Abstract
Natural forms of vitamin E are metabolized by ω-hydroxylation and β-oxidation of the hydrophobic side chain to generate urinary-excreted 2-(β-carboxyethyl)-6-hydroxychroman (CEHC) and CEHC conjugates (sulfate, glucuronide or glucoside). We have recently shown that sulfated long-chain carboxychromanols, the conjugated intermediate β-oxidation products, are formed from tocopherols and tocotrienols in human cells and in rats. CEHC conjugates have been quantified after being converted to its un-conjugated counterpart by sulfatase/glucuronidase. Although the enzymatic hydrolysis is critical for appropriate quantification of conjugated CEHC, it is not clear whether brief incubation of the plasma with sulfatases/glucuronidases results in complete de-conjugation of conjugated CEHC. Here we show that quantitative hydrolysis of the conjugated vitamin E metabolites in the plasma requires an extraction procedure using methanol/hexane (2ml/5mL) and an overnight sulfatase/glucuronidase hydrolysis. Using this procedure, we demonstrate that conjugated γ-CEHC and some sulfated long-chain carboxychromanols are fully deconjugated. In contrast, direct enzymatic hydrolysis of the whole plasma underestimates the conjugated metabolites by, at least, three fold. This protocol may be useful for the analysis of other conjugated phenolic compounds in complicated biological matrixes like plasma.
Keywords: CEHC, sulfatase, glucuronidase, tocopherol, tocotrienol, metabolism
Introduction
Vitamin E consists of eight structurally-related molecules, which are α-, β-, γ- and δ-tocopherol (α-T, β-T, γ-T and δ-T) and α-, β-, γ- and δ-tocotrienol (α-TE, β-TE, γ-TE and δ-TE). All vitamin E forms are metabolized via similar mechanism including ω-hydroxylation/oxidation and β-oxidation of their hydrophobic side chain to generate 2-(β-carboxyethyl)-6- hydroxychroman (CEHC), the terminal metabolite excreted in the urine [1; 2; 3; 4; 5] (Figure 1). Like many other phenolic compounds, CEHCs have been reported in free or conjugated forms including sulfate and glucuronide conjugates [6; 7; 8; 9; 10; 11; 12; 13], as well as a newly reported glucoside conjugate [14]. We recently found that sulfation of the intermediate metabolites occurs parallel to β-oxidation, as indicated by the formation of sulfated long-chain carboxychromanols when human A549 cells are incubated with tocopoherols and tocotrienols (Figure 1) [15; 16; 17]. Importantly, these metabolites are found in the plasma and tissues of rats supplemented with γ-tocopherol (γ-T) [16] and γ-tocotrienol (γ-TE) [15].
Figure 1. The metabolism of γ-T and the metabolites (modified based on ref. [16]).

Appropriate quantitation of the conjugated metabolites is important to evaluation of the bio-availability and bioactivity of different forms of vitamin E. Although a newly developed HPLC assay with fluorescent detection allows direct analysis of sulfated long-chain carboxychromanols along with their unconjugated counterparts [16], this method is not suitable for measuring CEHC conjugates. Despite readily detectable by mass spectrometer (MS), conjugated CEHCs are not feasibly quantified by MS due to the lack of authentic and stable-isotope labeled standards, such as 6-O-sulfate and 6-O-glucuronide α-, γ-, or δ-CEHC. For these reasons, conjugated CEHCs are commonly quantified after being enzymatically converted to CEHCs, which can be measured by gas chromatography coupled with MS, or liquid chromatography with fluorescent and electrochemical detection, or electrospray MS [10; 11; 16; 18; 19; 20].
It is conceivable that complete de-conjugation is essential to the appropriate quantitation of CEHC conjugates. Recently, several publications have reported that complete deconjugation of conjugated phenolic compounds in the plasma requires prolonged enzymatic hydrolysis [21; 22]. In this paper, we studied the hydrolysis of sulfated long-chain carboxychromanols and conjugated CEHC by a sulfatase from Helix promatia, which is known to have both sulfatase and glucuronidase activity. We find that in addition to the overnight incubation, a sample clean-up procedure prior to enzyme hydrolysis is necessary for complete deconjugation of CEHC conjugates and some sulfated long-chain carboxychromanols in the plasma.
Materials and Methods
Materials
α-T (99%), γ-T (97%-99%) and δ-T (97%) were purchased from Sigma (St Louis, MO). γ-CEHC (≥98%) was from Cayman Chemicals (Ann Arbor, MI). γ-TE was a gift from Klaus Kramer at BASF (Ludwigshafen, Germany). Tissue culture reagents were from Invitrogen (Rockville, MD). All sulfatases and glucuronidases, and other chemicals were purchased from Sigma. The sulfatases and glucuronidases tested include Type H-1 sulfatase from Helix pomatia (Cat# S9626), Type H-2 sulfatase from Helix pomatia (S9751), Type VI sulfatase from Aerobacter aerogenes (S1629), Type H-1 β-glucuronidase from Helix pomatia (G0751), Type B-1 β-glucuronidase from bovine liver (G0251), Type IX-A β-glucuronidase from Escherichia coli (G7396) and Type HP-2 β-glucuronidase from Helix pomatia (G7017).
Cell culture and conditioned media
The human alveolar epithelial cell line A549 was obtained from American Type Culture Collection (Manassas, VA). Cells were maintained routinely in RPMI-1640 with 10% fetal bovine serum (FBS). Vitamin E was first dissolved in dimethyl sulfoxide (DMSO) and then diluted in fatty-acid free bovine serum albumin (10mg/ml) prior to the addition to culture media. At the time of experiments, cells were seeded in RPMI-1640 with 10% FBS at a density of 8×105 cells per well in 6-well plates. Twenty-four hours later, cells were replenished with fresh Dulbecco's Modified Eagle Medium (DMEM) containing 1% FBS with vitamin E forms, or DMSO (0.05%) in controls and incubated for 24-72 h. Media were collected, frozen immediately and stored in -20°C until use.
Extraction of metabolites from cell-culture media
400 μL of cell-culture medium was added with 8 μL of ascorbic acid (60 mM), 10 μL of ethanol and 500 μL of hexane. The mixture was vortexed for 1 min and centrifuged at 13000 rpm for 2 min. The hexane layer was discarded and the aqueous phase was acidified to pH 3-4 using 14 μL of acetic acid. The aqueous phase was extracted twice with 1 mL of ethyl acetate. The combined ethyl acetate layers were dried under nitrogen gas. The residue was reconstituted in 200 μL of 70% MeOH/ 30% water and injected onto the HPLC column. This extraction procedure yielded a 97% or higher recovery of the metabolites [16].
Enzymatic digestion of metabolites in conditioned media
Metabolites extracted from conditioned media were dissolved in 10 μL ethanol and reconstituted in the enzyme solution. Samples were hydrolyzed by sulfatases or glucuronidases in 0.1 M NaAc at pH 5 for most enzymes, except for S1629 and G7396 which were used in 0.2 M Tris Buffer at pH 7.1. The enzyme amounts and buffers used for each enzyme were based on the recommendation by the manufacturer (Sigma). After 45- or 90-min incubation at 37 °C, samples were acidified to pH 3-4 by the addition of 5 μL of acetic acid. Metabolites were subsequently extracted twice with ethyl acetate, and analyzed by HPLC.
Analysis of free γ-CEHC in the plasma
One-hundred μL of plasma was mixed with 140 μl of methanol and kept on ice for 5 min, which was then added with 8 μl ascorbic acid (60 mM) and 200μl PBS. The mixture was acidified to pH 3-4 with 20 μL acetic acid. Metabolites were then extracted twice with 1 mL of ethyl acetate. After brief centrifugation, the combined ethyl acetate layers were dried under nitrogen gas. The residue was reconstituted in 100 μL of 70% MeOH/ 30% water and injected onto the HPLC column. This extraction procedure yielded a recovery of 90% spiked γ-CEHC in the plasma.
Analysis of total (free and conjugate) CEHC in the plasma
One-hundred μL of plasma was mixed with 8 μL of 60 mM ascorbic acid and 2 mL methanol, and was added with 100 μL of water and 5 mL of hexane. After the mixture was vortexed and centrifuged, 1.8 mL methanol layer was collected and dried under N2 stream. The residues were reconstituted in 100 μL water and 125 μL enzyme solution (pH 5), which was prepared using sodium acetate (9.45g) and glacial acetic acid (1.725 mL) in 500 mL water. Samples were hydrolyzed overnight by Helix pomatia Type H-1 sulfatase at 1.25 mg per sample at 37 °C. After the enzymatic hydrolysis, samples were acidified to pH 3-4 by the addition of 15 μL of acetic acid. Metabolites were subsequently extracted twice with ethyl acetate, and analyzed by HPLC.
Analysis of vitamin E metabolites by HPLC assay with fluorescent detection
Metabolites were quantified using a sensitive HPLC-fluorescent assay [16]. Briefly, the metabolites were separated on a 5 micron Supelcosil LC-18-DB column, 4.6 × 150 mm (Supelco, Bellefonte, PA) at a flow rate of 1.0 mL/min with the following gradient: maintaining 100% A (35% acetonitrile, 65% 10 mM ammonium acetate at pH 4.3) for 8 min, increasing to 100% B (96% acetonitrile, 4% 10 mM ammonium acetate at pH 4.3) from 8 to 30 min, maintaining 100% B until 55 min and then back to 100% A at 56 min. Metabolites were detected by a Shimadzu RF-10AXL spectrofluorometric detector (Shimadzu, Columbia, MD) with the excitation and emission at 292 and 327 nm, respectively. γ-CEHC was quantified using the authentic standard as the external standard. γ-CEHC stability during overnight incubation – To test the stability of γ-CEHC during the extraction and overnight incubation, fetal bovine serum (FBS) was spiked with γ-CEHC at final concentrations of 2 μM. The spiked FBS was then subjected to the methanol/hexane extraction and overnight enzyme hydrolysis as described under Analysis of total (free plus conjugate) CEHC in the plasma.
Animal Studies
All the animal studies were approved by Purdue Animal Care and Use Committee (PACUC). Male Wistar rats (230-250 g) were purchased from Charles River (Charles River, CA), and housed in Purdue Life Science Animal Facility for a week for adaptation. Rats were gavaged with γ-TE at 50 mg/kg body weight using tocopherol-stripped corn oil (0.5 mL) as the vehicle (n = 3 in each group). Control rats were fed 0.5 mL vitamin E-stripped corn oil. Six hours later, animals were sacrificed and plasma was collected. The plasma from γ-TE fed animals was used in the experiments to optimize the enzyme hydrolysis. The plasma samples from the corn-oil control group were used in the study to compare the quantitation of CEHC conjugates using our newly developed protocol with that using the un-extracted whole plasma.
Statistical analysis
Unpaired student's t-test was used in the statistical analysis. P < 0.05 was considered significant difference between compared groups. All results are expressed as mean ±SD.
Results and Discussion
The relative de-sulfation efficacy among sulfatases and glucuronidases
We recently showed that when γ-T or γ-TE were incubated with human A549 cells, long-chain carboxychromanols including 9′-, 11′- and 13′-COOH and their sulfated counterparts were accumulated in the culture media [15; 16]. On the other hand, no γ-CEHC or γ-CEHC conjugates were detected in this cellular system. In the preliminary study, we compared the relative efficacy of de-conjugation of sulfated long-chain metabolites in the conditioned media among various commercially-available sulfatases and glucuronidases (Materials and Methods). Although most tested enzymes are known to have both sulfatase and glucuronidase activities, the current study focuses on their ability to remove the sulfate group, as indicated by the conversion of 9′S, 11′S and 13′S to the corresponding 9′-, 11′- and 13′-COOH (Figure 2).
Figure 2.
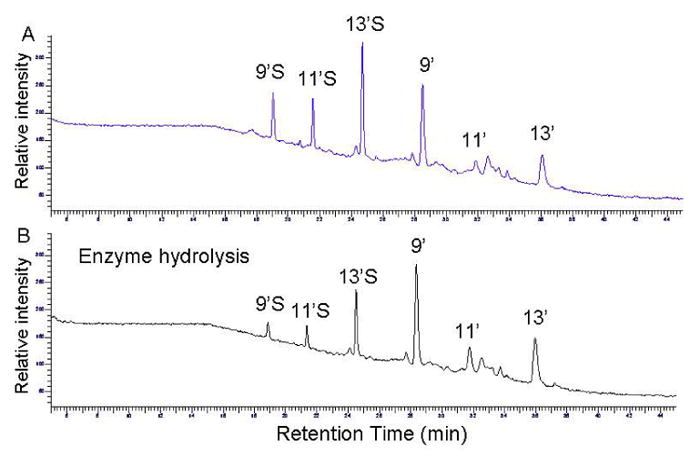
The HPLC-fluorescent chromatograms of the conditioned medium containing γ-T metabolites which were incubated in 0.1 M solidum acetate (pH 5) at 37 °C for 90 min in the absence (A) and presence (B) of the sulfatase. The detailed extraction and incubation conditions are described in Table 1. 9′, 11′, and 13′ represent 9′-COOH, 11′-COOH and 13′-COOH, and 9′S, 11′S and 13′S stand for sulfated 9′-COOH, sulfated 11′-COOH and sulfated 13′-COOH, respectively.
In a series of experiments, the sulfated metabolites in the culture medium were extracted by ethylacetate and then subjected to enzyme digestion. The efficacy of these enzymes was quantified by their de-conjugation of 9′ S after 45-min incubation. We found that two Type H-1 enzymes, i.e., S9626 and G0751, deconjugated 9′S by ∼65%. On the other hand, a TypeH-2 (S9751), Type VI (S1629) or Type HP-2 (G7017) enzyme appeared to be ineffective. A Type B-1 β-glucuronidase from bovine liver (G0251) showed modest efficacy by deconjugating 20% 9′S, which is consistent with a previous report indicating that this enzyme has 3% sulfatase activity [23]. These results are consistent with the fact that Type H-1 enzymes from Helix Promatia are the most popular enzymes used for deconjugation [21].
Between the two Type H-1 enzymes, we chose to use Type H-1 sulfatase (S9626) to further evaluate the de-sulfation efficacy with respect to the incubation time, the amount of enzyme and substrate specificity. This is because S9626 sulfatase is known to have slightly higher sulfatase activity (14.2U/mg for sulfatase and >330U/mg for β-glucuronidase activity) than G0751 (≥ 10 U/mg for sulfatase and 574 U/mg for glucuronidase activity). The hydrolysis efficiency of this sulfatase (at 29 U/mL) appeared to vary with the substrates in a relative order of 9′S > 11′S >13′S (Table 1). An increase of the incubation time from 45 to 90 min resulted in an enhanced deconjugation rate of all conjugated metabolites (Table 1). On the other hand, doubling the enzyme amount did not lead to any significant increase in the deconjugation rate (data not shown).
Table 1. Relative deconjugation efficacy of different substrates in culture media.
Metabolite-containing conditioned media were obtained by incubation of γ-T (50 μM) with A549 cells for 48 h. The metabolites from culture media were extracted by ethylacetate (Materials and Methods) and were incubated with Type H-1 sulfatase (S9626 at 0.2 mg per sample) in 0.1 M NaAc (pH 5.0) at 37 °C for the indicated times. Metabolites were quantified by HPLC. Deconjugation % = 100 × (Ci-Ca)/Ci, where Ci and Ca are the concentrations of conjugated metabolites before (Ci) and after (Ca) enzyme hydrolysis. 9′S, 11′S and 13′S stand for sulfated 9′-, 11′- and 13′-carboxychromanol.
| Deconjugation (%) | 9′S | 11′S | 13′S |
|---|---|---|---|
| 45 min | 63 ± 1 | 51 ± 1 | 32 ± 10 |
| 90 min | 79 ± 2 | 70 ± 2 | 47 ± 5 |
The different hydrolysis rate among 9′S, 11′S and 13′S may be due to the different hydrophobicity of these substrates. Although Dou C. et al [24] has shown that the electron donating or withdrawing groups play an important role in regulation of glucuronide hydrolysis, all the sulfated long-chain carboxychromanols here have the same chromanol ring structure. We reason that this sulfatase may be more effective to relatively hydrophilic compounds, i.g., sulfated 9′-COOH, than more hydrophobic ones, i.g., sulfated 13′-COOH.
De-conjugation of vitamin E metabolites in the plasma
Sulfated long-chain carboxychromanols including 9′S, 11′S and 13′S are found in the plasma of rats which are supplemented with γ-T or γ-TE [15; 16]. We evaluated the deconjugation of these metabolites in the plasma by the sulfatase. The plasma samples, which were obtained from rats supplemented with γ-TE at 50 mg/kg, were treated with the sulfatase (S9626) under the same condition as used with cell-culture media. The procedure includes extraction by ethylacetate after plasma samples were acidified to pH 3-4 and subsequent incubation with the enzyme for 90 min. The extent of de-conjugation was evaluated by the decrease of 11′S, which is the predominant long-chain metabolite in the plasma [15]. The results showed that only 30-40% of 11′S was deconjugated in the plasma, in contrast to 70% conversion in the culture media (Table 1).
Previous studies [21; 22] reported that a complete de-conjugation of phytoestrogen conjugates in the plasma requires overnight digestion. We decided to use the experimental conditions in these studies, including higher enzyme concentrations (1.25 mg per sample) and overnight hydrolysis of the whole plasma. However, this protocol resulted in only 20-30% deconjugation of 11′S. To our surprise, the hydrolysis revealed a substantial increase of γ-CEHC, suggesting that a high content of conjugated γ-CEHC exists in the plasma (data not shown).
It has been documented that conjugated metabolites are more difficult to be hydrolyzed in the plasma than in other biological fluid such as urine [21; 22]. Although some components in the plasma, such as high concentrations of lipids, proteins and inorganic ions, are proposed to inhibit the rate of enzymatic hydrolysis [21; 25], the exact reasons for the reduced efficacy is not known. To comprehensively remove potential interference, 100-200 μL plasma was mixed with 2 mL of methanol to completely precipitate proteins and with 5 mL hexane to eliminate neutral lipids (Materials and Methods). The processed plasma was then incubated with the sulfatase for 2, 4, 6, 16 and 20 h. The results showed that during the enzyme digestion, there was a marked increase of γ-CEHC (Figure 3). We also observed a time-dependent decrease of sulfated long-chain metabolites including 9′S and 11′S and the corresponding increase of 9′-COOH and 11′COOH, which were not present in the original plasma samples (Figure 3) [15; 16]. The deconjugation efficacy was quantitatively monitored by the increase of free γ-CEHC and the decrease of 11′S, respectively. A time-dependent increase of γ-CEHC was observed, which reached the plateau after 16 h incubation (Figure 4A). Meanwhile, 11′S gradually decreased and the hydrolysis was almost complete after 16-h incubation (Figure 4B). Doubling enzyme amount did not significantly improve the hydrolysis rate (data not shown). These data unambiguously demonstrate that both methanol/hexane extraction procedure and overnight enzyme digestion are necessary for complete deconjugation of the conjugated CEHC and sulfated long-chain carboxychromanols.
Figure 3.
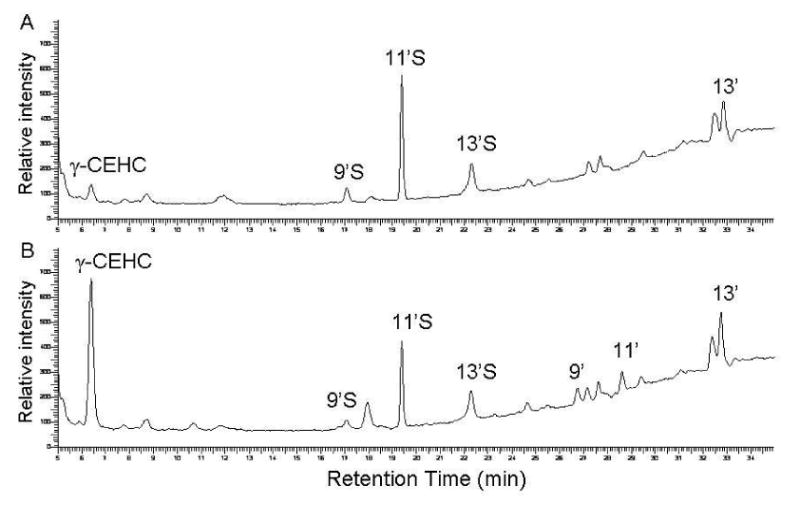
The HPLC-fluorescent chromatograms of the metabolites in the plasma from rats supplemented with γ-TE at 50 mg/Kg before (A) and after sulfatase hydrolysis (B). The plasma sample was extracted by methanol/hexane, incubated with the sulfatase (1.25 mg per sample) for 2 h and extracted by ethylacetate prior to HPLC analysis. The abbreviations of metabolites are the same as specified in Figure 2.
Figure 4. A time-dependent deconjugation of γ-CEHC conjugates (A) and 11′S (B) in the plasma.
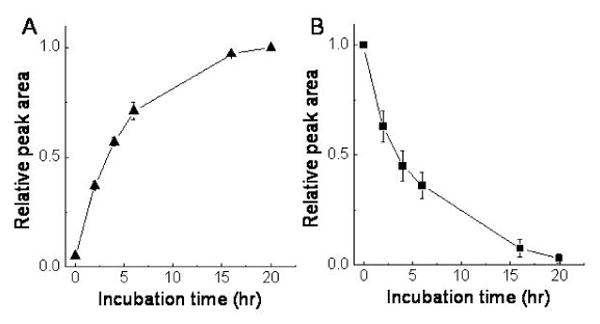
The plasma samples obtained from Wistar rats supplemented with γ-TE were extracted by methanol/hexane, and incubated with the sulfatase (1.25 mg per sample) in 0.1 M sodium acetate (pH 5) at 37 °C at indicated incubation times. The metabolites were then extracted by ethylacetate and analyzed by HPLC.
The stability of γ-CEHC during overnight incubation and assay precision
Like vitamin E, γ-CEHC is susceptible to oxidation. Therefore, it is important to examine whether this compound is stable during overnight incubation. To this end, γ-CEHC at a final concentration of 2 μM was spiked in fetal bovine serum, which was then processed by methanol/hexane extraction and treated with the sulfatase overnight. After overnight incubation, 81% γ-CEHC was recovered, which is similar to the extraction recovery rate of 87 ± 4%. These results indicate that γ-CEHC is stable during overnight incubation. We also evaluated inter-day and intra-day assay precision for the extraction of γ-CEHC during overnight incubation, which showed the coefficient of variation as 7.3% and 5.6 %, respectively.
Quantification of conjugated γ-CEHC in the plasma with and without the clean-up procedure
Our present study suggests that a brief incubation of the whole plasma with sulfatase may result in incomplete deconjugation and therefore under-estimate the concentrations of this metabolite in the plasma. We decided to compare the quantitation of CEHC conjugates using our newly developed protocol with that using the whole plasma. In this study, plasma samples were isolated from rats fed standard chow diet. The results showed that the use of methanol/hexane extraction to clean-up samples resulted in more than 3-fold higher levels of conjugated γ-CEHC than that from direct enzyme hydrolysis of the whole plasma (Table 2). The results also indicate that more than 97% CEHC is in the conjugated forms in the plasma. Consistent with these observations, using the same protocol, we found that conjugated CEHC is the predominant metabolite in the plasma of rats supplemented with γ-T and γ-TE, and more than 88-98% γ-CEHC is in the conjugated form [15].
Table 2. Comparison of the measurement of plasma conjugated γ-CEHC with and without the clean-up procedure.
To measure atotal γ-CEHC which includes free and conjugated form, plasma samples were either directly treated with the sulfatase (whole plasma), or were subject to enzyme hydrolysis after being cleaned-up with methanol/hexane extraction (extracted plasma). After overnight enzyme hydrolysis with S9626 sulfatase at 1.25 mg per sample at 37 °C, the metabolites were extracted and analyzed by HPLC. bFree γ-CEHC was measured after plasma was extracted by ethylacetate without sulfatase hydrolysis (No enzyme hydrolysis). cConjugated γ-CEHC was calculated by subtraction of free γ-CEHC from the total CEHC. *P < 0.01 indicates a significant difference between the extracted and whole plasma. Values are means ± SD, n = 3.
| γ-CEHC (μM) | Whole plasma | Extracted plasma | No enzyme hydrolysis |
|---|---|---|---|
| Sulfatase hydrolysis | |||
| aTotal | 0.22 ± 0.093 | 0.73 ± 0.16* | - |
| bFree | - | - | 0.022 ± 0.038 |
| cConjugated | 0.20 ± 0.056 | 0.71 ± 0.12* | - |
The observation that γ-CEHC exists primarily in the conjugated form in rat plasma is consistent with previous studies reporting that only conjugated CEHC but not free CEHC can be detected in rat urine [6; 26; 27]. Consistently, Tanabe et al. showed that γ-CEHC disappeared rapidly from plasma after intravenously administered and was found mainly as sulfated CEHC in the urine [27]. The high concentrations of conjugated CEHC found in the plasma suggests that CEHCs, upon generated from vitamin E by β-oxidation, may be readily conjugated in the liver and then excreted to the urine. A previous study reported that 30-40% CEHC was in the conjugated form in the liver [18]. This study, in light of our current finding, may underestimate the conjugated CEHC because sulfatase/glucuronidase hydrolysis was directly applied to the liver homogenate without any clean-up procedure. Further studies are warranted to examine the efficacy of sulfatase/glucuronidase hydrolysis toward tissue homogenates.
Although the present study focuses on γ-CEHC conjugates, the similar extraction and hydrolysis procedure may be used to deconjugate α-CEHC conjugates because both metabolite conjugates are similarly hydrolyzed by sulfatases/glucuronidase [9; 10; 18]. In addition, Cho et al [14] recently identified a new conjugated metabolite of γ-CEHC, i.e., γ-CEHC glucoside, in mouse urine. This metabolite appears to be readily deconjugated by Type H-1 β-glucuronidase from Helix pomatia in addition to glucosidase [14]. Thus, it is conceivable that the extraction procedure developed in the present study may be applied to deconjugate this metabolite in the plasma.
In summary, we showed that the complete deconjugation of conjugated CEHC and sulfated long-chain carboxychromanols in the plasma requires a clean-up procedure with methanol/hexane extraction to remove proteins and lipids, in addition to the subsequent overnight enzyme hydrolysis. Without the clean-up procedure, plasma conjugated CEHC is largely underestimated. This procedure may be useful for analysis of other conjugated metabolites in the plasma.
Acknowledgments
We would like to thank Xinmin Yin for the help with cell-culture and animal studies. This work was supported, in part, by National Institute of Health Grants R01AT001821 and NIH-P01AT002620.
Abbreviations
- α-T, β-T, γ-T, or δ-T
α,β,γ, or δ-tocopherol
- α-TE, β-TE, γ-TE, or δ-TE
α,β,γ, or δ-tocotrienol
- CEHC
2-(β-carboxyethyl)-6- hydroxychroman
- 9′-, 11′- and 13′-COOH
9′-, 11′- and 13′-carboxychromanol
- 9′S, 11′S, and 13′S
sulfated 9′-, 11′- and 13′-carboxychromanol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–8. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 2.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. Faseb J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 3.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–22. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 4.Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann N Y Acad Sci. 2004;1031:13–21. doi: 10.1196/annals.1331.002. [DOI] [PubMed] [Google Scholar]
- 5.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 6.Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. J Lipid Res. 1984;25:40–8. [PubMed] [Google Scholar]
- 7.Hattori A, Fukushima T, Yoshimura H, Abe K, Imai K. Production of LLU- alpha following an oral administration of gamma-tocotrienol or gamma-tocopherol to rats. Biol Pharm Bull. 2000;23:1395–7. doi: 10.1248/bpb.23.1395. [DOI] [PubMed] [Google Scholar]
- 8.Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG. Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids. 2001;36:43–8. doi: 10.1007/s11745-001-0666-z. [DOI] [PubMed] [Google Scholar]
- 9.Pope SA, Burtin GE, Clayton PT, Madge DJ, Muller DP. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic Biol Med. 2002;33:807–17. doi: 10.1016/s0891-5849(02)00974-7. [DOI] [PubMed] [Google Scholar]
- 10.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr. 1995;62:1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- 11.Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8- trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40:665–71. [PubMed] [Google Scholar]
- 12.Traber MG, Elsner A, Brigelius-Flohe R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145–8. doi: 10.1016/s0014-5793(98)01210-1. [DOI] [PubMed] [Google Scholar]
- 13.Wechter WJ, Kantoci D, Murray ED, Jr, D'Amico DC, Jung ME, Wang WH. A new endogenous natriuretic factor: LLU- Proc Natl Acad Sci U S A. 1996;93:6002–6007. doi: 10.1073/pnas.93.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho JY, Wook Kang D, Ma X, Ahn SH, Krausz KW, Luecke H, Idle JR, Gonzalez FJ. Metabolomics reveals a novel vitamin E metabolite and attenuated vitamin E metabolism upon PXR activation. J Lipid Res. 2009 doi: 10.1194/jlr.M800647-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxychroman and sulfated long-chain carboxychromanols in rats. J Nutr. 2009 doi: 10.3945/jn.108.103309. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221–30. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A. 2008;105:20464–9. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard SW, Gumpricht E, Devereaux MW, Sokol RJ, Traber MG. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J Lipid Res. 2005;46:1068–75. doi: 10.1194/jlr.D400044-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Lodge JK, Traber MG, Elsner A, Brigelius-Flohe R. A rapid method for the extraction and determination of vitamin E metabolites in human urine. J Lipid Res. 2000;41:148–54. [PubMed] [Google Scholar]
- 20.Stahl W, Graf P, Brigelius-Flohe R, Wechter W, Sies H. Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7, 8-tetramethyl-2-(2'-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl-2-(2'-carboxyethyl)-6-hydroxychroman in human serum. Anal Biochem. 1999;275:254–9. doi: 10.1006/abio.1999.4312. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JI, Grace PB, Bingham SA. Optimization of conditions for the enzymatic hydrolysis of phytoestrogen conjugates in urine and plasma. Anal Biochem. 2005;341:220–9. doi: 10.1016/j.ab.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Valentin-Blasini L, Blount BC, Rogers HS, Needham LL. HPLC-MS/MS method for the measurement of seven phytoestrogens in human serum and urine. J Expo Anal Environ Epidemiol. 2000;10:799–807. doi: 10.1038/sj.jea.7500122. [DOI] [PubMed] [Google Scholar]
- 23.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–94. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 24.Dou C, Bournique JS, Zinda MK, Gnezda M, McNally AJ, Salamone SJ. Comparison of the rates of hydrolysis of lorazepam-glucuronide, oxazepam-glucuronide and tamazepam-glucuronide catalyzed by E. coli beta-D-glucuronidase using the on-line benzodiazepine screening immunoassay on the Roche/Hitachi 917 analyzer. J Forensic Sci. 2001;46:335–40. [PubMed] [Google Scholar]
- 25.Shibasaki H, Tanabe C, Furuta T, Kasuya Y. Hydrolysis of conjugated steroids by the combined use of beta-glucuronidase preparations from helix pomatia and ampullaria: determination of urinary cortisol and its metabolites. Steroids. 2001;66:795–801. doi: 10.1016/s0039-128x(01)00118-0. [DOI] [PubMed] [Google Scholar]
- 26.Kiyose C, Saito H, Kaneko K, Hamamura K, Tomioka M, Ueda T, Igarashi O. Alpha-tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2 (2'-carboxyethyl)-6-hydroxychroman, gamma-tocopherol metabolite, in rats. Lipids. 2001;36:467–72. doi: 10.1007/s11745-001-0744-2. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe M, Fukushima T, Usui N, Aoyama N, Tsunoda M, Imai K. Intravenous administration of 2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxy chroman (gamma-CEHC) to rats and determination of its plasma concentration and urinary sodium excretion. Biomed Chromatogr. 2004;18:727–34. doi: 10.1002/bmc.385. [DOI] [PubMed] [Google Scholar]


