-17, as well as IL-4 and IFN-γ (Fig 5B). Based on the large amount of IL-17 secreted into these cocultures (Fig 5C), we hypothesized that inhibiting IL-6 signaling may restore Treg conversion. However, despite significant IL-17 abrogation, blockade of IL-6 (α-IL-6) with dual anti-receptor and neutralizing Abs (Fig 5D) or the use of IL-6−/− LpDCs (data not shown) failed to reverse the effect of CpG on Treg conversion. Moreover, additional measures to inhibit the IL-17 pathway, including using IL21R−/− T cells (Korn et al., 2007; Nurieva et al., 2007), and adding neutralizing antibodies against IL-12/23p40 (Zhou et al., 2007) and IL-27p28 (Stumhofer et al., 2006) in addition to α-IL-6 also failed to restore Treg conversion (data not shown). Intriguingly, blockade of IL-6 treatment consistently enhanced IFN-γ and IL-4 production (data not shown). In accord with this observation, adding neutralizing Abs against both IL-4 and IFN-γ, in addition to IL-6 blocking Abs, resulted in a ~70% rebound in the frequency of Treg (Fig 5D and 5E). The rescue afforded by IL-4 neutralization was greater than that resulting from IFN-γ neutralization. In the presence of LpDC, the addition of each cytokine alone blocked the induction of Foxp3 in vitro with IL-6 being the most potent. Maximal suppression was observed when cytokines were combined (Fig 5F). These findings complement a recent report demonstrating the antagonistic effect of Th1 and Th2 development on Treg conversion (Wei et al., 2007). Thus, in addition to limiting Treg conversion, TLR9 signaling in the gut may promote local Teff activity, which in turn could enhance immune responses in the gut.
Figure 5. Engagement of TLR9 limits Treg conversion and enhances Teff activity.
(A)5×104 purified LpDCs or SpDCs were stimulated for 18 hrs in the presence or absence of CpG (10 μg/ml) in complete media containing 40 ng/ml of GM-CSF (CM). Recovered supernatant was assessed for IL-6 by ELISA. (B) CD4+CD25−CD44loFoxp3− T cells from TLR9−/− Foxp3eGFP mice were cultured in Treg polarizing conditions in the presence or absence of CpG for 6 days and then restimulated for assessment of intracellular cytokine production. (C–E) Naïve CD4+CD25−CD44lo T cells from Myd88−/− mice were cultured in Treg polarizing conditions for 6 days in the presence or absence of CpG. In wells containing CpG, antibodies to IL-6 and IL-6 receptor α (αIL-6), IL-4 (α-IL-4) and IFN-γ (α-IFN-γ) were added at the start of culture as indicated. (C) Measurement of IL-17 by ELISA for previously described conditions. (D) Dot plots gated on viable CD4+ T cells show the percentages of Foxp3+ cells. (E) Summary of results of D normalized to baseline Treg conversion. Cross bars indicate the high and lows of duplicate cultures.. Data shown are representative of at least three independent experiments with similar results. (F) Blockade of LpDC induced Treg conversion by IL-6, IL-4 and IFN-γ. Naïve CD4+CD25−CD44lo T cells were cocultured with LpDCs in Treg polarizing conditions and various doses of IL-6, IL-4 or IFN-γ or a combination of all 3 cytokines. Cytokine concentration is provided on the x axis. In wells containing the combined cytokines, the same concentration of each cytokine was used. Data are representative of 2 independent experiments.
Engagement of TLR9 by commensally derived gfDNA inhibits Treg conversion in vitro
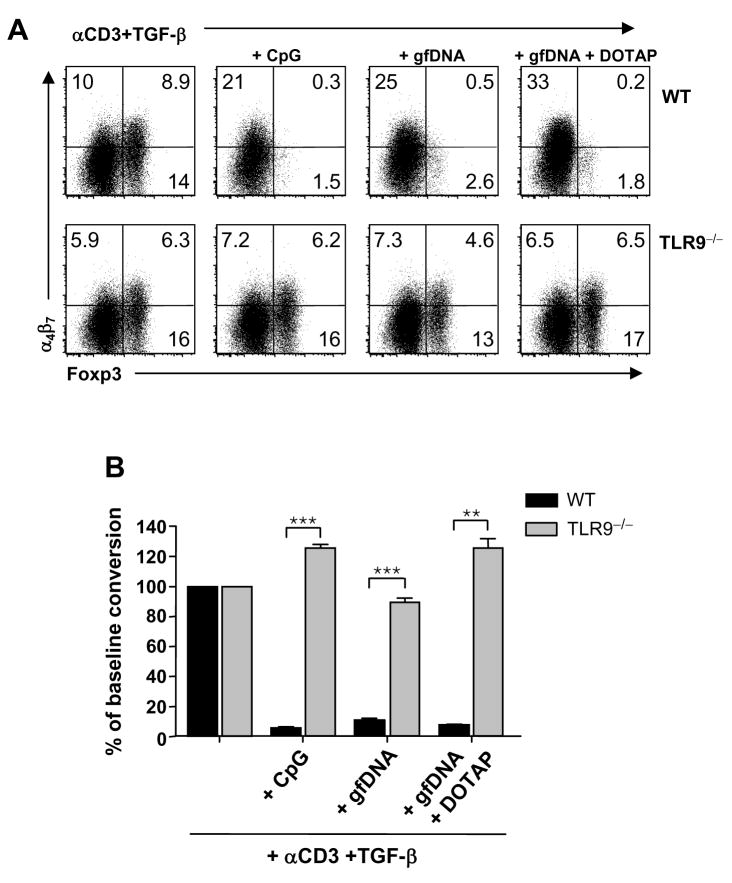
To test whether DNA derived from conventional gut flora (gfDNA) would have an effect on Treg conversion that was comparable to CpG, we extracted and purified gfDNA and added it to Treg polarizing cocultures. Without a defined sequence length of gfDNA, we included a control condition that would facilitate its access into the LpDC endosomal compartment, where TLR9 stimulation occurs (Schalasta and Doppler, 1990). In this condition, gfDNA was complexed to the monocationic lipid transfection reagent, DOTAP, prior to stimulation (Bucci et al., 1992). Regardless of whether DOTAP was present, gfDNA consistently and significantly inhibited Treg conversion in a manner similar to CpG, while still inducing α4β7 (Fig 6A and B). Thus, LpDC can efficiently take up gfDNA. Addition of CpG or gfDNA to splenic DC also inhibited Treg induction in vitro although not to the same extent as LpDC (FigS 6C). To assess if gfDNA inhibited Treg generation via TLR9, similar experiments were performed using LpDCs purified from TLR9−/− mice. Under these conditions, the suppressive effect of both CpG and gfDNA was largely abolished (Fig 6A and B). Thus gfDNA can inhibit Treg induction by LpDCs in a TLR9 dependent manner.
Figure 6. DNA enriched from the gut flora suppresses Treg conversion in a TLR9 dependent manner.
(A) CD4+CD25−CD44lo T cells isolated from WT or TLR9−/− mice were cultured in Treg polarizing conditions with LpDCs from WT or TLR9−/− mice, respectively. In some culture wells, 10μg/ml of CpG, DNA enriched from murine gut flora (gfDNA) or gfDNA formulated with the cationic liposome, DOTAP, was added. CD4, Foxp3 and α4β7 expression was analyzed on day 6. (B) Summary of results from (A) normalized to baseline Treg conversion. Error bars represent the SD of triplicate cultures (**, p<0.01; ***, p<0.001).
Gut flora DNA from conventional gut flora is a natural adjuvant of intestinal immune responses
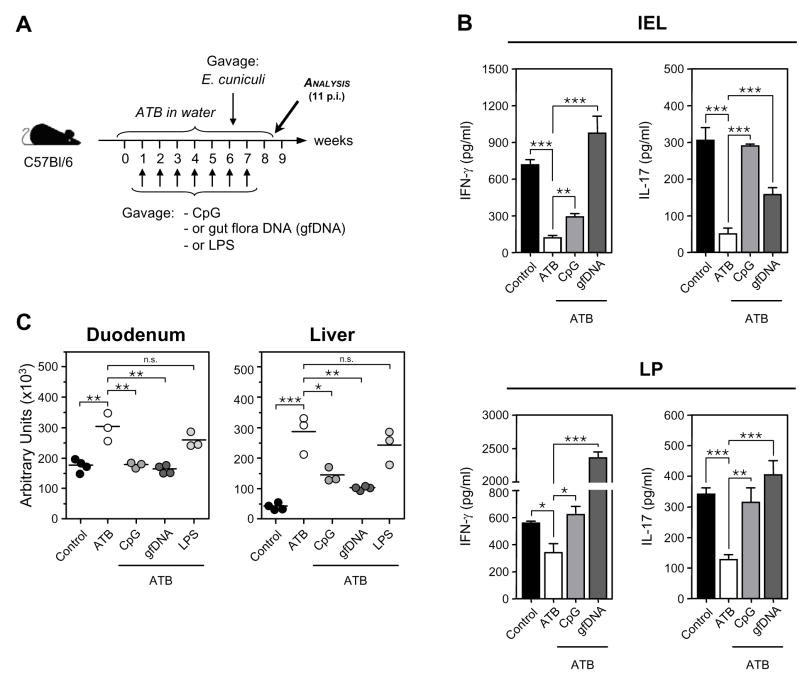
The preceding results suggested that gfDNA have a direct adjuvant effect on the induction of intestinal immune responses through engagement of TLR9. To test this, we first minimized the impact of other gut floral signals by placing mice on a cocktail of antibiotics (ATB) (Pasare and Medzhitov, 2004) while providing CpG, gfDNA or LPS (Fig. 7A). After 6 wk of ATB treatment, mice were orally infected with E. cuniculi. The frequencies and absolute number of CD4+ and CD8+ T cells were comparable between the different treatment groups (Fig S7A, B). The reduction of the gut flora via ATB treatment was sufficient to significantly impair IFN-γ and IL-17 responses in infected mice supporting the idea that the presence of gut flora is required to sustain gut immune responses (Fig 7B, C). The addition of CpG alone significantly corrected this impairment, arguing that TLR9 signals serve as preconditioning stimuli for immune responses. Strikingly, gfDNA also restored the intestinal response in ATB treated mice (Fig 7B). Impaired immune responses following ATB treatment led to increased parasite burden (Fig 7C). The increase of effector responses following CpG or gfDNA treatment correlated with restoration of parasite control (Fig 7C). In contrast, treatment of mice with LPS failed to restore immune response in the GI tract and parasite control (Fig S7C and Fig 7C). Similar data were obtained when ATB treated mice receiving CpG or gfDNA were subsequently vaccinated (Fig S8A). To test the specificity of gfDNA for TLR9, TLR9−/− mice were placed on ATB and received gfDNA prior to infection with E. cuniculi. Antibiotic treatment also reduced immune responses to oral infection in TLR9−/− mice but CpG or gfDNA did not rescue effector responses in these mice (Fig S8B). Thus, by means of TLR9 signaling, gfDNA serves as a natural adjuvant for priming intestinal immune responses.
Figure 7. Gut floral DNA restores immune responses in antibiotic treated mice orally infected with E. cuniculi.
(A) 3 weeks old mice received a cocktail of antibiotics (ATB) in the drinking water for 6 weeks in conjunction with oral weekly treatments of PBS alone or containing 100 μg of CpG, 500 μg gfDNA, or 25 mg/kg of lipopolysaccharide from Escherichia coli (LPS). Control mice received no ATB treatment. At 6 wks post-treatment, mice were infected orally with E. cuniculi. (B) ELISA of IFN-γ and IL-17 in supernatants of bulk leukocyte preparations from IEL and LP of 11 d post-infected WT and TLR9−/− mice restimulated with E. cuniculi-infected BMDC for 72 hrs. Histograms represent the mean cytokine concentration ± SD. This experiment is representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001). (C) 8 wk-old mice were treated and infected as described in (A) and parasite burden was evaluated in duodenum and liver 11 d post-infection by quantitative real-time PCR. Each dot represents one mouse and each bar the mean of three or four mice analyzed (*, p<0.05; **, p<0.01; ***, p<0.001; n.s., non significant).
Discussion
The tissues of the GI tract are constantly exposed to TLR ligands harbored by the commensal gut flora (Pamer, 2007). In our present study, we found that gut floral derived DNA (gfDNA) primed gastrointestinal immune responses to foreign antigen through an intrinsic adjuvant activity. TLR9 signaling lowered the activation threshold in the gut through negative and positive expansion of Treg and Teff, respectively, and favored development of protective responses upon oral infection or during oral vaccination. Concordant with this finding, gfDNA potently limited the Treg polarizing ability of LpDCs. Gut flora DNA was also sufficient to restore protective immune responses in antibiotic treated mice. To our knowledge, this study provides the first demonstration of a role for a gut flora component in initiating protective immune responses at sites of mucosal challenge.
During conditions of floral translocation, peripheral TLR9 signaling is a crucial mediator of polymicrobial sepsis (Plitas et al., 2008). Moreover, in other conditions where floral translocation occurs, during irradiation and HIV infection, for example, peripheral TLR4 signals enhance the activation status of both CD4+ and CD8+ T cells (Brenchley et al., 2006; Paulos et al., 2007). However, under most circumstances, it is the tissues of the GI tract that are constantly exposed to TLR ligands harbored by the commensal gut flora (Pamer, 2007). Regulatory T cells are crucial to the integrity of the GI tract (Izcue et al., 2006). Recent evidence suggests that TLR signaling can impact Treg homeostasis (Sutmuller et al., 2006b). Treg themselves selectively express TLRs, including 2, 4, 5 and 8 (Sutmuller et al., 2006b). Interaction with some of these ligands, such as those binding TLR2, can favor Treg expansion both in vitro and in vivo (Liu et al., 2006; Sutmuller et al., 2006a) Accordingly, in TLR2 deficient mice Treg frequencies in both gut and secondary lymphoid tissues are decreased (Liu et al., 2006; Sutmuller et al., 2006a). In TLR9 deficient mice, on the other hand, we observed increased Treg frequencies in all of the intestinal effector tissues. Together, these findings indicate that TLR ligands can exert differential effects on Treg homeostasis, which may explain why Myd88 deficient mice or germ free mice have unaltered Treg frequencies (Min et al., 2007; Rakoff-Nahoum et al., 2004; Sutmuller et al., 2006a). Our observation that Treg were selectively increased in gut effector sites was mirrored by a decrease in constitutive Teff proinflammatory secretion. In agreement with others, we found a significant frequency of T cells producing pro-inflammatory cytokines in small intestinal tissues of WT mice at steady state (Denning et al., 2007; Ivanov et al., 2006). This constitutive production, which included IL-17 and IFN-γ by CD4+ T cells in the LP and IFN-γ by CD8+ T cells in the IEL was markedly reduced in TLR9−/− mice. Thus, gfDNA interactions with TLR9 may be one of the factors that dictate the balance between Treg and Teff in the GI tract at steady state conditions.
Our finding that TLR9−/− mice mounted an impaired response to E. cuniculi only during the oral route of infection suggested that the disproportionate enhancement of intestinal Treg and or impaired Teff cell priming in these animals may predispose them to less effficient mucosal responses. A role for TLR9 has been previously reported against various infections including those that occur at mucosal sites such as Toxoplasma gondii or Herpes simplex virus (Lund et al., 2003; Minns et al., 2006). In contrast to WT mice, TLR9−/− mice are protected from immunopathology mediated lethal ileitis following T. gondii infection. Thus, while TLR9 may be critical for recognition of these microbes, we further propose that the dysregulated Treg/Teff ratio within the gut tissues of these mice would have additional bearing on the phenotype observed. Consistent with this hypothesis, Treg depletion effectively circumvented the requirement for intestinal TLR9 priming signals, and restored the capacity of TLR9 deficient mice to mount proper immune responses to oral infection with E. cuniculi and mucosal vaccination
The gut flora, by virtue of a vast repertoire of bacterial species, can engage TLR9 through differential expression of CpG motifs within its bacterial genomes (Dalpke et al., 2006). Yet it is still unclear by what means gfDNA become accessible for interaction with TLR9. Epithelia in the GI tract basally express TLR9 on their surface (both apical and basolateral) (Ewaschuk et al., 2007; Lee et al., 2006). However, TLR9 signaling on the apical side can mediate anti-inflammatory effects, linking lumenal signals from gfDNA to intestinal homeostasis (Lee et al., 2006). In support of this, oral administration of CpG was shown to ameliorate the severity of dextran sodium sulfate (DSS)-induced colitis via TLR9 (Katakura et al., 2005). Along these lines, the absence of TLR9 exacerbated the severity of DSS-induced colitis (Lee et al., 2006). Thus in a context of a highly inflammatory setting, TLR9 signaling on epithelial cells can mediate a protective function. In contrast, at steady-state conditions or during oral exposure to pathogens, our results demonstrate that luminal gfDNA may also have potent immunogenic potential. To best address this, we utilized an antibiotic regimen that dramatically reduces floral density (Rakoff-Nahoum et al., 2004) and creates an environment in which the specific contribution of microbial ligands to intestinal immunity can be assessed. To avoid possible effects of floral depletion on immune system development, we also used this treatment on adult mice. While CpG and gfDNA similarly restored protective immune responses to infection with E. cuniculi in ATB treated mice, the impact of LPS conditioning was minor, with bug burdens comparable to those observed in ATB treated mice and recall responses significantly attenuated relative to non-ATB treated controls. The inability of LPS to efficiently adjuvant intestinal immune responiveness is reminiscent of a recent report which found that LPS tolerance in the intestine is established shortly after birth (Lotz et al., 2006).
Our results obtained using chimera mice suggested that TLR9 expression by the hematopoietic compartment was sufficient to constrain intestinal Treg frequencies and enhance protective immune response against mucosal infection with E. cuniculi. Thus, gfDNA makes essential contributions to intestinal homeostasis through two seemingly disparate pathways with the establishment of this dichotomy potentially resting on the cellular target (e.g. epithelial cells versus antigen presenting cells), the intensity of the tissue disruption or even the gut compartment involved (small versus large intestine).
We have previously shown that peripheral conversion of CD4+ T cells to Treg occurs primarily in gut associated lymphoid tissue. Dendritic cells purified from the lamina propria of the small intestine were found to promote a high level of Treg conversion. Enhanced conversion of Treg by LpDCs was dependent on TGF-β and retinoic acid, a vitamin A metabolite highly expressed in GALT. One possible explanation for the phenotype observed in TLR9−/− mice could be associated with a dysregulation of this pathway. Indeed, we found that TLR9 signaling by gfDNA potently limited Treg induction by LpDCs. Notably, other representative bacterial TLR ligands including 2, 4 and 5, did not markedly affect LpDC induced Treg conversion in vitro. This is consistent with a recent report finding that LpDCs express low levels of TLR2 and TLR4 by message. TLR5, on the other hand, was reported to be quite high in these cells (Uematsu et al., 2008). We also found that overnight incubation of LpDCs with a TLR5 ligand strongly enhanced CD40, CD80 and CD86 expression. Nevertheless, when added to Treg polarizing cocultures, TLR5, TLR4 or TLR2 ligation had little effect on conversion supporting previous repports suggesting regulatory properties for TLR4 and 5 in this environment (Bashir et al., 2004; Rakoff-Nahoum et al., 2004; Vijay-Kumar et al., 2007). Several proinflammatory signals have been shown to limit Foxp3 induction (Bettelli et al., 2006; Korn et al., 2007; Nurieva et al., 2007; Stumhofer et al., 2006; Veldhoen et al., 2006; Zhou et al., 2007). For instance IL-6 and TGF-β in tandem can direct the production of IL-17 secreting T cells (Th17) over Treg (Bettelli et al., 2006; Veldhoen et al., 2006). We found that LpDCs responded robustly to TLR9 stimulation, and secreted several proinflammatory cytokines, including large amounts of IL-6 and IL-12p40, but undetectable levels of IL-12p70. Accordingly, in addition to restricting Treg conversion, TLR9 activation induced Teff cytokines including IL-17, IFN-γ and IL-4. The difficuty in reversing this effect underscored TLR9’s potent antagonizing influence on Treg conversion. Effective rescue required inhibition of IL-6 in conjunction with neutralization of IL-4 and IFN-γ. These findings complement a recent report demonstrating the antagonistic effect of Th1 and Th2 development on Treg conversion (Wei et al., 2007). These data are in line with the augmented Treg/Teff ratio that we observed in the intestinal tissues of TLR9−/− mice.
In addition to limiting Treg conversion, TLR9 signaling may be required for sustaining effector activity and/or priming in the GI tract. Interestingly, temporary ablation of LpDCs was recently shown to reduce constitutive Th17 production in the lamina propria (Denning et al., 2007). The reduction of both IFN-γ and IL-17 in TLR9 deficient mice suggested that gfDNA signaling on LpDC was involved in this basal cytokine production. In addition to cytokine induction, we propose that TLR9 signals may also promote Teff migration into the gut via retinoic acid release from DCs. Although our data suggest that RA contributes to tolerance mechanisms through reinforcement of Treg conversion, RA is also required for migration of lymphocytes into intestinal effector sites via induction of α4β7 and CCR9 (Johansson-Lindbom et al., 2005; Johansson-Lindbom et al., 2003; Mora et al., 2006; Siewert et al., 2007). Additionally, a recent repport demonstrated that low doses of RA have a proinflammatory effect on T cell polarization when coupled with microbial stimulus (Uematsu et al., 2008). We observed strong α4β7 induction on Teff in cocultures exposed to CpG and gfDNA. The downstream signals associated with these outcomes are still unclear (Svensson et al., 2007), though we propose that they are distinct. Synthesizing these findings, constitutive TLR9 signaling in DCs derived from the lamina propria may therefore create a negative-regulatory circuit that mediates sensitivity to intestinal pathogens through modulation of Treg/Teff proportions in intestinal effector sites and intestinal homing. Previously, Pasare et al demonstrated that CpG could render Teff resistant to Treg regulation following DC activation (Pasare and Medzhitov, 2003). In this regard, the absence of TLR9 may subject Teff to more regulation by Treg in the GI tract. Treg were previously shown to limit the homeostatic expansion and cytokine production by Teff (Shen et al., 2005). Consequently, TLR9−/− Treg may exert more stringent regulation on the homeostatic proliferation of T cells. This scenario could also manifest in higher proportions of Treg, and fewer cytokine producing Teff in the gut.
The adult human intestine harbors up to 100 trillion microorganisms (Backhed et al., 2005). These microflora play a central role in the maintenance and control of host homeostasis. In addition to promoting development of the immune system and control of metabolic functions intestinal microflora play a major protective role by displacing pathogens and enhancing barrier fortification (Hooper and Gordon, 2001; O’Hara and Shanahan, 2006). In particular, gut flora interactions with specific TLRs can protect against gut injury (Fukata et al., 2005; Katakura et al., 2005; Rachmilewitz et al., 2002; Rachmilewitz et al., 2004; Rakoff-Nahoum et al., 2004) or mediate oral tolerance against dietary antigens (Bashir et al., 2004). Alteration to the structural integrity of TLR signaling components is often associated with profound clinical outcome and susceptibility to various infections or autoimmune disorder (Uematsu and Akira, 2006). The structure and composition of the gut flora reflect natural selection at both the microbial and host levels. One possibility could be that the absence of TLR9 would alter the gut flora composition. Modification of gut flora was shown in areas of inflamed gut in IBD patients (Barnich et al., 2003; Darfeuille-Michaud et al., 2004; Martin et al., 2004; Masseret et al., 2001; Ott et al., 2004; Seksik et al., 2003; Swidsinski et al., 2005). Furthermore, the presence of certain bacteria can aggravate small intestinal immunopathology following oral infection with mice (Heimesaat et al., 2006). It is tempting to speculate that alteration of Treg homeostasis mediated by TLR9 signaling, either because of genetic polymorphism or changes in gut flora composition, could also have consequences on development of gut inflammatory disorders. Indeed, gut flora bacteria are not all equal in their capapacity to stimulate TLR9 and do so with various levels of efficiency that correlate with their frequency of [CG] dinucleotides (Dalpke et al., 2006). Thus, control of Treg ratio and Teff function in the GI tract is likely to be differentially regulated by specific gut flora species.
In some instances, Treg level has to be limited to favor efficient immune responses, as in the case of oral vaccination or control of acute infection, while in other cases, Treg ratio or function has to be increased to limit immunopathology. Manipulation of Treg numbers or functions offers promising therapeutic avenues; however, systemic control of Treg function could be potentially harmful to the host by leading to autoimmune disorders or disease reactivation. Our present data offer the possibility to target the ratio between Treg and Teff in a site-specific manner. We propose to exploit our current observations to test how manipulation of gut flora or gut flora signaling could become part of a rational therapeutic strategy against oral infections or to favor oral vaccination.
MATERIALS AND METHODS
Mice
C57BL/6 (WT) and B6.SJL mice were purchased from Taconic Farms or bred in house. B6.129P2-Tlr9tmAki (TLR9−/−) (Hemmi et al., 2000) mice were obtained from Dr. S. Akira (Osaka University) via Dr. R. Seder (Vaccine Research Center, NIH) and backcrossed 11 generations onto the C57BL/6 background (Taconic). B6.129S6-Il6tm1Kopf (IL-6−/−) were purchased from the Jackson Laboratory and B6.129P2-Myd88tmAki (Myd88−/−) were kindly provided by Dr A. Sher. Foxp3 eGFP reporter mice (Foxp3eGFP) were obtained from Dr. M. Oukka (Bettelli et al., 2006). TLR9−/− Foxp3eGFP mice were generated by crossing the F1 progeny of TLR9−/− x Foxp3 eGFP breeders. TLR9−/− B6.SJL were generated by crossing the F1 progeny of TLR9−/− x B6.SJL breeders. All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee. In some experiments mice were co-housed. Mice between 8 and 12 wk of age were used. Mice were gender and age-matched for each experiment.
Phenotypic analysis
Cells from spleen, MLN, IEL, LP and PP were prepared as previously described (Sun et al., 2007). Single-cell suspensions were incubated with anti-FcεIII/II receptor. Cells were stained with fluorochrome-conjugated antibodies against surface markers CD4 (RM4-5), CD8α (53–6.7), CD25 (PC61.5), TCR-β chain (H57-597), CD45.1 (A20), CD45.2 (104), and/or α4β7 (DATK32) in PBS containing 1% FBS for 20 min on ice and then washed. In some experiments, 7-amino-actinomycin D (7-AAD, eBioscience) was used to exclude dead cells. For Foxp3 staining, cells were subsequently stained using the Foxp3 staining set (eBioscience) according to the manufacturer’s protocol. Nuclear Ki-67 staining was performed using an antibody against human Ki-67 (clone B56) from BD Pharmingen. Cell acquisition was performed on an LSRII machine using FACSDiVa software (BD Biosciences). For each sample, at least 300,000 events were collected. Data were analyzed using FlowJo software (TreeStar).
Intracellular cytokine detection
For basal cytokine detection, IEL, LP and PP single cell suspensions were stained with the CD90 positive selection kit and enriched for T cells using an autoMACs (Miltenyi Biotec). Cells were then cultured in triplicate at 1×106 cells/ml in a 96-well U-bottom plate and stimulated with 50 ng/ml PMA (Sigma) and 5 μg/ml ionomycin (Sigma), in the presence of brefeldin A (GolgiPlug, BD Biosciences) at 37°C in 5% CO2. After 5 hrs, cells were fixed in 4% paraformaldehyde for 10 min at room temperature or using the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Cells were then stained with fluorochrome-conjugated antibodies against CD4 (RM4-5), CD8α (53–6.7), IFN-γ (XMG1.2), IL-10 (JES5-16E3), IL-4 (11B11) and IL-17 (clone TC11-18H10), or isotype controls: rat IgG1 (clone R3-34), rat IgG2a (clone eBR2a), rat IgG2b (A95-1), mouse IgG2a (eBM2a), and hamster IgG2 (Ha4/8) for 30 min in FACS buffer containing 0.1% saponin or permabilization buffer supplied with the BD Cytofix/Cytoperm kit. All antibodies were purchased from eBioscience or BD Biosciences.
Vaccination and infection protocol
C57BL/6 and TLR9−/− mice were immunized orally with OVA protein (grade V; Sigma-Aldrich; 1 mg/mouse for each immunization) on day 0 and 7 in combination with 20 μg/mouse of the mutant form of E. coli LT(R129G) (Chong et al., 1998). Both OVA and LT(R129G) were prepared in an isotonic bicarbonate buffer. Immune responses were analyzed two weeks after the first immunization. A rabbit isolate of E. cuniculi obtained from Waterborne Inc. (New Orleans, USA) was used throughout the study. The parasites were maintained by continuous passage in rabbit kidney (RK13) cells obtained from the American Type Culture Collection (ATCC #CCL37) and maintained as previously described (Bouladoux et al., 2003). Spores were resuspended in sterile PBS, and immediately used for inoculation of mice or cell cultures. Mice were infected by intragastric gavage with 5×106 fresh spores in a volume of 200 μl. In some experiments, mice were infected i.p. using the same number of spores of E. cuniculi.
For CD25+ Treg depletion, C57BL/6 and TLR9−/− mice were injected i.p. with 0.5 mg of anti-CD25 mAb (clone PC61.5) or the corresponding isotype control (clone GL113) 3 days before the first oral immunization with OVA and then on the day of each immunization.
In vitro restimulation
Single-cell suspensions of spleen, MLN, IEL, LP, and PP of vaccinated or E. cuniculi-infected mice were prepared as described above. BMDC were generated as previously described (Lutz et al., 1999). Leukocytes (5×105) were incubated with 1×105 BMDC in 250 μl RPMI, 55 mM 2-β mercaptoethanol, and 10% FBS per well of a 96-well U-bottom plate. BMDC were previously incubated overnight with or without recombinant vaccinia virus expressing OVA protein (10 pfu/BMDC), E. cuniculi spores (parasite:BMDC ratio, 10:1) or OVA peptide (1 μg/ml) in the presence of 20 ng/ml GM-CSF (Peprotech) and washed before culture with leukocytes. After 3 days at 37°C in 5% CO2, culture supernatants were collected for cytokine assays. IFN-γ, IL-4, IL-6, IL-10, and IL-17 were quantitated in culture supernatants of restimulated leukocytes using the DuoSet ELISA system (R&D Systems) according to the manufacturer’s protocol.
Quantitation of parasite tissue loads
Duodenum and liver were removed from infected mice and digested with proteinase K (Invitrogen or Qiagen) after homogenization. DNA was subsequently extracted either with phenol-chloroform-isoamyl alcohol followed by ethanol precipitation or with the DNeasy Tissue kit from Qiagen. Quantitative real-time PCR was performed in triplicates with 50 ng of total tissue DNA, the iQ SYBR Green Supermix (BioRad), and the following primers specific for a 268-bp DNA sequence of the SSU rRNA gene from E. cuniculi (Weiss and Vossbrinck, 1998): forward 5′-GTGAGACCCTTTGACGGTGT-3′ and reverse 5′-CTCAGACCTTCCGATCTTCG-3′. Real-time PCR was conducted on a Bio-Rad iCycler under the following conditions: 3 min at 95°C; 40 cycles of 45 s at 95°C; 60 s at 60°C; and 45 s at 72°C. Genomic DNA were extracted from known amounts of E. cuniculi with the QIAamp DNA stool mini-kit (Qiagen) and used as PCR standards. A standard curve was generated by linear regression on plotted cycle threshold (CT) values of the standards against the logarithms of parasite numbers using iCycler iQ Optical System software (version 3.1; Bio-Rad).
Lamina propria DC (LpDC) and T cell purification
After LP digests were passed through 70- and 40-μm cell strainers, cells were resuspended in 1.077 g/cm3 iso-osmotic NycoPrep medium (Accurate Chemical & Scientific Corp.), and overlayed with RPMI 1640. The low-density fraction was collected after centrifugation at 1,650 g for 15 min. Cells were washed and incubated with a mixture of mAb containing α-CD16/32 (2.4G2), 7-AAD viability staining solution, α-CD11c (HL-3), α-MHCII (AF6-120.1), as well as the non-DC components α-DX5 (DX5), α-NK1.1 (PK136), and α-B220 (RA3-6B2) (all from eBioscience). DCs were defined as CD11chiMHCII+ cells and non-DCs were excluded when sorted by flow cytometry on a FACSAria. CD11c+MHCII+ cells were > 90% and used for in vitro conversion assays.
For T cell purification, single-cell suspensions of peripheral LNs extracted from Foxp3eGFP, TLR9−/− Foxp3eGFP, WT, TLR9−/−, Myd88−/− or IL21R−/− mice were enriched for CD4+ T cells by a negative selection kit using an autoMACs (Miltenyi Biotec). The enriched fraction was further labeled with fluorescent dye-conjugated mAb, including CD4 (RM4-5), CD25 (7D4), CD44, (IM7) (all from eBioscience) and sorted by flow cytometry on a FACSVantage or FACSAria (BD Biosciences). Purified CD4+CD25−CD44loFoxp3− T cells or CD4+CD25−CD44lo (<1% Foxp3+) were used for in vitro conversion assays. In proliferation assays, purified CD4+ T cells were labeled with 1.25 μM CFSE (Invitrogen) in HBSS (Mediatech Inc.) for 5 min at room temperature. Cells were washed twice in media containing 3% FBS.
In vitro conversion assay
Our conversion protocol was performed as previously described (Bettelli et al., 2006) with T cells and LpDCs obtained as described above. In brief, FACS purified LpDCs and CD4+ T cells were cocultured at a 1:10 ratio (1×105 CD4+ T cells) in complete medium (RPMI-1640 containing 10% FBS, 20 μM of 2-β mercaptoethanol and antibiotics) and Treg polarizing conditions (soluble α–CD3 mAb (1 μg/ml) (BD Bioscience) and human rTGF-β (0.6 ng/ml) (Cell Science)). 5 ng/ml of IL-2 was supplemented in cocultures every 2 days. In experiments detailed in Fig 4 the following were added to co-coculture conditions: a) peptidoglycan (PGN), ultra-pure lipopolysaccharide (LPS), flagellin (Flgn), CpG ODN 1826 (CpG) (all from Invivogen) at the indicated concentrations. In experiments detailed in Fig 5, various combinations of mAb including α-IL-6 (MP5-20F3), α-IL-6R (D7715A7), α-IL12/23p40 (C17.8), α-IL27p28 (RαD) and/or α-IFN-γ (XMG1.2 or 11B11), or isotype controls α-IgG1κ (R3-34) and/or α-IgG2α (R35-95) were added at the start of cocultures in Treg polarizing conditions containing CpG (10 μg/ml). All antibodies were purchased from BD Biosciences, excluding α-IL27p28, which was purchased from R&D Systems. On day 5, cells were surface stained with antibodies to CD4 (RM4-5), α4β7 (DATK32), CD25 (PC61.5), and the viability marker 7-AAD (all from eBioscience). Foxp3+ cells were detected on an LSRII either by eGFP expression and/or α Foxp3 mAb (FJK-16) following fixation and permeabilization with the kit provided by eBioscience in accordance with the manufacturer’s protocol. For intracellular cytokine staining, supernatants were removed and replaced with complete media containing PMA, ionomycin and brefeldin A, as described above.
Gut flora DNA extraction
Gut contents from the caecum and the colon of naïve C57BL/6 mice were collected and washed in cold PBS. The pellet was resuspended in lysis buffer (10 mM Tris/HCl, 50 mM EDTA, pH 8.0) containing lysozyme (0.5 mg/ml; Sigma-Aldrich). After incubation at 37°C for 2 h, 2 mg/ml of proteinase K and 1% SDS were added and the sample incubated at 60°C for 3 h. DNA was then purified by a series of 7 consecutive phenol-chloroform-isoamyl alcohol affinity extractions.
Antibiotic treatment
Male 3-wk or 8-wk-old C57BL/6 mice were provided ampicillin (1 g/l), vancomycin (500 mg/l), neomycin trisulfate (1 g/l), and metronidazole (1 g/l) in drinking water as previously described (Rakoff-Nahoum et al., 2004). All antibiotics were purchased from Sigma-Aldrich. Mice also received orally 100 μg CpG ODN 1826 in sterile PBS (Coley Pharmaceutical) or 500 μg of DNA from the gut content in sterile water or 25 mg/kg of LPS from Escherichia coli (serotype 026:B6 from Sigma-Aldrich) once weekly from the start of the antibiotic course.
Statistical analysis
Groups were compared with Prism software (GraphPad) using the unpaired or paired Student’s t test.
Supplementary Material
Supplementary Figure 1. (A) Absolute number of CD4+TCR-β+Foxp3+ Treg in spleen, MLN, IEL, LP and PP compartments of naïve WT and TLR9−/− mice. Each dot represents the result from one experiment (three mice pooled per group) and each bar the mean of three independent experiments (*, p<0.05, **, p<0.01 compared with WT mice). (B) Percentages of CD4+TCR-β+Foxp3+ in the LP of the colon from naïve WT (^) and TLR9−/− (○) mice. (C) Percentages of CD4+TCR-β+Foxp3+ and CD4+TCR-β+Foxp3− expressing Ki-67 in the LP and PP of naïve WT (^) and TLR9−/− (○) mice. For B and C, each dot represents one mouse and each bar the mean of three mice analyzed. Data shown are representative of two independent experiments with similar results. (D-E) IL-10 and IL-4 production by bulk preparation of leukocytes from WT and TLR9−/− mice 11 d after oral infection with E. cuniculi. Histograms represent the mean cytokine concentration from triplicate wells ± SD. This experiment is representative of three independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001; ND, not detected). (F) Percentage of CD4+TCR-β+Foxp3+ in spleen, MLN, IEL, LP and PP compartments of WT (^) and TLR9−/− (○) mice 11 d post-infection with E. cuniculi. Each dot represents the result from one experiment (three mice pooled per group) and each bar the mean of three independent experiments (*, p<0.05 compared with WT mice). (G) WT and TLR9−/− mice were orally immunized with a mixture of OVA and the mutant E. coli LT(R129G) on d 0 and d 7, and treated with anti-CD25 mAb or isotype control antibody. On d 14, IFN-γ and IL-17 secretion by IEL cells were evaluated by ELISA after in vitro restimulation with BMDC infected with recombinant vaccinia virus expressing OVA.
Supplementary Figure 2. IFN-γ and IL-17 secretion by IEL and LP cells from orally vaccinated WT and TLR9−/− mice after restimulation with OVA peptide-pulsed BMDC. WT and TLR9−/− mice were orally immunized with a mixture of OVA and the mutant E. coli LT(R129G) on d 0 and d 7. On d 14, IFN-γ and IL-17 secretion by IEL and LP compartments were evaluated by ELISA. Histograms represent the mean cytokine concentration of triplicate wells ± SD and are representative of two independent experiments with similar results (**, p<0.01; ***, p<0.001).
Supplementary Figure 3. Depletion of CD25+ Treg by anti-CD25 antibody treatment increases Th1 immune responses in E. cuniculi orally infected TLR-9−/− mice. (A) At 14 d postinfection, IFN-γ production by leukocytes from spleen and LP compartments was measured by ELISA after in vitro restimulation with E. cuniculi-infected BMDC. Histograms represent the mean cytokine concentration from triplicate wells. Data shown are representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001 compared with mice treated with isotype control mAb). (B) Parasite burden was evaluated in duodenum and liver 11 d post-infection by quantitative real-time PCR. Each dot represents one individual mouse and each bar the mean value of the results from five mice analyzed (**, p<0.01; ***, p<0.001; n.s., non significant).
Supplementary Figure 4. Efficiency of anti-CD25 mAb treatment. The phenotype of CD4+TCR-β+ cells purified from spleen, MLN, IEL, LP, and PP compartments was analyzed by flow cytometry in WT and TLR9−/− mice (three mice per group) treated with anti-CD25 mAb or the corresponding isotype control mAb. Cell phenotype was analysed by flow cytometry 2 wks after oral infection with E. cuniculi. All plots are gated on TCR-β+CD4+ cells. Numbers refer to the percentages of each subset. Representative dot plots are shown from one representative experiment of two with similar results.
Supplementary Figure 5. Expression of TLR9 by hematopoietic cells is sufficient to promote parasite control following oral infection. BM chimeric mice, in which the hematopoietic or the nonhematopoietic compartment lacks TLR9 expression, were orally infected with E. cuniculi. At 11 d post infection, parasite loads in the duodenum and liver were measured by quantitative real-time PCR. Each dot represents one individual mouse and each bar the mean value of the results from three mice analyzed.
Supplementary Figure 6. CpG and gfDNA limit Treg conversion through engagement of TLR9 on Lp and SpDCs. (A) Dose effect of CpG on LpDC induced Treg conversion. Naïve CD4+CD25−CD44lo T cells from TLR9−/− mice were cocultured with LpDCs from WT mice in Treg polarizing conditions at a ratio of 10 to 1. Cells were harvested on day 6 and assessed for intracellular Foxp3. (B) Engagement of TLR9 on LpDCs potently limits Treg conversion. FACS sorted naïve CD4+CD25−CD44lo T cells isolated from Myd88−/− mice were cultured in Treg polarizing conditions with WT LpDCs. PGN (TLR2), LPS (TLR4), Flgn (TLR5) or CpG (TLR9) were also added in the culture at starting concentrations of: 2 μg/ml, 10 μg/ml, 1 μg/ml and 10 μg/ml for PGN, LPS, Flgn and CpG, respectively. Two subsequent five-fold dilutions of ligand were tested (gray wedge). On day 6, cells were harvested and the percentage of Foxp3+ T cells among CD4+7-AAD− T cells was then determined by flow cytometry. Results in bar graph were normalized to conditions plated in the absence of TLR ligands. Cross bars indicate the high and lows of duplicate cultures. (C) Same as in A with additional conditions containing splenic DCs (SpDC) as well as gfDNA (10μg/ml). Arrows point to the percentage of Foxp3+ T cells in each condition.
Supplementary Figure 7. (A–B) 3 wks old WT mice received ATB in their drinking water for 6 wks in conjunction with oral weekly treatments of CpG, gfDNA or LPS (three mice per group). Control mice received no ATB and no treatment. At 6 wks post-treatment, mice were infected orally with E. cuniculi. Frequencies of TCRβ+CD4+ and TCRβ+CD8α+ cells (A) and total cell numbers (B) from the spleen, the IEL and LP compartments were analyzed at 11 d post infection for each group of mice. For A and B, data shown are representative of three independent experiments with similar results. (C) 3 wk-old WT mice were treated and infected as in (A) and (B). At 11 d post infection, IFN-γ in supernatants of bulk leukocyte preparations from WT and TLR9−/− mice was measured by ELISA after restimulation with E. cuniculi-infected BMDC (inf DC) for 72 hrs. Histograms represent the mean cytokine concentration ± SD. This experiment is representative of two independent experiments with similar results (*, p<0.05; **, p<0.01, ***; p<0.001).
Supplementary Figure 8. gfDNA favors effector immune responses following oral vaccination. (A) WT mice received ATB in their drinking water for 6 wks in conjunction with oral weekly treatments of PBS alone or containing CpG or gfDNA. Control mice received no ATB treatment. 6 wks post-treatment, all mice were orally immunized with two doses of OVA on d 0 and d 7 in combination with the mutant E. coli LT(R129G). On d 14, IFN-γ and IL-17 production by leukocytes isolated from the different groups was assayed by ELISA after in vitro restimulation with BMDC infected with recombinant vaccinia virus expressing OVA. Histograms represent the mean cytokine concentration ± SD. This experiment is representative of two distinct experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001). (B) gfDNA cannot rescue immune responses following oral infection in TLR9−/− mice. TLR9−/− mice were treated as described in (A) and all mice were orally infected with E. cuniculi. At 11 d post-infection, IFN-γ production by cells isolated from the different groups was assayed by ELISA after in vitro restimulation with E. cuniculi-infected BMDC. Histograms represent the mean cytokine concentration ± SD. A and B are representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001; n.s., non significant).
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank the NIAID sorting facility and Kim Beacht for technical assistance. We would like to thank Drs Alan Sher and David Chou for critical reading of the manuscript.
Abbreviations used
- BMDC
BM-derived DC
- CpG
unmethylated cytosine phosphate guanosine dinucleotide
- Flgn
flagellin
- GALT
gut-associated lymphoid tissue
- GI
gastrointestinal tract
- gfDNA
gut flora DNA
- IBD
inflammatory bowel disease
- IEL
intraepithelial lymphocyte
- inf. DC
infected DC
- LP
lamina propria
- LT
labile toxin
- LpDC
lamina propria DC
- MLN
mesenteric lymph node
- PGN
peptidoglycan
- PP
Peyer’s patch
- TLR
Toll-like receptor
- Teff
effector T cell
- Treg
regulatory T cell
- SpDC
spleen DC
- RA
retinoic acid
- LPS
Lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barnich N, Boudeau J, Claret L, Darfeuille-Michaud A. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn’s disease. Mol Microbiol. 2003;48:781–794. doi: 10.1046/j.1365-2958.2003.03468.x. [DOI] [PubMed] [Google Scholar]
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bouladoux N, Biligui S, Desportes-Livage I. A new monoclonal antibody enzyme-linked immunosorbent assay to measure in vitro multiplication of the microsporidium Encephalitozoon intestinalis. J Microbiol Methods. 2003;53:377–385. doi: 10.1016/s0167-7012(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C, Friberg M, Clements JD. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine. 1998;16:732–740. doi: 10.1016/s0264-410x(97)00255-7. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- Dalpke A, Frank J, Peter M, Heeg K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–946. doi: 10.1128/IAI.74.2.940-946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–2579. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Moretto M. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect Immun. 1999;67:1887–1893. doi: 10.1128/iai.67.4.1887-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa DF, Gelman AE, Rahman AH, Zhang J, Turka LA, Walsh PT. CpG DNA inhibits CD4+CD25+ Treg suppression through direct MyD88-dependent costimulation of effector CD4+ T cells. Immunol Lett. 2007;108:183–188. doi: 10.1016/j.imlet.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Masseret E, Boudeau J, Colombel JF, Neut C, Desreumaux P, Joly B, Cortot A, Darfeuille-Michaud A. Genetically related Escherichia coli strains associated with Crohn’s disease. Gut. 2001;48:320–325. doi: 10.1136/gut.48.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Moretto M, Weiss LM, Khan IA. Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol. 2004;172:4402–4409. doi: 10.4049/jimmunol.172.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to commensal and environmental microbes. Nat Immunol. 2007;8:1173–1178. doi: 10.1038/ni1526. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells.[comment] Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Schalasta G, Doppler C. Inhibition of c-fos transcription and phosphorylation of the serum response factor by an inhibitor of phospholipase C-type reactions. Mol Cell Biol. 1990;10:5558–5561. doi: 10.1128/mcb.10.10.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Ding Y, Tadokoro CE, Olivares-Villagomez D, Camps-Ramirez M, Curotto de Lafaille MA, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. J Clin Invest. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Floess S, Campbell DJ, Hamann A, Huehn J. Induction of organ-selective CD4(+) regulatory T cell homing. Eur J Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature immunology. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006a;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006b;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Svensson M, Johansson-Lindbom B, Zapata F, jaensson E, Austenaa L, Blomhoff R, Agace W. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+T cells. Mucosal immuunology. 2007;1:38–45. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Torok HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. Crohn’s disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127:365–366. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S. The role of Toll-like receptors in immune disorders. Expert Opin Biol Ther. 2006;6:203–214. doi: 10.1517/14712598.6.3.203. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Vossbrinck CR. Microsporidiosis: molecular and diagnostic aspects. Adv Parasitol. 1998;40:351–395. doi: 10.1016/s0065-308x(08)60127-x. [DOI] [PubMed] [Google Scholar]
- Wu W, Weigand L, Belkaid Y, Mendez S. Immunomodulatory effects associated with a live vaccine against Leishmania major containing CpG oligodeoxynucleotides. Eur J Immunol. 2006;36:3238–3247. doi: 10.1002/eji.200636472. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Absolute number of CD4+TCR-β+Foxp3+ Treg in spleen, MLN, IEL, LP and PP compartments of naïve WT and TLR9−/− mice. Each dot represents the result from one experiment (three mice pooled per group) and each bar the mean of three independent experiments (*, p<0.05, **, p<0.01 compared with WT mice). (B) Percentages of CD4+TCR-β+Foxp3+ in the LP of the colon from naïve WT (^) and TLR9−/− (○) mice. (C) Percentages of CD4+TCR-β+Foxp3+ and CD4+TCR-β+Foxp3− expressing Ki-67 in the LP and PP of naïve WT (^) and TLR9−/− (○) mice. For B and C, each dot represents one mouse and each bar the mean of three mice analyzed. Data shown are representative of two independent experiments with similar results. (D-E) IL-10 and IL-4 production by bulk preparation of leukocytes from WT and TLR9−/− mice 11 d after oral infection with E. cuniculi. Histograms represent the mean cytokine concentration from triplicate wells ± SD. This experiment is representative of three independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001; ND, not detected). (F) Percentage of CD4+TCR-β+Foxp3+ in spleen, MLN, IEL, LP and PP compartments of WT (^) and TLR9−/− (○) mice 11 d post-infection with E. cuniculi. Each dot represents the result from one experiment (three mice pooled per group) and each bar the mean of three independent experiments (*, p<0.05 compared with WT mice). (G) WT and TLR9−/− mice were orally immunized with a mixture of OVA and the mutant E. coli LT(R129G) on d 0 and d 7, and treated with anti-CD25 mAb or isotype control antibody. On d 14, IFN-γ and IL-17 secretion by IEL cells were evaluated by ELISA after in vitro restimulation with BMDC infected with recombinant vaccinia virus expressing OVA.
Supplementary Figure 2. IFN-γ and IL-17 secretion by IEL and LP cells from orally vaccinated WT and TLR9−/− mice after restimulation with OVA peptide-pulsed BMDC. WT and TLR9−/− mice were orally immunized with a mixture of OVA and the mutant E. coli LT(R129G) on d 0 and d 7. On d 14, IFN-γ and IL-17 secretion by IEL and LP compartments were evaluated by ELISA. Histograms represent the mean cytokine concentration of triplicate wells ± SD and are representative of two independent experiments with similar results (**, p<0.01; ***, p<0.001).
Supplementary Figure 3. Depletion of CD25+ Treg by anti-CD25 antibody treatment increases Th1 immune responses in E. cuniculi orally infected TLR-9−/− mice. (A) At 14 d postinfection, IFN-γ production by leukocytes from spleen and LP compartments was measured by ELISA after in vitro restimulation with E. cuniculi-infected BMDC. Histograms represent the mean cytokine concentration from triplicate wells. Data shown are representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001 compared with mice treated with isotype control mAb). (B) Parasite burden was evaluated in duodenum and liver 11 d post-infection by quantitative real-time PCR. Each dot represents one individual mouse and each bar the mean value of the results from five mice analyzed (**, p<0.01; ***, p<0.001; n.s., non significant).
Supplementary Figure 4. Efficiency of anti-CD25 mAb treatment. The phenotype of CD4+TCR-β+ cells purified from spleen, MLN, IEL, LP, and PP compartments was analyzed by flow cytometry in WT and TLR9−/− mice (three mice per group) treated with anti-CD25 mAb or the corresponding isotype control mAb. Cell phenotype was analysed by flow cytometry 2 wks after oral infection with E. cuniculi. All plots are gated on TCR-β+CD4+ cells. Numbers refer to the percentages of each subset. Representative dot plots are shown from one representative experiment of two with similar results.
Supplementary Figure 5. Expression of TLR9 by hematopoietic cells is sufficient to promote parasite control following oral infection. BM chimeric mice, in which the hematopoietic or the nonhematopoietic compartment lacks TLR9 expression, were orally infected with E. cuniculi. At 11 d post infection, parasite loads in the duodenum and liver were measured by quantitative real-time PCR. Each dot represents one individual mouse and each bar the mean value of the results from three mice analyzed.
Supplementary Figure 6. CpG and gfDNA limit Treg conversion through engagement of TLR9 on Lp and SpDCs. (A) Dose effect of CpG on LpDC induced Treg conversion. Naïve CD4+CD25−CD44lo T cells from TLR9−/− mice were cocultured with LpDCs from WT mice in Treg polarizing conditions at a ratio of 10 to 1. Cells were harvested on day 6 and assessed for intracellular Foxp3. (B) Engagement of TLR9 on LpDCs potently limits Treg conversion. FACS sorted naïve CD4+CD25−CD44lo T cells isolated from Myd88−/− mice were cultured in Treg polarizing conditions with WT LpDCs. PGN (TLR2), LPS (TLR4), Flgn (TLR5) or CpG (TLR9) were also added in the culture at starting concentrations of: 2 μg/ml, 10 μg/ml, 1 μg/ml and 10 μg/ml for PGN, LPS, Flgn and CpG, respectively. Two subsequent five-fold dilutions of ligand were tested (gray wedge). On day 6, cells were harvested and the percentage of Foxp3+ T cells among CD4+7-AAD− T cells was then determined by flow cytometry. Results in bar graph were normalized to conditions plated in the absence of TLR ligands. Cross bars indicate the high and lows of duplicate cultures. (C) Same as in A with additional conditions containing splenic DCs (SpDC) as well as gfDNA (10μg/ml). Arrows point to the percentage of Foxp3+ T cells in each condition.
Supplementary Figure 7. (A–B) 3 wks old WT mice received ATB in their drinking water for 6 wks in conjunction with oral weekly treatments of CpG, gfDNA or LPS (three mice per group). Control mice received no ATB and no treatment. At 6 wks post-treatment, mice were infected orally with E. cuniculi. Frequencies of TCRβ+CD4+ and TCRβ+CD8α+ cells (A) and total cell numbers (B) from the spleen, the IEL and LP compartments were analyzed at 11 d post infection for each group of mice. For A and B, data shown are representative of three independent experiments with similar results. (C) 3 wk-old WT mice were treated and infected as in (A) and (B). At 11 d post infection, IFN-γ in supernatants of bulk leukocyte preparations from WT and TLR9−/− mice was measured by ELISA after restimulation with E. cuniculi-infected BMDC (inf DC) for 72 hrs. Histograms represent the mean cytokine concentration ± SD. This experiment is representative of two independent experiments with similar results (*, p<0.05; **, p<0.01, ***; p<0.001).
Supplementary Figure 8. gfDNA favors effector immune responses following oral vaccination. (A) WT mice received ATB in their drinking water for 6 wks in conjunction with oral weekly treatments of PBS alone or containing CpG or gfDNA. Control mice received no ATB treatment. 6 wks post-treatment, all mice were orally immunized with two doses of OVA on d 0 and d 7 in combination with the mutant E. coli LT(R129G). On d 14, IFN-γ and IL-17 production by leukocytes isolated from the different groups was assayed by ELISA after in vitro restimulation with BMDC infected with recombinant vaccinia virus expressing OVA. Histograms represent the mean cytokine concentration ± SD. This experiment is representative of two distinct experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001). (B) gfDNA cannot rescue immune responses following oral infection in TLR9−/− mice. TLR9−/− mice were treated as described in (A) and all mice were orally infected with E. cuniculi. At 11 d post-infection, IFN-γ production by cells isolated from the different groups was assayed by ELISA after in vitro restimulation with E. cuniculi-infected BMDC. Histograms represent the mean cytokine concentration ± SD. A and B are representative of two independent experiments with similar results (*, p<0.05; **, p<0.01; ***, p<0.001; n.s., non significant).